Translate this page into:
Liquid-based cytology: Technical aspects

*Corresponding author: Manjiri Milind Makde, Department of Pathology, Government Medical College, Nagpur, Maharashtra, India. majiri0288@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Makde MM, Sathawane P. Liquid-based cytology : Technical aspects. CytoJournal 2022;19:41.
Abstract
Liquid-based cytology (LBC) is a monolayer slide preparation technology that has outperformed conventional Pap smears because of improved fixation, decreased obscuring factors, and standardized cell transfer. In LBC, samples are collected by completely immersing the sampling device into the company vial containing preservative fluid, whereby the cells are preserved and fixed simultaneously unlike conventional smears where the sample is smeared onto the glass slide and fixed separately. To date, two major liquid-based preparation methods are known – ThinPrep and SurePath. These two methods are different in their principles of cell harvesting but produce similar preparations. SurePath works on the principle of density gradient sedimentation. In this, a sample is vortexed and strained to break the mucus and large cell groups and then is treated through a density gradient centrifugation process to remove blood and debris. The cell pellet is resuspended and is allowed to sediment onto a glass slide. This is followed by staining on the PrepStain instrument. Government Medical College and Hospital, Nagpur, India, uses the SurePath method which was approved by FDA in the USA in 1999. Our institution uses Rovers Cervex-Brush to collect the cells from the transformation zone. This chapter describes the principle of SurePath and the processing of cervicovaginal specimen using the fully automated system in the laboratory.
Keywords
Liquid-based cytology
SurePath
Cervex-Brush
Monolayer preparation

For many years, pathologists have worked to increase the sensitivity and specificity of the “conventional Pap smear.” Automated screening devices were introduced in cervical screening in the 1950s as an attempt to reduce the tedium faced by screeners in this error-prone task. However, the first few attempts failed as it was very difficult to measure dimensions of single cells manually and that too in a complex background. Hence, the pathologists emphasized on to create automated machines which would make representative monolayer cell samples on standardized slides readable by the automated screening devices. Deep research into this concept gave birth to the concept of liquid-based cytology (LBC). In addition to monolayer preparation, LBC resulted in complete and standardized cell transfer, decreased obscuring factors, improved fixation, and reduced screening time.
To date, two major preparation methods are known – ThinPrep and SurePath. In 1996, the FDA approved the use of ThinPrep as an alternative to the cervicovaginal smear in the United States. This was followed by approval to use Autocyte Prep (also called as SurePath or CytoRich Prep) in 1999. These two methods are different in their principle but produce similar preparations.
In LBC, samples are collected by completely immersing the sampling device into the company vial containing preservative fluid [Figure 1] unlike conventional smears where the sample is smeared on the slide and fixed separately. The preservative fluid-containing sample is then processed in a laboratory (using ThinPrep or SurePath) [Flowchart 1] and a smear representing monolayer cell sample with a clean background is formed. The Government Medical College and Hospital (GMCH), Nagpur, Maharashtra, makes use of BD SurePath™ machine for LBC preparation.
- Flowchart showing working principle of SurePath.

- Liquid-based cytology (SurePath) vial.
SUREPATH
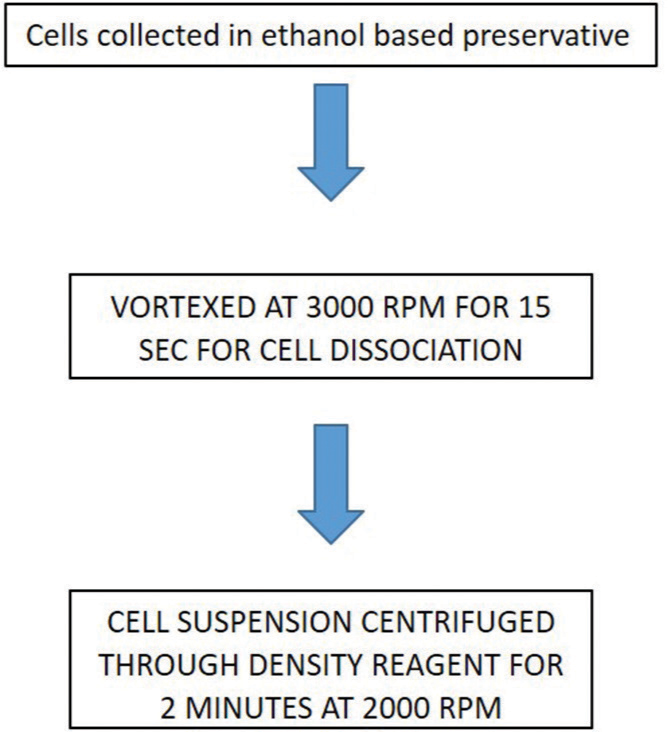
Our setup (GMCH) uses Rovers Cervex-Brush [Figure 2] to collect cervical samples. The clinician snips the tip of the collection device into the vial containing ethanol-based preservative [Figure 3] which preserves and fixes the sample simultaneously. BD SurePath™ works on the principle of density gradient sedimentation. In this, the cell/sample is vortexed and strained to break the mucus and large cell groups and then is treated through a density gradient centrifugation process to remove blood and debris. The cell pellet is resuspended and is allowed to sediment onto a glass slide.

- Rovers Cervex-Brush.

- Tip of collection device (Cervex-Brush) in the vial containing ethanol.
The steps followed in our laboratory are as follows:
Sample vials along with a requisition form containing complete details of the patient are received at the laboratory. Vials should be checked for labeled details and should be matched with the requisition form.
SPECIMEN HANDLING AND CELL ENRICHMENT
During this phase of the BD SurePath™ slide preparation process, the collected cervicovaginal material is randomized and the cellular content of the sample is enriched. In this process, the sample first goes through a series of manual steps that include:
Sample randomization: Received vials are vortexed at 3000 rpm for 30 s on the vortex machine [Figure 4]. This step allows the cells to get free from one another, and also, the cell clusters from the specimen collection device are set free
Mixing and layering: This process is accomplished automatically using PrepMate [Figure 5]. It is an automated accessory to the PrepStain system. The specimen rack of the PrepMate is loaded with a plunger, sample vial, and a centrifuge tube containing 4 ml of density reagent. PrepMate density reagent is a polysaccharide solution with sodium azide added as a preservative. The PrepMate automates the initial enrichment process of mixing and dispensing the specimen over the density reagent. A single specimen rack holds 12 samples at a time. Total three specimen racks are there for the machine (i.e., 48 samples are run at a time)
The PrepMate mixes and removes the specimen from the preservative vial and layers it onto the density reagent in the centrifuge tube. The PrepMate automated process handles from 1 to 12 specimens per cycle. Layering is the process in which 8 ml of sample is mixed with 4 ml of density reagent in a centrifuge tube present in the rack
The racks are removed from the PrepMate after mixing and layering the samples. The centrifuge tubes containing the sample and density reagent are transferred to the centrifuge machine [Figure 6a and b]. The tubes are centrifuged for 2 min and the supernatant is discarded. Small particulates and debris which are trapped above the interface between the supernatant preservative fluid and the density reagent are removed, enriching the clinical materials in the sample
The tubes are centrifuged again for 10 min and the supernatant is discarded by inverting tubes at 180°. This step concentrates the diagnostic cellular material at the bottom of the tube (pellet)
The pellet is vortexed for 30 s and placed on the PrepStain machine for further processing.

- Vortex device.

- PrepMate device.

- (a and b) Centrifugation machine.
SPECIMEN PROCESSING WITH THE PREPSTAIN SYSTEM
The PrepStain system performs the automated slide preparation and staining steps for the thin layer preparation of cytologic material on a BD SurePath™ pre-coat slide. The parts of the PrepStain system are as follows:
PrepStain settling chamber [Figure 7]: It serves as a vessel to contain resuspended cellular material while they settle onto the coated microscope slide
Computer workstation: The PrepStain instrument is linked to a DOS-based computer system. The software that runs the sample processor is started by typing command at the DOS prompt and then controlled using various menus
PrepStain instrument [Figure 8]: This performs automated sample transfer and staining steps
-
Robotic sample processor: It is a fully automated base instrument of the PrepStain system. It is a system of microprocessor-controlled liquid handling components controlled by system software located on the hard disk of a personal computer. This instrument resuspends the pelleted cell samples in buffered deionized water (DI) and transfers aliquots of cell suspensions settling chambers mounted on the BD SurePath™ pre-coated slides. After a particular incubation period, the processor performs a sequence of washings and staining steps to stain the slides by the Papanicolaou method. The principal components of the processor are listed below:
Syringe pump: It is a microprocessor-controlled syringe with a pump and two-way valve that connects to the quad tubing and a reagent container through the tubing. The PrepStain instrument has four syringe pumps to control aspiration and dispensing of the programmed volume of reagents and samples with high accuracy and at variable speeds
X/Y/Z movement mechanism (robotic arm): The robotic arm moves in the X (left-right), Y (forwards – backward), and Z (up and down) directions. It has Z-rod [Figure 9] which moves up and down
Disposable tip (DiTi) assembly: This is for aspiration and pipetting samples. PrepStain instrument uses a fresh pipetting tip to mix and aspirate each sample and transfer it to the settling chamber. The DiTi is mounted on the Z-rod.
Quad arm [Figure 10]: It is a system of pipettes, tubing, and manifolds that is mounted on the robotic arm of the PrepStain instrument. There are four pipette bundles on the quad arm that are positioned by the action of the robotic arm into four settling chambers in the slide rack. Each pipette bundle has four dispensing tips and one large vacuum tip. The vacuum tip empties the supernatant fluid in the settling chamber. The four dispensing tips can then apply one of four reagents to the chambers for stain and rinse sequence [Figure 11].
Waste station [Figure 12]: It is present on the left side of the instrument. During priming or cleaning of the system tubing, excess liquids are dispensed to the waste trough, which drains into a waste container for easy and safe disposal. After use, DiTi is also discarded into the waste container.
Slide racks and work platform [Figure 13]: The work platform holds the slide racks and mounts to the right of the waste station. Each slide rack has a four-row by a three-column array of glass slide positions. The work platform and slide racks are numbered 1–4 and pinned so that each slide rack will fit only into its correspondingly numbered position. Each slide is mounted on the rack under a settling chamber. The settling chamber seal and the slide form a barrier that prevents leaking when the chamber is filled with liquid. The sedimentation of the cells onto the slide and staining takes place in the settling chamber. After staining, the settling chamber is removed and discarded. The slide is cleaned and the cover slipped for screening.

- PrepStain settling chambers.

- (a and b) PrepStain device.

- Z-rod (arrow) on the robotic arm.

- Quad arm with four pipette bundles.

- Stain and rinse cycle.

- Waste station with pipetting tips (blue arrow) and waste container (yellow arrow).

- Slide racks and work platform.
STAINING WITH PREPSTAIN INSTRUMENT
Slides labeled with respective numbers of the vial are arranged on the staining rack of PrepStain machine
Priming of PrepStain machine is done before giving the command for staining
First, 1000 µl of buffered DI water is added to the cell pellet
Then, the instrument picks up a DiTi and mixes the resulting solution by flushing it in and out of the disposable pipette tip 8 times
Next, the instrument aspirates 200 µl of the sample from the centrifuge tube and injects it into the settling chamber, and then washes out the tip with 600 µl of buffered DI water. The tube and the remaining specimen can be discarded or retained for adjunctive testing
The sample is allowed to settle onto the slide for a minimum of 10 min. During this time, cells bonding with the PrepStain slide coating form a thin layer of cells
The instrument then adds a 600 µl alcohol wash to the sample and evacuates all remaining fluids
The sample is then allowed to dry for approximately 60 s
The last part of the automated processing is a sequence of stain and rinse cycles
The PrepStain system produces a uniform layer of stained cells in a 13 mm diameter circle [Figure 14]. The sample layer contains single cells or small clusters
After completion of staining, staining racks are removed.

- BD SurePath slide with monolayered 13 mm diameter circle smear.
THINPREP
Principle
In this procedure, samples are collected in a vial containing a filter and methanol-based preservative (provided by the company) [Figure 15]. The vials are placed one at a time on the semi-automated ThinPrep machine. The filter within the vial rotates mechanically dispersing cells, mucus, blood, and debris. This suspension of cells is passed through a filter made up of neutral polycarbonate. The flow of this suspension is constantly monitored to get an optimal quantity of cells. The cells trapped onto the filter surface are automatically transferred to a glass slide and fixed immediately.

- ThinPrep vial.
TP-2000 processor [Figure 16] is a semi-automated device, which processes one specimen at a time. A newer version, TP-5000 [Figure 17a and b], is a fully automated bench-top instrument that processes specimens in batches of 20. Multiple preparations can be made from a single vial. Fixation of sample collected by the clinician is done in CytoLyt solution (methanol-based fixative, which is both hemolytic and mucolytic). The specimen is labeled and transported to the cytology laboratory. The microscopic slides are provided by the company and are marked with a 20 mm diameter circle. The vial and a labeled slide are placed into the ThinPrep processor. Preparatory steps include specimen dispersion, collection, transfer, and staining.

- TP-2000 processor.

- (a and b) TP-5000 processor.
Dispersion
A disposable cylinder with a polycarbonate filter attached to one end is introduced into the vial. The pore size of the filter is 8 µm. The instrument is rotated creating a current that disaggregates blood, mucus, and other debris and breaks up large cell clusters, mixes, and homogenizes the cell suspension [Figure 18a].

- (a-c) ThinPrep cell dispersion, cell collection, and cell transfer.
Collection
A gentle vacuum is applied to the cylinder, which aspirates the cell suspension through the filter [Figure 18b]. Most of the broken red blood cells and debris are allowed to pass through while the diagnostic cells attach to the external surface of the filter. The instrument monitors cell density across the filter and the flow rate decreases when cells are evenly distributed on the filter with minimal overlap.
Transfer
The cylinder moves out of the specimen, is inverted 180°, gently pressed against a positively charged slide, and with slight positive pressure transfers the cells to the glass slide [Figure 18c]. The result is a 20 mm circular smear with even distribution of cells and minimal overlap.
Staining
Papanicolaou staining is either performed manually or in an automatic stainer. The staining process takes 30 min. Papanicolaou stain of fixed samples offers the best option of judging the fine details of cell structure.
Residual LBP specimen
The shelf life of the residual specimen for SurePath and ThinPrep is 3 weeks and 3 months, respectively, at room temperature. A residual specimen can be used for immunochemistry, molecular tests, or processed as a cell block.
ABBREVIATIONS IN ALPHABETICAL ORDER
DI – deionized water
DiTi – Disposable tip
GMCH – Government Medical College and Hospital
LBC – liquid-based cytology
RPM – Revolutions per minute
s – second.
References
- Liquid based cytology for cervical screening In: Underwood J, Pignatelli M, eds. In Recent Advances in Histopathology. Vol Vol 22. London: The Royal Society of Medicine Press Limited; 2007. p. :127-44.
- [Google Scholar]
- Automated screening of cervical cytology specimens. Hum Pathol. 1996;27:468-81.
- [CrossRef] [Google Scholar]
- Operator’s Manual-prepstain Slide Processor- 780-13000-00 Rev D. United States: Scribd Inc.; 2014.
- [Google Scholar]








