Translate this page into:
A case of lung adenocarcinoma expressing carbonic anhydrase IX and cluster of differentiation 10 antigen combined with renal clear cell carcinoma


*Corresponding author: Guohua Yu, Department of Pathology, Yantai Yuhuangding Hospital, Yantai, Shandong Province, China. guohuayu@qdu.edu.cn
Hengming Zhang, Department of Pathology, Weifang People’s Hospital, Weifang, Shandong Province, China. Email: 13696364618@163.com
-
Received: ,
Accepted: ,
How to cite this article: Wang Y, Wang T, Zhang H, Yu G. A case of lung adenocarcinoma expressing carbonic anhydrase IX and cluster of differentiation 10 antigen combined with renal clear cell carcinoma. CytoJournal. 2024;21:62. doi: 10.25259/Cytojournal_106_2024
Abstract
Few reports exist on dual primary lung and kidney tumors. Lung adenocarcinoma and renal clear-cell carcinoma are usually distinguished based on histological morphology and characteristic immunohistochemistry. This study describes a patient with concurrent lung adenocarcinoma and clear cell renal cell carcinoma, notable for their shared expression of both carbonic anhydrase IX (CA9) and cluster of differentiation 10 (CD10) markers, a phenomenon that has remained unreported in existing literature. We made a correct diagnosis based on histological morphology and immunohistochemical results, discussed the significance of CA9 and CD10 expression in lung adenocarcinoma, and provided insights for differential diagnosis, intending to offer pathologists relevant insights for diagnosing multiple primary malignant neoplasms.
Keywords
carbonic anhydrase IX
cluster of differentiation 10 antigen
adenocarcinoma of lung
renal cell carcinoma
multiple primary neoplasms
INTRODUCTION
In the field of oncology, multiple primary malignant neoplasms (MPMNs) have emerged as a complex clinical phenomenon with an increasing incidence rate in recent years that has gained attention. Among these, the combination of lung adenocarcinoma and renal clear cell carcinoma represents a relatively rare type of multiple primary cancer, posing challenges in diagnosis, treatment, and prognostic evaluation.
Carbonic anhydrase IX (CA9), a transmembrane protein, is often overexpressed in hypoxic and acidic tumor microenvironments and is widely recognized as an important biomarker for various tumors, particularly clear cell renal cell carcinoma (ccRCC).[1] Studies have shown that high CA9 expression is closely associated with tumor progression, recurrence, and poor prognosis in ccRCC.[2] Cluster of differentiation 10 (CD10), another important immunohistochemical marker, is almost always positively expressed in renal cells and holds great value in diagnosing ccRCC. However, the expression patterns and clinical significance of CA9 and CD10 in lung adenocarcinoma require further investigation.[3]
This study reports a rare lung adenocarcinoma concomitant with renal clear cell carcinoma, focusing on the expression of CA9 and CD10 in tumor tissues of patients through immunohistochemical analysis. The patient was diagnosed with both lung adenocarcinoma and renal clear cell carcinoma, which originated from different epithelial tissues but exhibited distinct expression patterns of CA9 and CD10. This study aimed to deepen the understanding of the differences in biomarker expression among different tumors in multiple primary cancers through a thorough analysis and discussion of this patient, providing a reference for the formulation of clinical diagnosis and treatment strategies.
CASE REPORT
The patient was a 75-year-old male. He presented with paroxysmal cough and coughing of blood for no apparent reason 3 months before presentation. He sought medical attention at a local hospital, where imaging studies revealed a lung mass. The patient was transferred to another hospital on April 21st, 2023, to pursue additional treatment. On physical examination, no notable abnormalities were detected. Still, tumor marker tests showed elevated levels of carcinoembryonic antigen at 246 ng/mL, neuron-specific enolase 30.2 ng/mL, cytokeratin 21–1 20.8 mL/mg, carbohydrate antigen 125 at 971 U/mL, and carbohydrate antigen 72–4 37.1 U/mL, all above the reference values. Enhanced chest computed tomography (CT) revealed an irregular soft-tissue density shadow adjacent to the right hilum of the upper lobe of the right lung, with indistinct borders measuring 7.9 cm × 5.4 cm [Figure 1a]. Multiple enlarged lymph nodes were observed in the right supraclavicular fossa, right axilla, mediastinum, and right hilum, with the largest node having a short diameter of 1.9 cm. This finding was suggestive of lung cancer with lymph node metastases in the right supraclavicular fossa, right axilla, mediastinum, and right hilum (Tumor stage: T4N2M0). Abdominal CT showed a slightly hyperdense mass with clear borders measuring 5 cm × 4.6 cm in the left kidney, with patchy areas of low density. During the arterial phase of contrast enhancement, there was obvious heterogeneous enhancement, whereas the degree of enhancement was reduced during the venous and delayed phases, indicating possible renal clear-cell carcinoma [Figure 1b]. No abnormalities were observed. The patient underwent a CT-guided biopsy of the right lung and left kidney tumors. Smoking History: The patient had a history of smoking for 20 years, with an average daily consumption of 20 cigarettes. The patient had successfully abstained from smoking for the past 10 years. Family History of Genetic Conditions: The patient’s parents had deceased, and the patient denied any familial history of genetic conditions.
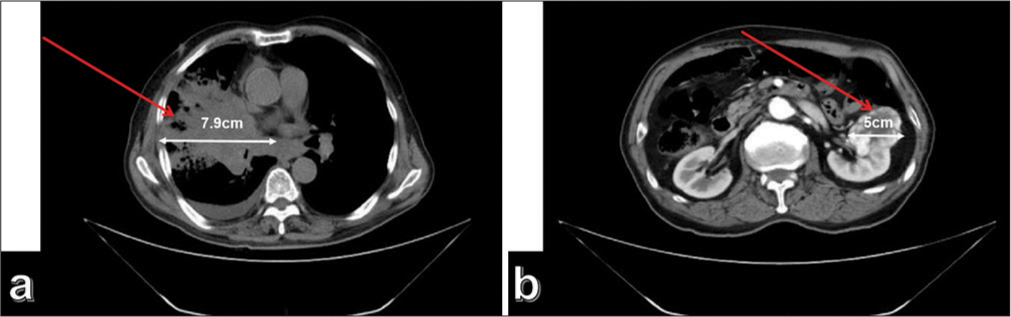
- (a) Computed tomography (CT) shows a space-occupying lesion measuring approximately 7.9 cm × 5.4 cm in the right upper lobe of the lung near the hilar region (The red arrow indicates the location of the tumor); (b) CT showed a space-occupying lesion measuring approximately 5 cm × 4.6 cm in the left kidney. The arrows in the image indicate the location and extent of the tumor (The red arrow indicates the location of the tumor).
Pathological examination: (1) Lung biopsy revealed tumor cells arranged in a nest-like pattern under a microscope. The tumor cells had large cell volume, abundant eosinophilic cytoplasm, irregularly shaped, dark-stained nuclei with visible nucleoli, coarse chromatin particles, and occasional nuclear division [Figure 2a]. Immunohistochemical staining results showed positive expression of thyroid transcription factor-1 (TTF-1) [Figure 2b], napsin A [Figure 2c], CA9 [Figure 2d], and CD10 [Figure 2e] in the tumor cells, while Paired box 8 (PAX-8)[Figure 2f] was not expressed. (2) Renal biopsy results indicated that the tumor cells were arranged in a cribriform pattern under a microscope, with thin-walled capillaries around them. Hemorrhage and cystic changes were observed in the tumor tissues. The tumor cells had large cell volume, translucent cytoplasm, well-defined cell boundaries, round or oval-shaped nuclei, and moderately sized nucleoli [Figure 3a]. Immunohistochemical staining results showed positive expression of CA9 [Figure 3b], PAX-8 [Figure 3c], and CD10 [Figure 3d] in the tumor cells, whereas TTF1 [Figure 3e], napsin A [Figure 3f], Cytokeratin 7 [Figure 3g], and P504S [Figure 3h] were not expressed.
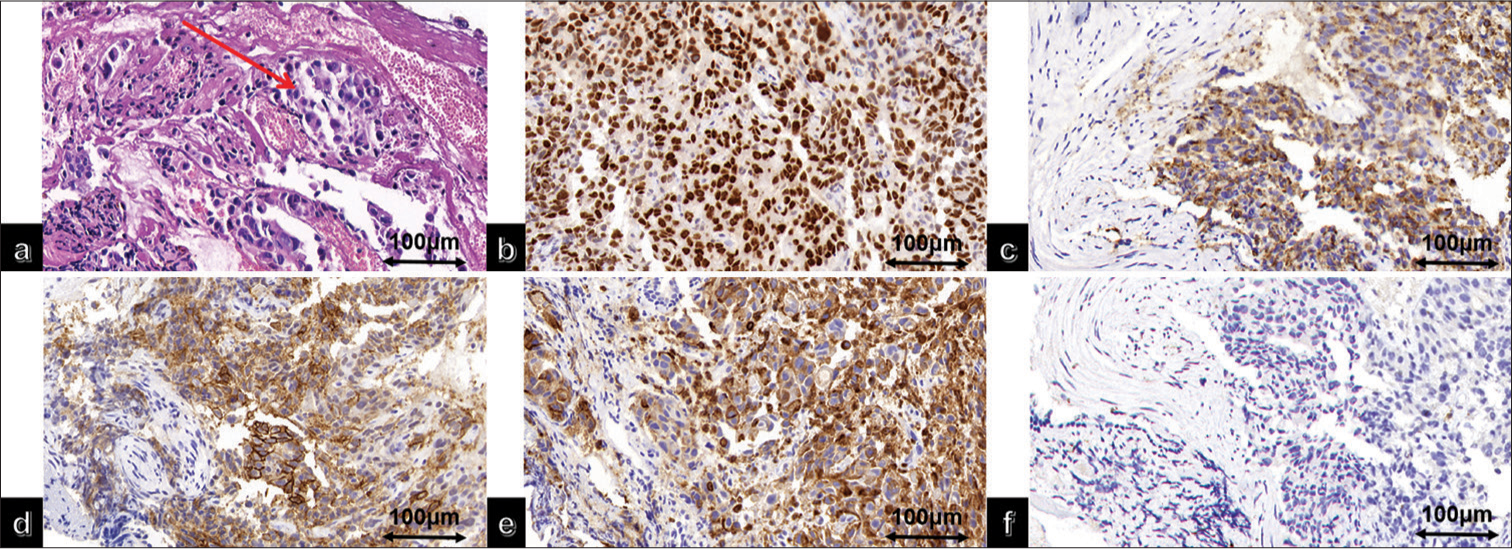
- (a) High magnification of lung adenocarcinoma hematoxylin and eosin (The red arrow indicates tumor cells); immunohistochemical staining of lung adenocarcinoma showed positive (b) thyroid transcription factor-1, (c) Napsin A, (d) carbonic anhydrase IX, (e) cluster of differentiation 10, (f) and negative Paired box gene 8 (PAX-8).
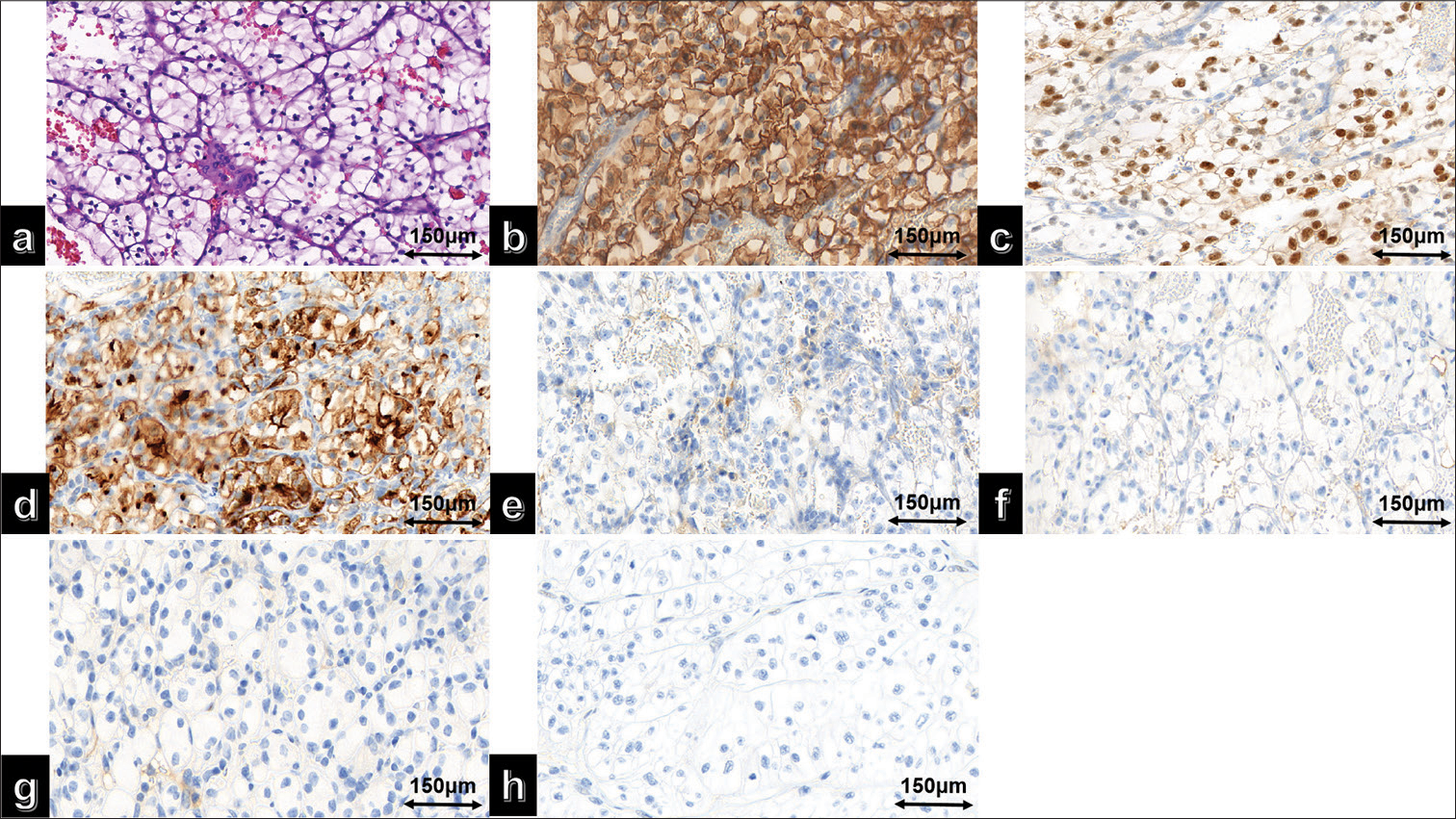
- (a) High magnification of clear cell renal cell carcinoma hematoxylin and eosin; (b) immunohistochemical staining results showed positive expression of carbonic anhydrase IX, (c) Paired box gene 8 (PAX-8), (d) and cluster of differentiation 10 in the tumor cells. In contrast, (e) thyroid transcription factor-1, (f) Napsin A, (g) Cytokeratin 7 (CK7), and (h) Alpha-Methylacyl-CoA Racemase (P504S) were not expressed.
Treatment and follow-up: The patient did not undergo surgery and was discharged with targeted treatment using gefitinib and self-administered traditional Chinese medicine, the details of which are unknown. Follow-up examinations were not performed. Six months after discharge, the patient developed symptoms of hemoptysis and hematuria, died of respiratory failure 1 month later, and signed an informed consent form before discharge expressing their willingness to contribute to this study and authorizing the publication of the research findings in the form of a comprehensive case report.
Ethics committee approval was obtained for the manuscript. Patients provided informed consent, which was reviewed by the ethics committee, and it was certified that the study was performed following the ethical standards laid down in the 2013 Declaration of Helsinki.
DISCUSSION
CA9, known as CA9, belongs to the carbonic anhydrase enzyme family and is expressed in the human gastric mucosa, bile ducts, and small intestine. Moreover, it is expressed in malignant tumors such as renal cell carcinoma, colorectal cancer, breast cancer, and lung cancer. CA9 is a transmembrane protein composed of 459 amino acids with four main domains: an N-terminal proteoglycan-like domain, a CA catalytic domain (CA), a single-pass helix transmembrane region, and a short intracellular tail. CA9 is primarily localized on the surface of tumor cell membranes, particularly overexpressed on the surfaces of hypoxic solid tumor cells. As a downstream molecule of the hypoxiainducible factor pathway, CA9 can convert carbon dioxide into bicarbonate and protons in the body, acidifying the extracellular environment and leading to poor prognosis in patients with non-small cell lung cancer.[2] Other studies have reported that CA9 helps maintain tumor cell proliferation in a hypoxic microenvironment and significantly upregulates tyrosine kinase inhibitor resistance in patients with lung cancer, possibly through CA9 inhibiting tumor cell iron release under hypoxic conditions.[4] Chang et al.[5] showed in a preclinical study the ability of human anti-CA9 antibodies to mediate immune cell inhibition in renal cell carcinoma. They demonstrated that fixation of human anti-CA9 mAbs fixation on CA9 expressive renal clear cell carcinoma cells led to immune-mediated destruction of tumor cells in vitro by antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent cellular phagocytosis.
CD10 is a type II transmembrane glycoprotein known as membrane metalloendopeptidase or neutral endopeptidase. It is mainly localized in the cell membrane and expressed in various normal tissues and tumor cells. In the kidney, CD10 is abundant and present on the brush border of proximal tubules and glomerular epithelia.[3] Studies have shown that CD10 expression is significantly lower in lung cancer than in normal lung tissues. Patients with non-small cell lung cancer and CD10-positive expression have a better prognosis than those with CD10-negative expression, making it a potential prognostic marker.[6] No reports exist on the co-expression of CA9 and CD10 in lung adenocarcinoma. In this case, tumors were simultaneously found in the lungs and kidneys, and the primary concern was to differentiate between dual primary tumors and tumor metastases. In clinicopathological diagnosis, CA9 and CD10 are commonly used for the differential diagnosis of renal cell carcinoma. In lung biopsy, it is important not to misdiagnose it as renal clear cell carcinoma lung metastasis based solely on the expression of CA9 and CD10. The combination of TTF-1 and napsin A, which are markers of lung adenocarcinoma, can help to establish a definitive diagnosis.
MPMNs are commonly encountered in clinical practice. According to the time of occurrence, they can be classified as synchronous multiple primary tumors (within 6 months of diagnosis) or metachronous multiple primary tumors (>6 months between diagnoses). Metachronous multiple primary tumors are more common than synchronous multiple primary tumors.[7] The proportion of combined kidney cancers in MPMN was relatively low, accounting for approximately 2%.[8] The etiology of MPMN remains unclear; however, it is generally believed to be associated with factors such as genetics, environmental exposure, personal lifestyle habits, and chemotherapy drug use. The treatment of MPMN requires the selection of surgical and radiotherapy/chemotherapy methods based on the tumor location, pathological staging, and clinical conditions. For synchronous multiple primary tumors, a comprehensive evaluation based on the patient’s situation is necessary, with tumor staging being an important prognostic factor. The overall prognosis depends mainly on the highest stage of the multiple primary tumors.[9,10] Factors such as tumor location, degree of malignancy, and histopathological classification should be considered. The treatment plans for both tumors should not be combined. Multiple primary tumors in the same patient may be driven by different carcinogenic events, requiring consideration of a combination of targeted therapies during the treatment decision-making process.
At present, most patients with lung adenocarcinoma combined with ccRCC are treated with surgical resection and post-operative chemotherapy. The principles of surgery are similar to those for single primary cancers, but a comprehensive treatment plan, primarily involving surgery, should be adopted based on the clinical stage. The primary focus of treatment should be on lung adenocarcinoma as opposed to renal tumors. Given the patient’s lung adenocarcinoma coupled with extensive lymph node metastasis, surgical intervention was not indicated. At present, chemotherapy is the preferred treatment. Pursuing kidney surgery blindly would fail to extend the survival of patient and necessitate the discontinuation of targeted therapy during the perioperative period, which could potentially expedite the progression of lung adenocarcinoma.
In this report, we describe an unusual case of a patient concurrently diagnosed with lung adenocarcinoma and renal clear-cell carcinoma, in which both malignancies exhibited immunohistochemical co-expression of CA9 and CD10 markers. The findings of our immunohistochemical analysis highlight the complexity and diversity of biomarker expression in multiple primary malignancies. Immunohistochemical staining, which is an auxiliary method for pathological diagnosis, has certain limitations. In the process of diagnosis by pathologists, it is crucial to prioritize histological morphology and avoid overreliance on immunohistochemical results.
This case report has certain limitations, as the death of the patient resulted in a lack of information regarding the treatment and prognosis. This case highlights the challenges in diagnosing and managing multiple primary malignancies. Distinct histogenesis and the potential for distinct treatment responses require a multidisciplinary approach incorporating expertise from various specialties to ensure accurate diagnosis and individualized treatment plans. The recognition of biomarker expression patterns such as those of CA9 and CD10 may aid in guiding therapeutic decisions and predicting treatment responses.
SUMMARY
The patient was diagnosed with right lung adenocarcinoma and stage II ccRCC with hemorrhagic cystic changes. This case report contributes to the growing body of knowledge regarding the heterogeneity of biomarker expression in multiple primary cancers. Further research is needed to elucidate the mechanisms underlying biomarker expression in different tumor types and to explore their potential as therapeutic targets or prognostic markers. In addition, continued attention to the complexities of managing multiple primary malignancies is crucial for optimizing patient outcomes.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author on reasonable request.
ABBREVIATIONS
CA9 – Carbonic anhydrase IX
CD10 – Cluster of differentiation 10 antigen
MPMNs – Multiple primary malignant neoplasms
ccRCC – Clear cell renal cell carcinoma
CT – Computed tomography
TTF-1 – Thyroid transcription factor-1
PAX-8 – Paired box 8
CK7 – Cytokeratin 7
CA – CA catalytic domain
AUTHOR CONTRIBUTIONS
YJW and GHY: Designed the study; all authors conducted the study; TW and HMZ: Collected and analyzed the data; YJW and GHY: Participated in drafting the manuscript; and all authors contributed to critical revision of the manuscript for important intellectual content. All authors gave final approval of the version to be published. All authors participated fully in the work, took public responsibility for appropriate portions of the content, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or completeness of any part of the work were appropriately investigated and resolved.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The patient has signed the informed consent form and the study has been approved by the Ethics Committee of Yantai Yuhuangding Hospital: NO.43 (November 10, 2023). The study was performed in accordance with the ethical standards as laid down in the 2013 Declaration of Helsinki.
CONFLICT OF INTEREST
Given his role as editorial member, Guohua Yu had no involvement in the peer-review of this article and has no access to information regarding its peer-review. The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Carbonic anhydrase IX in renal cell carcinoma, implications for disease management. Int J Mol Sci. 2020;21:7146.
- [CrossRef] [PubMed] [Google Scholar]
- Carbonic anhydrase 9 (CA9) expression in non-small-cell lung cancer: Correlation with regulatory FOXP3+T-cell tumour stroma infiltration. Br J Cancer. 2020;122:1205-10.
- [CrossRef] [PubMed] [Google Scholar]
- Concise review: Neutral endopeptidase (CD10): A multifaceted environment actor in stem cells, physiological mechanisms, and cancer. Stem Cells. 2011;29:389-96.
- [CrossRef] [PubMed] [Google Scholar]
- Carbonic anhydrase IX controls vulnerability to ferroptosis in gefitinib-resistant lung cancer. Oxid Med Cell Longev. 2023;2023:1367938.
- [CrossRef] [PubMed] [Google Scholar]
- Human anti-CAIX antibodies mediate immune cell inhibition of renal cell carcinoma in vitro and in a humanized mouse model in vivo. Mol Cancer. 2015;14:119.
- [CrossRef] [PubMed] [Google Scholar]
- CD10 immunostaining of bile canaliculi in liver biopsies: Change of staining pattern with the development of cirrhosis. Histopathology. 2004;45:335-42.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of myoferlin expression for prediction of subsequent primary malignancy in patients with clear cell renal cell carcinoma. In Vivo. 2019;33:1103-8.
- [CrossRef] [PubMed] [Google Scholar]
- The relative risk of second primary cancers in Switzerland: A population-based retrospective cohort study. BMC Cancer. 2020;20:51.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment and prognosis of multiple primary malignant neoplasms complicated with renal cell carcinoma. Beijing Da Xue Xue Bao Yi Xue Ban. 2022;54:680-5.
- [Google Scholar]
- Clinical characteristics and prognostic analysis of multiple primary malignant neoplasms in patients with lung cancer. Cancer Gene Ther. 2019;26:419-26.
- [CrossRef] [PubMed] [Google Scholar]








