Translate this page into:
Utility of anticoagulation, pre-smearing and post-smearing hemolytic techniques on morphological assessment and reproducibility in fluid cytology

*Corresponding author: Sana Ahuja, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India. sanaahuja11@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Agrawal M, Lata P, Singh M, Lal MK, Gupta B, Shamsunder S, et al. Utility of anticoagulation, pre-smearing and post-smearing hemolytic techniques on morphological assessment and reproducibility in fluid cytology. CytoJournal 2024;21:9. doi: 10.25259/Cytojournal_51_2023
Abstract
Objective:
Knowledge of proper collection, storage, preservation, and processing techniques is critical to ensuring proper handling and analysis of fluid cytology specimens. This study was conducted to determine the effect of anticoagulation, pre-smearing acetic acid treatment technique, and saline rehydration technique on morphological assessment, reproducibility, and reporting in fluid cytology.
Material and Methods:
The study was carried out in the cytopathology laboratory over 2 months (April–May 2022), where 100 effusion samples were analyzed. At least 20–40 mL of fluid was collected in heparinized and non-heparinized containers for each patient. Samples were processed in cytospin and stained with Giemsa and Papanicolaou stains. For 70 hemorrhagic specimens, an extra smear was prepared from the sediment and subjected to the saline rehydration technique as per the Indian Academy of Cytologists (IAC) guidelines. Seventy-three hemorrhagic specimens whose quantity received was more than 35 mL were subjected to the pre-smearing technique. These smears were evaluated for (a) the presence or absence of blue background/any other background staining, (b) cellularity, (c) cell morphology and (d) the presence/absence of microclots.
Results:
Heparinized samples showed no compromise in cellular morphology or cellularity although a blue background was observed in an occasional case. The pre-smearing technique had less background hemorrhage and preserved cell characteristics. The post-smearing saline rehydration technique did not compromise the cellularity but distorted morphology and showed background staining.
Conclusion:
The pre-smearing acetic acid treatment showed better-preserved cellularity and cytomorphology with the absence of background staining when compared to the post-smearing saline rehydration technique.
Keywords
Heparinized
Glacial acetic acid
Saline rehydration
Fluid cytology
INTRODUCTION
Fluid cytology is an indispensable investigation in the workup of body cavity effusion specimens. It is helpful in both benign (including infectious etiology) and malignant effusions. Although these have been among the most common specimens, yet there was no consistent and reproducible reporting system earlier. The Indian Academy of Cytologists (IAC) published guidelines for the collection, preparation, interpretation, and reporting of serous effusion specimens.[1] The three main categories of these guidelines are essential, optimal, and optional.
The essential guidelines are absolute and non-negotiable; thus, they are followed at whichever center processes and accepts cytology specimens. However, the “optimal” guidelines are still not practiced as a routine. Further, as more and more centers are becoming aware of quality implementation and undergoing accreditation (including ours), the application of optimal guidelines will soon be inevitable. This study was conducted to determine the effect of anticoagulation, pre-smearing acetic acid treatment technique, and saline rehydration technique on morphological assessment, reproducibility, and reporting in fluid cytology.
MATERIAL AND METHODS
The study was carried out in the Cytopathology Laboratory over 2 months (April–May 2022) where 100 effusion samples were analyzed. These included both exudates and transudates-pleural fluid, ascitic fluid, and peritoneal washings. Both neoplastic and non-neoplastic effusions were included in the study.
At least 20–40 mL of fluid was collected for each patient in heparinized containers. Heparin of 3 units/mL was prepared as per standard guidelines[2] and pre-added to the sample collection containers to ensure prompt anticoagulation. About 20 mL of fluid was added to these containers. Any additional fluid collected was sent in separate nonheparinized containers.
Samples were processed in cytospin.[3] Centrifugation was done for 6 RPM/RCF-X 100 for 10 min. Smears were stained with Giemsa and Papanicolaou stains, mounted, and examined.
For 70 hemorrhagic specimens, an extra smear was prepared from the sediment and subjected to the saline rehydration technique as per the IAC guidelines.[1]
The Indian academy of cytology (IAC) guidelines are divided into three categories as follows:
Essential: These are absolute and non-negotiable guidelines, and if a laboratory cannot achieve this, then the sample may be referred to a center which is equipped to fulfill these criteria
Optimal: This is achievable in any good laboratory and should be part of the protocol and can be made mandatory for accreditation
Optional: This recommendation is resource-dependent and is left to the choice of the individual laboratory.[1]
73 specimens whose quantity received was more than 35 mL were subjected to a pre-smearing technique as per the IAC guidelines. Briefly, 1 mL of 1% acetic acid was added to the specimens which were then centrifuged. The sediment was washed with phosphate-buffered saline and the smears prepared were evaluated as follows.
These smears were independently reviewed by two pathologists and evaluated for the following parameters: (a) The presence or absence of blue background/any other background staining, (b) cellularity, (c) cell morphology, and (d) the presence or absence of microclots.
RESULTS
A total of 100 specimens were included in the study. Of these, 57 and 43 were transudates and exudates, respectively. Heparinized samples were available for all the cases. Of the smears from 100 specimens, 73 were hemorrhagic, and pre-smearing acetic acid treatment was applied to these specimens. The post-smearing saline rehydration technique could be applied to 70 specimens due to the limitation of sample volume.
Heparinized specimens: In the smears prepared from heparinized specimens, only one case showed the presence of a significant blue background. In all other smears, there was no compromise in cellularity or cellular morphology between heparinized and non-heparinized samples. In addition, 16/100 (16%) non-heparinized specimens showed the presence of microclots with entrapped cells. These included both neoplastic and non-neoplastic samples. However, an accurate concordant diagnosis was achieved in all these cases due to the presence of multiple smears and/or cell blocks [Figure 1].
![Non-heparinized versus heparinized samples in fluid cytology (a) Heparinized samples show a considerable reduction in the microclots although a blue background (marked with yellow arrow) was seen in few of the heparinized samples. [May Grunwald Giemsa, 400x] (b) Heparinized samples show a considerable reduction in the microclots. [May Grunwald Giemsa, 100x] (c) Non-heparinized samples show more microclots as compared to the heparinized samples. [May Grunwald Giemsa, 100x].](/content/105/2024/21/1/img/Cytojournal-21-9-g001.png)
- Non-heparinized versus heparinized samples in fluid cytology (a) Heparinized samples show a considerable reduction in the microclots although a blue background (marked with yellow arrow) was seen in few of the heparinized samples. [May Grunwald Giemsa, 400x] (b) Heparinized samples show a considerable reduction in the microclots. [May Grunwald Giemsa, 100x] (c) Non-heparinized samples show more microclots as compared to the heparinized samples. [May Grunwald Giemsa, 100x].
Pre-smearing glacial acetic acid treatment: Out of the 100 specimens, the pre-smearing technique was applied in 73 cases. In most of these cases (70/73, 96%), there was less background hemorrhage, and cell characteristics were preserved. Furthermore, the work could be easily incorporated into the routine workflow of the laboratory in terms of the processing time per case. In contrast to saline rehydration, there was less background haziness, cell morphology was better preserved and reduced eye strain considerably [Figure 2].

- Pre-smearing acetic acid treatment (a) showed hemolysis of RBCs with a clean background as compared to the control slide (May Grunwald Giemsa, 400x) (b) which shows debris in the background (May Grunwald Giemsa, 400x).
Saline rehydration technique: Among the 70 specimens which were subjected to saline rehydration, all the smears showed lysis of red blood cell (RBC); however, there was haziness, background staining, and disruption of cell morphology in 19/70 (27%) cases. In all of these cases, the processing time per specimen was increased, and dedicated manpower was needed for consistent coordination. Thus, the stringent time control added to the workload of the laboratory. The remaining 51 (73%) smears showed a reduction in background RBCs, relative preservation of cell morphology, and thus reduced eye strain to some extent [Figure 3 and Table 1].
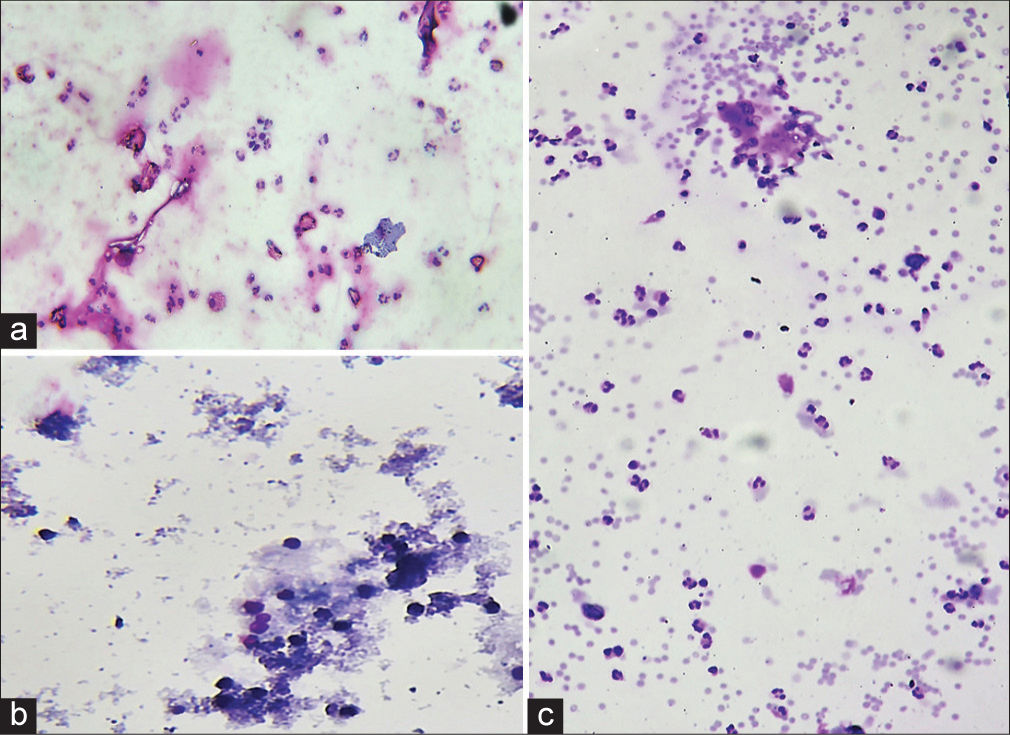
- Saline rehydration technique showed hemolysis of RBCs, however, background haziness and distortion of cells (a) (May Grunwald Giemsa, 400x), (b) (May Grunwald Giemsa, 100x) as compared to (c) control slide (May Grunwald Giemsa, 100x).
| Heparinized Samples (n=100) |
Pre-smearing acetic acid technique (n=73) | Post-smearing saline rehydration technique (n=70) | |
|---|---|---|---|
| Positive observations | • Accurate cell morphology • Reduced number of microclots in 16/100 (16%) samples • No compromise on cellularity in cases |
• Preserved cell morphology in most cases • No background staining • No compromise on cellularity • No microclots • Easier to do |
• Lysis of RBC; so less hemorrhagic background • No compromise on cellularity • No microclots |
| Negative observations | • Blue Background in 01/100 (0.1%) cases | - | • 19/70 (27%) cases show distorted morphology • Background staining present in most cases |
RBC: Red blood cell
DISCUSSION
Knowledge of proper collection, storage, preservation, and processing techniques is critical to ensuring proper handling and analysis of fluid cytology specimens. In this regard, techniques to analyze and process the specimens such as direct smears, cytospin, liquid-based cytology, and cell blocks are well described. In this context, guidelines provide an important framework, based on which quality of patient care services can be improved.
The IAC guidelines for the collection, preparation, interpretation, and reporting of serous effusion fluid samples were published in January 2020.[1] These provide a systematic, point-by-point approach and a standard format for a fluid report.
The guidelines have been categorized into essential, optimal, and optional categories. The essential guidelines are a basic requirement for any laboratory that accepts, processes, and reports these specimens. However, for a center like ours which is also a large institutional laboratory and is now rigorously following national accreditation protocols, following the “optimal” category of guidelines with the same rigor is the need of the hour.
It is well known that body cavity fluids have a higher concentration of thrombin and fibrinogen than blood.[2] As a consequence, cells often get entrapped in microclots, thus leading to a substantial loss of diagnostic cells. Our laboratory has been processing and analyzing non-anticoagulated fluid specimens.[3] The number of specimens being received earlier was fewer, transportation time was faster, and structured guidelines were not available. With the drastic increase in the workload, availability of structured guidelines, and the laboratory itself being accredited it becomes mandatory for us to follow standard set guidelines for better patient care services and better quality implementation.
In this study, the addition of 3 units per mL of heparin to fluid specimens, showed a reduction in microclots in 16 out of 100 (16% cases). At the same time, there was no significant change in cell morphology or compromise in cellularity in any case.
However, in all of these cases, a required concentration of heparin had to be prepared and the containers had to be present to the clinician. In a few instances, when these containers were not available, there was no means of accurate dilution in the wards failing which an arbitrary concentration of heparin was frequently added to the containers.
Pre-treatment of specimens with 1% glacial acetic acid and subsequent washing of cells yielded good results in hemorrhagic specimens. There was an improvement in the ease of reporting in both transudative and exudative effusions, as observed by the reporting pathologists. There could be two possible reasons for this: One, interpreting cellular details without background hemorrhage was easier. Second, the simultaneous evaluation and comparison of two categories of smears-those with and without acetic acid pre-treatment would have added to the confidence in reporting doubtful smears.
Smears which underwent saline rehydration technique showed preserved cell morphology in 51/70 (73%) cases. Although it reduced eye strain to some extent, it disrupted cell morphology in 19/70 (27%) cases. A fair fraction of these cases showed damage to the diagnostic cells, in addition to damaging the RBCs. A plausible explanation for this is the strict time, treatment, and processing control that is needed with these smears. Ng et al. also assessed 11 grossly hemorrhagic samples and observed complete lysis in all cases.[4]
For reliable cytopathological evaluation, the Papanicolaou stain would be critical for the evaluation of malignancy. Although Romanowski stain is critical in addition to Papanicolaou stain for evaluation of effusion fluids, rehydration for eluting out hemoglobin in contaminant erythrocytes is not applicable to Romanowski staining such as Giemsa evaluated in the present study.
About lysis of blood contamination, acetic acid would compromise the immunoreactivity so a better approach is to lyse the blood contamination with an ammonium chloride-based lysis agent. In most cases, cell-block is recommended and if prepared, immunocytochemistry may be indicated. However, ammonium chloride-based lysis used for flow cytometry analysis will not compromise the immunoreactivity.[4]
SUMMARY
Overall, the use of “optimal” guidelines as and when applicable has added to the ease and comfort of reporting fluid cytology. Some practical problems encountered in the application of the same are regular availability of heparinized containers with appropriate concentration at the bedside, maintenance of strict timing and control for saline rehydration technique, and random errors which may be related to intrinsic fragility of cells. These problems can be easily overcome by being aware of the guidelines and regular implementation of the same.
AVAILABILITY OF DATA AND MATERIALS
The data is available from the corresponding author on request.
ABBREVIATIONS
IAC - Indian Academy of Cytologists
RBC - Red blood cells
AUTHOR CONTRIBUTIONS
MA: Conceptualization, data curation, formal analysis, methodology, writing original draft; LP: Data curation, methodology; MS: Conceptualization, data curation, formal analysis, supervision; ML: Data curation, resources; BG: Data curation, resources; SS: Data curation, resources; Si: data curation, resources; NKM: Data curation, resources; SA: Data curation, writing review and editing; SR: Data curation, resources, supervision. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The analysis was done retrospectively hence it was exempted from Ethical approval.
Patient’s consent is not required as there are no patients in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Indian academy of cytologists guidelines for collection, preparation, interpretation, and reporting of serous effusion fluid samples. J Cytol. 2020;37:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-analytical issues in effusion cytology. Pleura Peritoneum. 2016;1:45-56.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation of cells for microscopy using cytospin. Methods Enzymol. 2013;533:235-40.
- [CrossRef] [PubMed] [Google Scholar]
- Rehydration in air-dried smears with normal saline application in fluid cytology. Acta Cytol. 1994;38:56-64.
- [Google Scholar]








