Translate this page into:
Exploring the efficacy and safety of anti-BCMA chimeric antigen receptor T-cell therapy for multiple myeloma: Systematic review and meta-analysis

*Corresponding author: Xiaoxiao Ding, Department of Hematology, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, China. 13634090669@163.com
-
Received: ,
Accepted: ,
How to cite this article: Zhang J, Ding X, Ding X. Exploring the efficacy and safety of anti-BCMA chimeric antigen receptor T-cell therapy for multiple myeloma: Systematic review and meta-analysis. CytoJournal 2023;21:13. doi: 10.25259/Cytojournal_64_2023
Abstract
Objective:
Multiple myeloma (MM) is a bone marrow cancer that profoundly affects plasma cells involved in the immune response. Myeloma cells alter the average production of cells in the bone marrow. Anti-B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy allows genetic modifications of an individual’s T-cells to increase the expression of CARs used to identify and attach BCMA proteins to the malignant cells. Our main objective is to perform a systematic review and meta-analysis to explore the efficacy and safety of anti-BCMA CAR T-cell therapy for MM.
Material and Methods:
We searched five databases, PubMed, CNKI, EMBASE, Cochrane, Web of Science, and CNKI, for studies published on anti-BCMA,CAR-T-cell treatment for MM. Inclusion criteria involved prospective single-arm studies either single or multi-center, in various MM phases and studies that reported anti-BCMA,CAR-T-cell treatment for MM. We excluded non-English publications and conference papers. All statistical analyses were performed in R software and Review Manager 5.4.1.
Results:
Thirteen articles were included in the analysis. We found that the overall response survival complete response increase was statistically significant. Similarly, the reduction in cytokine release syndrome grades 3 and 4 and neurotoxicity after follow-up was statistically significant. However, the reduction in minimal residual disease negativity (MRDN) was not statistically significant.
Conclusion:
Using anti-BCMA CAR T-cell therapy in MM was highly efficacious and safe in lowering the adverse outcomes and improving the survival outcomes, complete response, and overall response.
Keywords
Anti-B-cell
Maturation antigen
Chimeric antigen receptor
T-cell therapy
Multiple myeloma
Plasma cells and Survival
INTRODUCTION
Multiple myeloma (MM) is a hematological malignancy associated with the proliferation of plasma cells in the bone marrow, making it a complex and challenging disease to treat.[1] Over the years, advancements in research have led to the development of therapeutic approaches, including chemotherapy, immunomodulatory drugs, and stem cell transplantation.[2,3] However, despite these therapeutic options, MM remains incurable in most cases, necessitating the exploration of novel therapies.[4]
A promising and emerging treatment approach with significant potential for patients with MM is the anti-B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy.[5,6] The therapy revolves around genetically modifying the patient’s T-cells to express CARs, allowing them to identify and eradicate myeloma cells[5] efficiently. The ability to target BCMA lies in its high expression on the surface of malignant plasma cells, making it suitable for CAR T-cell therapy in MM.[6] The rationale behind targeting BCMA lies in its selective expression on myeloma cells while sparing healthy tissues, making it a therapeutic target.[7] Moreover, BCMA has a crucial role in the survival and proliferation of MM cells.[7] Modifying T-cells to express CARs specific to BCMA allows researchers to understand the immune system’s capacity to eradicate myeloma cells while minimizing off-target toxicity.
Early clinical trials evaluating anti-BCMA CAR T-cell therapy for MM have yielded consistent results[7,8] in examining various CAR designs. These designs encompass second and third generations, incorporating distinct signaling domains and T-cell functionalities. The therapy’s effectiveness has been observed in refractory MM compared to traditional treatments with limited efficacy and outcomes.[9] D’Agostino and Raje[9] reported positive anti-BCMA CAR T-cell therapy outcomes. They showed that patients experienced significant improvements, including higher response rates, extended remission periods, and enhanced overall survival. Similarly, Anderson[10] evaluated idecabtagene vicleucel, a BCMA-targeting CAR T-cell therapy in MM. Their outcomes revealed a promising overall response rate of 73%, with 33% of patients attaining complete response.[10] These findings strongly indicate the potential efficacy of anti-BCMA CAR T-cell, positioning it as a viable and promising treatment option for MM.
According to Chekol Abebe et al.,[11] anti-BCMA CAR T-cell therapy has demonstrated manageable safety profiles in clinical trials.[11] Cytokine release syndrome (CRS) and neurotoxicity can be effectively managed with appropriate supportive care measures.[12] However, researchers continue to refine the therapy’s administration and dosing strategies to minimize toxicity while maximizing its anti-tumor effects. Nam et al.[13] suggested that despite these promising results, challenges remain in adopting anti-BCMA CAR T-cell therapy for MM. The manufacturing process of CAR T-cell therapies is complex and time-consuming, making it logistically demanding and costly.[13] In addition, the durability of responses and long-term safety data require further investigation to establish the therapy’s role in treating MM. To address these challenges, ongoing research is dedicated to enhancing the efficacy and safety of anti-BCMA CAR T-cell, focusing on optimizing the accessibility and affordability of the therapy. This involves streamlining manufacturing processes, reducing turnaround times, and ensuring cost-effectiveness.[13] In addition, synergistic approaches are explored by combining anti-BCMA CAR T-cell therapy with other treatments like immunomodulatory drugs or monoclonal antibodies to amplify therapeutic outcomes and broaden the therapy’s safety and efficacy.[2,3]
Our main objective is to perform a systematic review and meta-analysis to explore the efficacy and safety of anti-BCMA CAR T-cell therapy for MM.
MATERIAL AND METHODS
Information sources
Our study was performed according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines[14] and the Cochrane Handbook for systematic reviews and interventions.[15] We searched five databases: Web of Science (https://clarivate.com/products/scientific-and-academic-research/research-discovery-andworkflow-solutions/webofscience-platform/), CNKI (https://en.cnki.com.cn/index/), EMBASE (https://www.embase.com/landing?status=gray), PubMed (https://pubmed.ncbi.nlm.nih.gov/), and Cochrane (https://www.cochranelibrary.com/), for studies published on anti-BCMA CAR-T-cell treatment for MM. The databases were searched from their inception to 2023 using the following keywords; “Myeloma cells,” “CAR,” and “BCMA.”
Eligibility criteria
Our study adopted the participants, intervention, comparison, and outcomes approach to determine eligible studies for inclusion. Moreover, the following inclusion and exclusion criteria were adopted;
Inclusion criteria
The inclusion criteria encompassed prospective single-arm studies conducted in single- or multi-center settings involving patients exclusively diagnosed with MM, including refractory cases. These studies were required to report on the intervention of anti-BCMA CAR T-cell treatment for MM and provide outcomes related to the efficacy, safety, or both aspects of this treatment.
Exclusion criteria
The exclusion criteria involved studies that combined anti-BCMA CAR T-cell treatment with other regimens and observational studies, conference papers, or reviews. In addition, studies reporting outcomes unrelated to anti-BCMA CAR T-cell treatment parameters were excluded. Finally, studies not published in English were also excluded from the study.
Data extraction and selection
Our study adopted two independent reviewers who conducted the study selection and data extraction. These reviewers scrutinized the selected databases using the keywords and search terms. The articles were retrieved by screening their titles and abstracts. Furthermore, the full texts were screened to ensure sufficient information available based on the study population, assessment of outcomes, and other confounding factors. A third reviewer and majority vote resolved any conflicts between the reviewers.
We obtained the following information: Year of publication, author’s surname, features of patients, features of anti-BCMA CAR-T administered, and the outcomes of safety and efficacy of the intervention. The measures of efficacy outcomes were assessed based on the rate of overall, complete, and duration of responses. Furthermore, progression-free and overall survival was determined. The rates of overall response ranged from complete to partial responses. Our safety outcomes measures were based on the grades of CRS, CAR-T encephalopathy syndromes, and other secondary infections such as anemia (Neurotoxicity).
Quality assessment
Our study adopted the Cochrane risk of bias tool (version 2.0, Oxford, England: The Cochrane Collaboration, 2003) to assess the risk of bias based on five domains; adequate random sequence generation ensures unbiased participant allocation, while allocation concealment prevents biased group assignments. Blinding of participants and personnel minimized performance and detection bias. Handling incomplete outcome data helps reduce attrition bias, and reporting all planned outcomes and analysis protects against selective reporting bias. Furthermore, we only included studies meeting the inclusion criteria and had a relatively low risk of bias based on all assessment domains. In addition, the quality of the selected publications was assessed using the Newcastle-Ottawa scale (NOS).[16] The NOS consisted of eight items that were subdivided into three main categories: Selection of cases (0–4 points), compatibility of groups (0–2 points), and clinical outcomes (0–3 points). High-quality studies are those studies that attained a score of 6 and above on the NOS scale. We found it essential to assess the risk of bias in selected publications because it minimizes false reporting and increases the accuracy of our study.
Statistical analysis
Our study used Review Manager (RevMan) (version 5.4.1 RevMan (Computer program) Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003) and R software (version 4.2.3, The R core team, Boston, USA) to perform all statistical analyses. We obtained forest plots, publication bias plots, and risk of bias plots. We extracted data on the response rates and incidences of adverse outcomes to perform comparisons and subgroup analyses. Furthermore, all the analyses were produced with their corresponding 95% confidence intervals (CIs). Heterogeneity was assessed using the Higgins I^2 statistic and Chi-square statistic. Heterogeneity was inferred at an I^2 ≥ 50% with P < 0.1. In such cases, the random effects model was used for analysis. Furthermore, we carried out a subgroup analysis to examine sources of heterogeneity and a sensitivity analysis with the heterogeneous studies being omitted. Our subgroup analyses were conducted based on the overall response, complete response, grades of CRS, antigens targeted by CAR, and neurologic toxicities.
RESULTS
Search results
The literature search produced 678 studies, of which 650 were screened based on their titles and abstracts. After applying the eligibility criteria, 400 full-text articles were reviewed, with 387 full-text articles excluded. Finally, 13 articles were eligible for analysis [Figure 1 and Table 1].
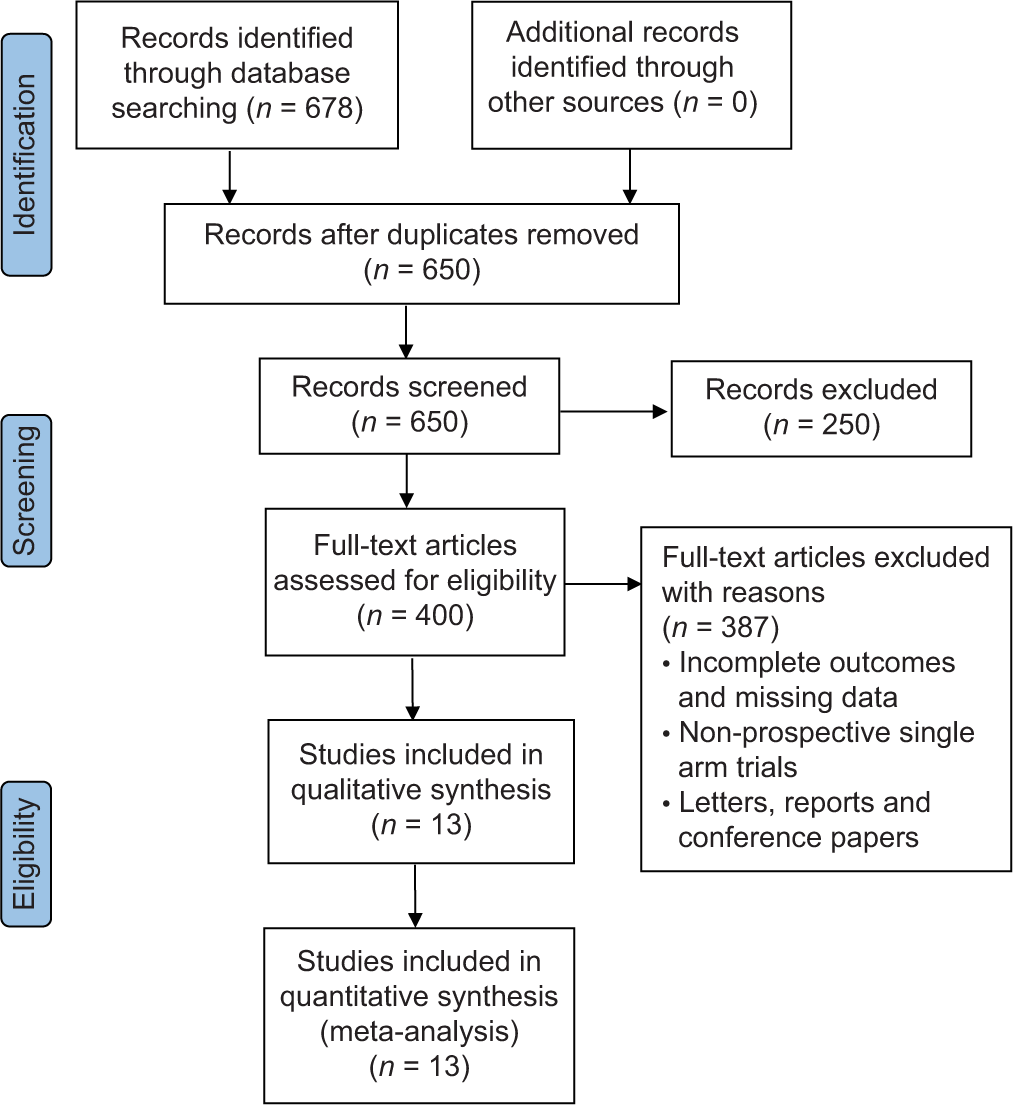
- Preferred reporting items for systematic reviews and meta-analysis flow chart of selected studies.
| Author’s surname | Year of publication | No. of Patients | Age/gender | Prior treatment, ASCT, and lymphodepletion | Antigen positivity, the origin of T-cells, and subset of T-cells | CAR: Vector/co-stimulatory molecule/scFvspecies | CAR-T dose and follow-up |
|---|---|---|---|---|---|---|---|
| Brudno et al.[17] | 2018 | 26 | Age: 18–70 years. 13 males and 13 females |
Ten lines of prior treatment ranged from 3 to 19, with an 85% ASCT before CAR-T. Lymphodepletion at the rate of Flu/Cy of 300 mg/m^2 for 3–5 days. | BCMA at 50%, Autologous T-cells of CD4/CD8 | Retrovirus/CD28/murine | 0.3–9.0 *10^6/kg with a median follow-up of 20 weeks |
| Zhao et al.[18] | 2018 | 57 | Age: 54 years, ranging from 27 years to 72 years |
Three lines of prior treatment ranged from 1 to 9, with an 18% ASCT before CAR-T. Lymphodepletion at Cy: 300 mg/m^2 between 3 and 5 days. | BCMA, Autologous T-cells | Lentivirus/4–1BB/Llama | Median dose of 0.5 *10^6/kg, with a median follow-up of 32 weeks (2.8–82.8 weeks) |
| Li et al.[19] | 2019 | 28 | N/A | No prior treatment, Lymphodepletion at Flu/Cy | BCMA, Autologous T-cells | Lentiviral/4–1BB/murine | 5.4–25.0*10^6 with a median follow-up of 40 weeks |
| Raje et al.[20] | 2019 | 33 | Age: 60 years (37–75), with 21 males and 12 females |
Seven lines of prior treatment, ranging from 3 to 23, with an ASCT before CAR-T of 97%, Lymphodepletion at Flu/Cy: 30 mg/m^2/300 mg/m^2 daily for 3–5 days | BCMA at 50%, Autologous T-cells of CD4/CD8 | Lentiviral/4–1BB/murine | 50–800*10^6 with a median follow-up of 45 weeks (24.8 weeks–91.2 weeks) |
| Xu et al.[21] | 2019 | 17 | Age: 55 years, ranging from 35 years to 73 years, with 11 males and six females |
Five lines of prior treatment, ranging from 3 to 11, with an ASCT before CAR-T of 47%, Lymphodepletion at Flu/Cy: 25 mg/m^2 for 3 days and 300 mg/m^2 daily | BCMA, autologous T-cells of CD4/CD8 | Lentiviral/4–1BB/Llama | 0.21–1.52*10^6/kg with a median follow-up of 60 weeks (1.7 weeks–76.4 weeks) |
| Li et al.[22] | 2021 | 9 | N/A | Four lines of prior treatment, ranging from 3 to 5 and Lymphodepletion at Flu/Cy Two lines of prior treatment, with an ASCT before CAR-T of 17%, Lymphodepletion at Flu/Cy: 25 mg/m^2/250 mg/m^2 for 3–5 days |
BCMA CD38, | Lentiviral/4–1BB/human | 1.0–6.0*10^6/kg with a median follow-up of 9 weeks Median dose of 2.17*10^6, and follow-up duration of 22 weeks |
| Cohen et al.[23] | 2019 | 25 | Age: 58 years, ranging from 44 to 75 years, with 17 males and eight females | Seven lines of prior treatment, ranging from 3 to 13, with an ASCT before CAR-T of 92%, Lymphodepletion at Cy: 1.5mg/m^2 or none | BCMA, Autologous T-cells of CD4/CD8 | Lentiviral/4–1BB/human | 1–50*10^7 with a median follow-up of 54 weeks |
| Garfall et al.[24] | 2018 | 10 | Age: 61 years, 48–68 years, with four males and six females | Six lines of prior treatment, ranging from 2 to 10, with an ASCT before CAR-T of 100%, Lymphodepletion at Melphalan: 140–200 mg/m^2 | CD19 with Autologous T-cells | Lentiviral/4–1BB | 1–5 *10^7 with a median follow-up of<14 weeks |
| Guo et al.[25] | 2016 | 5 | Age: 58 years, ranging from 48 to 68 years, with one male and four females | 11 lines of prior treatment, ranging from 5 to 18, with an ASCT before CAR-T of 20%, No Lymphodepletion | CD138 with Autologous T-cells of CD8 | Lentiviral/4–1BB/human | Median dose of 7.56 *10^6 with a median of 12 weeks |
| Ramos et al.[26] | 2016 | 8 | Age: 56.5 years, ranging from 43 to 69 years, with three males and five females | N/A | k light chain with Autologous T-cells of CD4/CD8 | Retroviral/CD28/murine | 0.2–2*10^8/m^2 with a median follow-up of 6–96 weeks |
| Baumeister et al.[27] | 2018 | 5 | Age: 70 years, ranging from 44 to 79 years, with three males and two females | Five lines of prior treatment, with an ASCT before CAR-T of 100%, No Lymphodepletion | NKG2D-ligands with Autologous T-cells of CD4/CD8 | Retroviral/NKG2D-CAR | 1*10^6–3*10^7 with a median follow-up of 2–20 weeks |
| Yan et al.[28] | 2021 | 8 | Age: 57 years, ranging from 43 to 69 years, with six males and two females | Four lines of prior treatment, ranging from 2 to 7, Lymphodepletion at Flu/Cy: 30 mg/m^2/750 mg/m^2 for 3–5 days | CD 19, BCMA, Autologous T-cells | Lentiviral/CD28 and OX40 | 25–82 *10^6/kg with a median follow-up of 5 weeks |
| Yan et al.[29] | 2019 | 21 | Age: 58 years, ranging from 49.5 to 61 years | Six lines of prior treatment, ranging from 5 to 8, with an ASCT before CAR-T of 14%, Lymphodepletion at Flu/Cy: 30 mg/m^2 for 3 days and 750 mg/m^2 daily | CD 19, BCMA, Autologous T-cells | Lentiviral/4–1BB/human | CD19 (1*10^6/kg), BCMA (1*10^6/kg) with a median follow-up of 25.6 weeks |
BCMA: B -cell maturation antigen, CAR: Chimeric antigen receptor, BCMA : B-Cell maturation antigen, ASCT: Autologous stem cell transplant, CAR-T: Chimeric antigen receptor T cell therapy, Flu/Cy: Fludarabine/cyclophosphamide (Flu/Cy), CD28, CD4, CD8, CD38: Cluster of differentiation 28, Cluster of differentiation 4, Culster of differentiation 8, Cluster of differentiation 38, 4-1BB: 4-1 B Cells, N/A: Not Applicable
Characteristics of included studies
We included studies published from 2016 to 2022 and were single-arm studies.[17-29] The participants were middle-aged, about 40 years and had an average oncology performance. In all the studies, the patients were diagnosed with MM or cases of refractory MM. We observed that patients were subjected to prior treatments using proteasome inhibitors, autologous stem cell transplantation (ASCT), or immunotherapy regimens. In most studies, patients were subjected to ASCT before treatment, while some studies did not report on the outcomes of ASCT. Lymphodepletion was mainly carried out using melphalan, cyclophosphamide, and fludarabine. However, some studies did not use this technique, and others reported incomplete outcomes.
In these studies, the extracted T-cells underwent genetic modifications to express levels of CAR in the presence of retroviruses or transposons. Most T-cells were autologous, with a single study reporting from both autologous and autologous T-cells.
Risk of bias
According to Figure 2, most studies had a low risk of bias in all five domains. There was a high unclear risk of bias in blinding participants and personnel because it was impossible to blind the medical professional and personnel who offered the anti-BCMA CAR-T treatment regimen to the participants. Blinding reduces the risk of bias by minimizing the influence of participant’s and personnel’s expectations or preferences on the study outcomes. Lack of blinding can introduce performance or detection bias, affecting the trial’s internal validity. Double-blind designs are ideal, where participants and investigators are unaware of the assigned interventions. Moreover, some studies reported incomplete outcomes, such as the duration of follow-up and the number of participants in the experimental and control conditions. Nonetheless, we observed moderate to high methodological qualities of all selected publications based on the ratings of the NOS Scale. Despite some incomplete outcomes, all the included studies had sufficient information for meta-analysis.
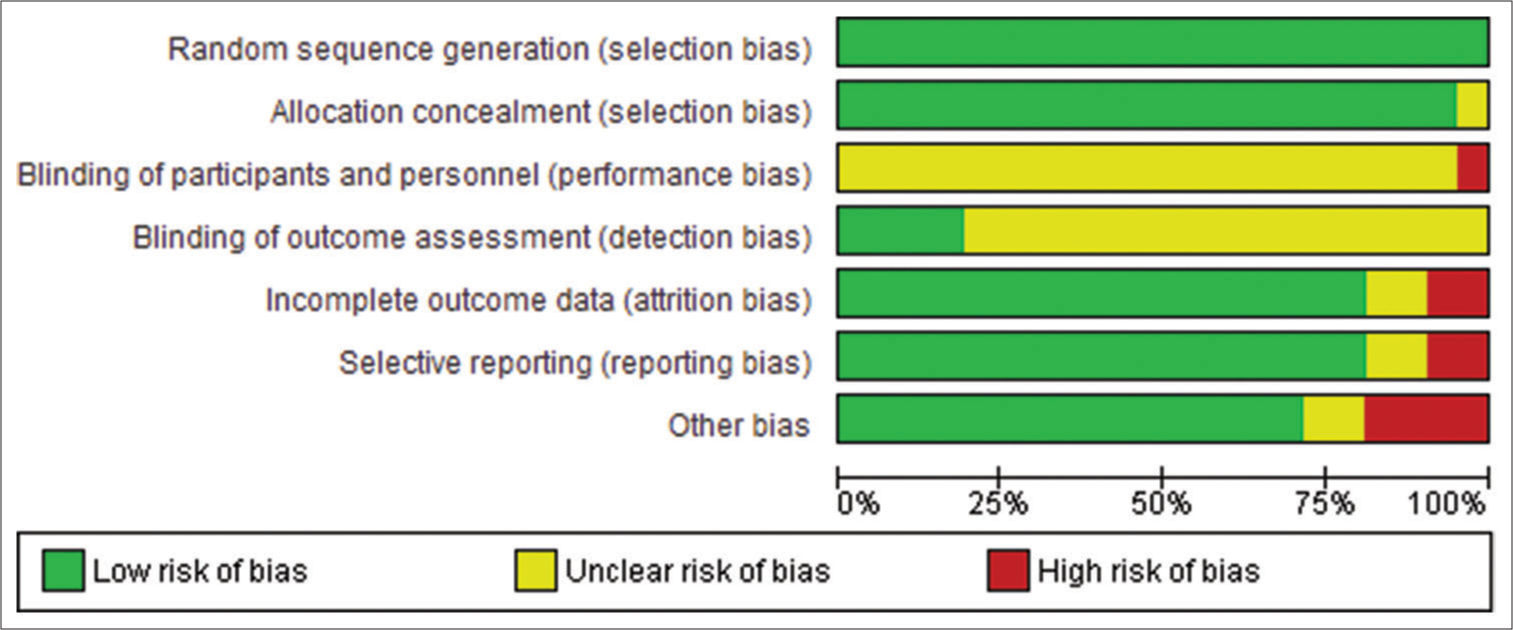
- Assessment of the risk of bias.
Analysis of outcomes
According to Figure 3, the pooled proportion of the overall response rate was 82% observed from 185 patients in seven studies, with a median response within 10 months in 151 patients. The increase in overall response was statistically significant (95% CI: 75–87; I^2 = 69%; P < 0.001).
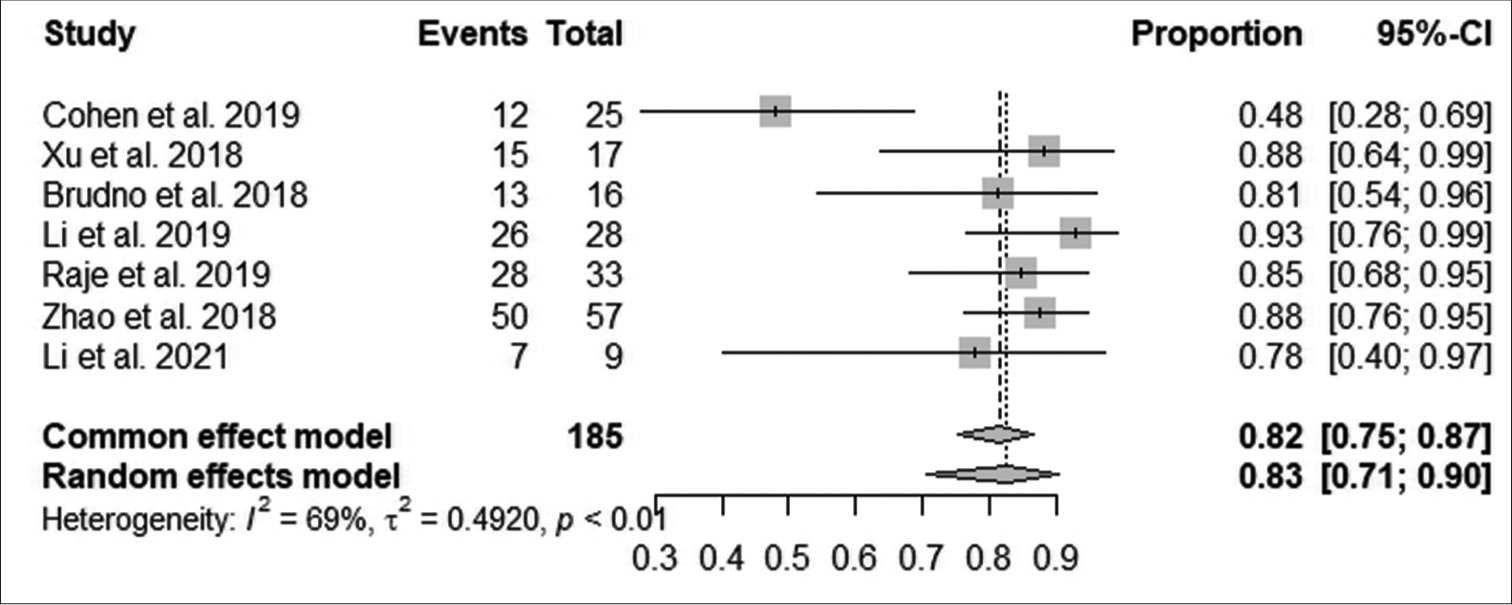
- Effect of anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy on overall response. (CI: Confidence interval).
According to Figure 4, the pooled proportion of complete response rate was 45% observed from 185 patients in 7 studies, with 83 patients having complete responses. The increase in complete response was statistically significant (95% CI: 38–52; I^2 = 81%; P < 0.001).

- Effect of anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy on complete response. (CI: Confidence interval).
According to Figure 5, the pooled proportion of reduction in minimal residual disease negativity (MRDN) was 80% observed from 79 patients in three studies, with 63 patients having reductions in MRDN. The reduction in MRDN was not statistically significant (95% CI: 69–87; I^2 = 0%; P > 0.05).
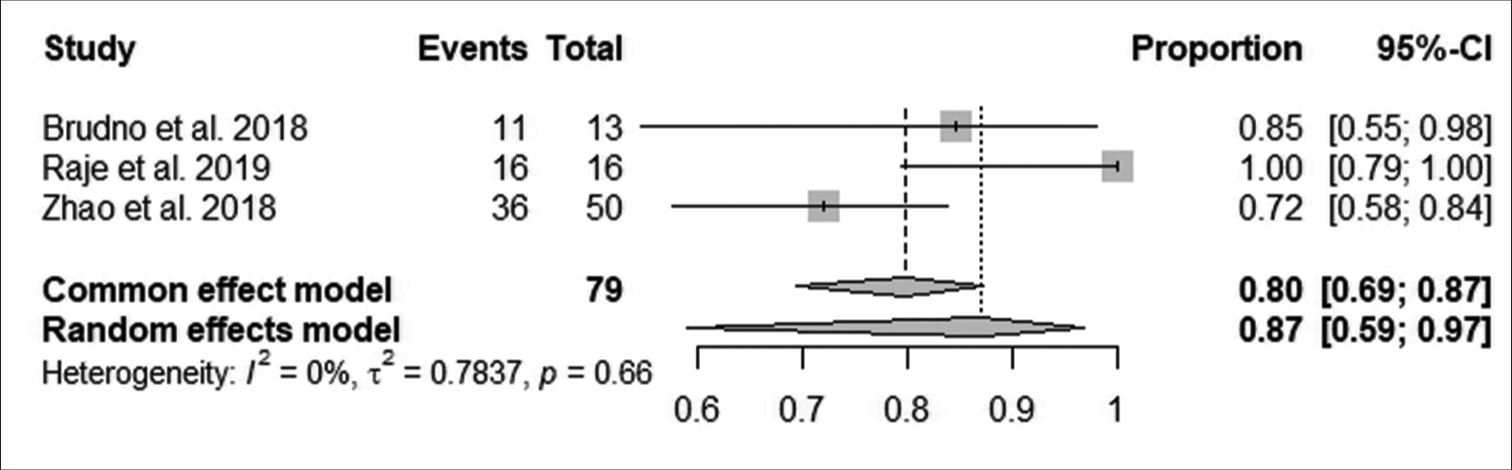
- Effect of anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy on minimal residual disease negativity. (CI: Confidence interval).
According to Figure 6, the pooled proportion of overall survival rate after follow-up was 83% observed from 148 patients in five studies, with 123 improving after 11 months. The increase in overall survival after follow-up was statistically significant (95% CI: 76–88; I^2 = 80%; P < 0.001).

- Effect of anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy on overall survival of patients. (CI: Confidence interval).
According to Figure 7, the pooled proportion of CRS grades 3 and 4 after follow-up was 17% observed from 185 patients in seven studies, with 31 having CRS grades 3 and 4. CRS grade 3 and 4 reduction after follow-up was statistically significant (95% CI: 12–23; I^2 = 68%; P < 0.05).

- Effect of anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy on cytokine release syndrome grade 3 and 4. (CI: Confidence interval).
According to Figure 8, the pooled proportion of neurotoxicity after follow-up was 22% observed from 131 patients in four studies, with 29 having neurotoxicity. The reduction in neurotoxicity after follow-up was statistically significant (95% CI: 16–30; I^2 = 76%; P < 0.05).
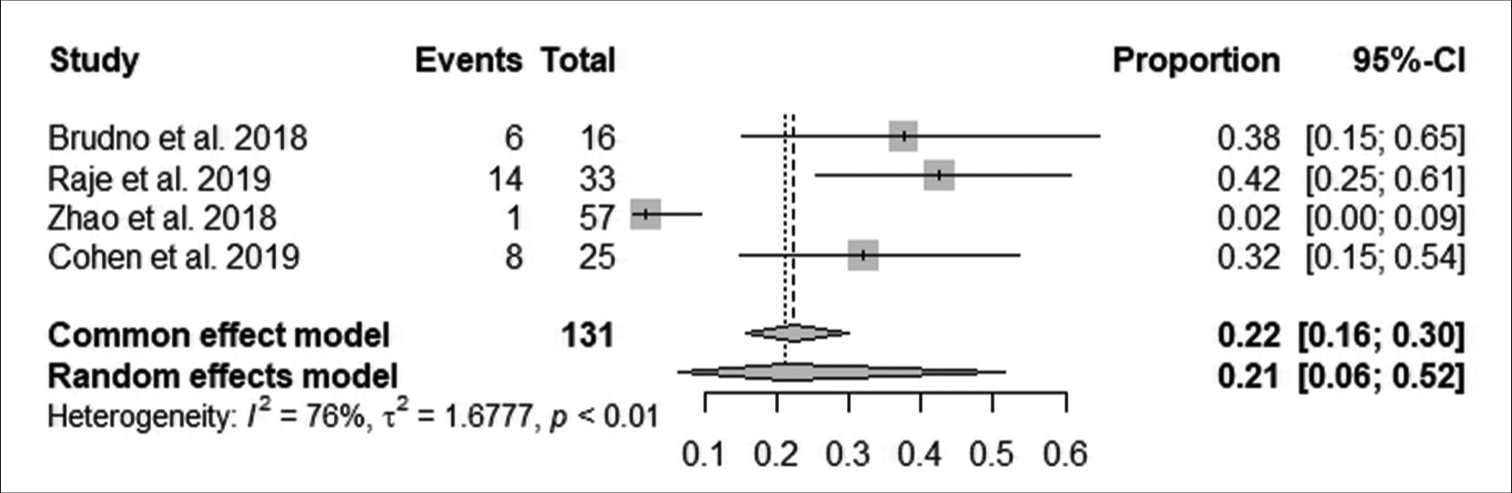
- Effect of anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy on neurotoxicity. (CI: Confidence interval).
DISCUSSION
CAR-T therapy has revolutionized cancer treatment, offering a novel approach to addressing malignancies like MM. This therapy has shown remarkable promise in improving clinical outcomes by genetically modifying patients’ T-cells to express CARs targeting specific antigens on cancer cells.
Our study comprehensively evaluated anti-BCMA CAR T-cell therapy’s efficacy by examining overall response rates, complete response rates, MRDN, and overall survival. The outcomes revealed a statistically significant improvement in overall, complete, and survival response rates. However, the reduction in MRDN was not significant. The reduction in MRDN underscores the therapy’s ability to control the disease robustly. Moreover, the observed reduction in neurotoxicity further accentuates the promising potential of anti-BCMA CAR T-cell therapy in MM.[30] Anti-BCMA CAR T-cell therapy enhanced the overall response rate in MM through a series of interconnected mechanisms. These CAR T-cells are initially designed to precisely recognize BCMA, highly expressed on malignant plasma cells in MM. This specificity ensures that CAR T-cells selectively target MM cells while sparing healthy tissue, minimizing collateral damage.[30]
The mechanism of anti-BCMA CAR T-cell therapy lies in the design of CARs. These synthetic receptors consist of an extracellular domain designed to recognize BCMA expressed on malignant plasma cells in MM. The binding of CAR T-cells to BCMA initiates a cascade of intracellular signaling events, leading to the activation of cytotoxic molecules such as perforin, granzymes, interferon-gamma, and tumor necrosis factor, contributing to a robust immune response against MM cells.[31] Perforin plays a critical role by forming pores in the cell membrane of the MM cells, creating channels for granzymes to enter. Once inside the target MM cells, granzymes trigger biochemical events, leading to apoptosis or programmed cell death.[31] This apoptosis induction is a fundamental mechanism by which anti-BCMA CAR T-cells effectively eliminate MM cells.
Our study’s results aligned with Cornell et al.,[32] who outlined the genetic modification process in anti-BCMA CAR T-cell therapy. The improvement in complete and overall response rates is attributed to its persistence in the patient’s body. The ability of CAR T-cells to recognize BCMA-expressing myeloma cells increases its efficacy as a therapeutic target in MM.[31] Incorporating CD28 signaling domains in CAR design enhances T-cell activation and persistence, with cytokine production sustaining the anti-tumor immune response.[33-37] These results reaffirm anti-BCMA CAR T-cell therapy’s potential to advance MM treatment[31] significantly.
The strategic inclusion of CD28 signaling domains within CAR design amplifies the activation and persistence of T-cells. This approach stimulates cytokine production, sustaining the anti-tumor immune response, and fostering improved overall patient survival.[37] Honikel and Olejniczak[36] showed that illuminating the significance of CD28 allows an understanding of its role as a potent costimulatory signal for T-cell activation and function. The interactions between CAR design and T-cell biology outline the therapy’s multifaceted effectiveness in combating MM. The presence of CD28 signaling domains within the CAR design significantly enhances the activation and persistence of CAR T-cells. This heightened activation leads to a more effective immune response against MM cells. As a result, patients treated with anti-BCMA CAR T-cells containing CD28 signaling domains experience improved overall responses, with a more significant proportion of MM cells being targeted and eliminated.[37,38]
The effective management of neurotoxicity is a critical concern in CAR T-cell therapy, particularly the emergence of CAR T-cell-related encephalopathy syndrome (CRES). Our findings are similar to Kotch et al.,[39] who advocate for a methodical approach encompassing accurate dose optimization and a comprehensive evaluation of risk factors. The multifaceted strategy is pivotal in mitigating the potential impact of CRES. Central to this approach is the concept of patient-centric customization, where a tailored administration of CAR T-cell doses coupled with a deep understanding of individual patient attributes and vigilant response monitoring synergistically contribute to a substantial reduction in both the likelihood and severity of neurotoxicity. Anti-BCMA CAR T-cells are designed to recognize and target MM cells expressing BCMA. This targeted approach ensures that CAR T-cells selectively attack MM cells, particularly those that may be resistant to conventional therapies. As a result, the number of remaining MM cells is reduced, contributing to MRD negativity. Furthermore, the therapy is designed to minimize off-target effects on healthy tissues. This design reduces the risk of CRES that can occur when CAR T-cells affect non-tumor brain tissue. Thus, by focusing their activity primarily on BCMA-expressing MM cells, anti-BCMA CAR T-cells aim to spare healthy brain tissue, thus lowering the incidence and severity of CRES.
Dose optimization is crucial in precision medicine, ensuring proper alignment of therapeutic intervention with individual patient needs. Balancing efficacy and minimizing adverse effects lowers neurotoxicity risks and enhances tolerability during treatment. This approach advances personalized medicine, reflecting a profound understanding of CAR T-cell therapy.[39] Interventions such as tocilizumab, an interleukin-6 receptor antagonist, emerge as potential factors in minimizing adverse effects. Tocilizumab’s mechanism is associated with immune response and targeting the interleukin-6 receptor. Tocilizumab intercepts a critical signaling pathway in CRS development with a positive outcome in improved immune response modulation and dampening the intensity of CRS.[40]
The diverse strategies for managing neurotoxicity and CRS are critical for cancer treatment. Balancing therapeutic efficacy and patient well-being is central to effective and safe treatment. In CAR T-cell therapy, proactive measures such as precise dosing and targeted tocilizumab protect against adverse effects. Thus, by carefully administering these strategies, Healthcare professionals optimize the therapy while prioritizing patient safety. The previous studies[41-44] showed that anti-BCMA CAR T-cell therapy demonstrated a marked reduction in MRDN, primarily achieved through the targeted elimination of malignant plasma cells. This approach ensures the direct and efficient killing of myeloma cells, even those resistant to conventional therapies.[42] The persistence of CAR T-cells and continuous targeting of residual myeloma cells contributes to sustained disease control, minimizing the risk of relapse.[40-42]
Strengths and limitations
Our study was robust because it was performed based on the Cochrane guidelines and PRISMA protocols. We used an extensive search strategy and the NOS scale to assess the methodological quality of selected publications. Nonetheless, our study was limited by heterogeneous data of the selected publications and variations in the doses administered. In addition, some of the studies had significant variations in the duration of follow-up. Further studies should adopt randomized controlled trials involving experimental and control groups in MM.
SUMMARY
Our study suggested that using anti-BCMA CAR T-cell therapy in MM has high efficacy and safety in mitigating adverse outcomes such as MRDN, neurotoxicity, and CRS. In addition, this therapy improved survival outcomes, complete response rates, and overall response rates in MM patients. We found that the extracellular domain of CARs allows the CAR T-cells to recognize and bind specifically to BCMA on the surface of myeloma cells. Once infused back into the patient’s body, the modified CAR T-cells circulate and encounter BCMA-expressing myeloma cells.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article or its supplementary materials.
ABBREVIATIONS
MM - Multiple Myeloma
BCMA: B-cell maturation antigen
CD28: Cluster of Differentiation 28
CD14: Cluster of Differentiation 14
ASCT: Autologous stem cell transplantation.
CAR-T: Chimeric antigen receptor T-cell therapy
Flu/Cy: Fludarabine and cyclophosphamide.
4-1BB (CD137): Cluster of Differentiation 137
AUTHOR CONTRIBUTIONS
JZ, XD and XD designed the research study, performed the research. XD and XD provided help and advice on the experiments. XD analyzed the data. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was granted exemption from the Institutional Review Board.
The authors certify that they have obtained all appropriate patient consent.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
SUPPLEMENTARY MATERIAL
Supplementary material associated with this article can be found doi: 10.25259/Cytojournal_64_2023
FUNDING
Not applicable.
References
- Enumeration and characterization of circulating multiple myeloma cells in patients with plasma cell disorders. Br J Haematol. 2018;180:71-81.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple myeloma therapy: Emerging trends and challenges. Cancers (Basel). 2022;14:4082.
- [CrossRef] [PubMed] [Google Scholar]
- The role of high-dose melphalan with autologous stem-cell transplant in multiple myeloma: Is it time for a paradigm shift? Br J Haematol. 2020;191:692-703.
- [CrossRef] [PubMed] [Google Scholar]
- Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020;10:17.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-B-cell maturation antigen chimeric antigen receptor T cell function against multiple myeloma is enhanced in the presence of lenalidomide. Mol Cancer Ther. 2019;18:2246-57.
- [CrossRef] [PubMed] [Google Scholar]
- Chimeric antigen receptor-modified T cell therapy in multiple myeloma: Beyond B cell maturation antigen. Front Immunol. 2019;10:1613.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term follow-up of combination of B-cell maturation antigen and CD19 chimeric antigen receptor T cells in multiple myeloma. J Clin Oncol. 2022;40:2246-56.
- [CrossRef] [PubMed] [Google Scholar]
- Chimeric antigen receptor T cell targeting B cell maturation antigen immunotherapy is promising for multiple myeloma. Ann Hematol. 2019;98:813-22.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-BCMA CAR T-cell therapy in multiple myeloma: Can we do better? Leukemia. 2020;34:21-34.
- [CrossRef] [PubMed] [Google Scholar]
- Idecabtagene vicleucel (ide-cel) CAR T-cell therapy for relapsed and refractory multiple myeloma. Future Oncol. 2021;18:277-89.
- [CrossRef] [PubMed] [Google Scholar]
- Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. 2022;13:991092.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-BCMA CAR T-cell therapy CT103A in relapsed or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders: Phase 1 trial interim results. Signal Transduct Target Ther. 2023;8:5.
- [CrossRef] [PubMed] [Google Scholar]
- Driving the next wave of innovation in CAR T-cell therapies New York: McKinsey and Company; 2019.
- [Google Scholar]
- A guide for systematic reviews: PRISMA. Turk Arch Otorhinolaryngol. 2019;57:57-8.
- [CrossRef] [PubMed] [Google Scholar]
- Updated guidance for trusted systematic reviews: A new edition of the Cochrane handbook for systematic reviews of interventions In: Cochrane Database Syst Rev. Vol 10. 2019. p. :ED000142.
- [CrossRef] [PubMed] [Google Scholar]
- A modified Newcastle-Ottawa scale for assessment of study quality in genetic urological research. Eur Urol. 2021;79:325-6.
- [CrossRef] [PubMed] [Google Scholar]
- T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36:2267-80.
- [CrossRef] [PubMed] [Google Scholar]
- A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11:141.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical responses and pharmacokinetics of fully human BCMA targeting CAR T-cell therapy in relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19:e23-4.
- [CrossRef] [Google Scholar]
- Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726-37.
- [CrossRef] [PubMed] [Google Scholar]
- Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Nat Acad Sci U S A. 2018;116:9543-51.
- [CrossRef] [PubMed] [Google Scholar]
- A phase I study of anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma and plasma cell leukemia. Clin Transl Med. 2021;11:e346.
- [CrossRef] [PubMed] [Google Scholar]
- B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129:2210-21.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight. 2018;3:120505.
- [CrossRef] [PubMed] [Google Scholar]
- CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. 2016;2:28-35.
- [CrossRef] [Google Scholar]
- Clinical responses with T lymphocytes targeting malignancy-associated k light chains. J Clin Investig. 2016;126:2588-96.
- [CrossRef] [PubMed] [Google Scholar]
- Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. 2018;7:100-12.
- [CrossRef] [PubMed] [Google Scholar]
- Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma. Cancer Med. 2021;10:563-74.
- [CrossRef] [PubMed] [Google Scholar]
- A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019;6:e521-9.
- [CrossRef] [PubMed] [Google Scholar]
- sBCMA plasma LEVEL dynamics and anti-BCMA CAR-T-cell treatment in relapsed multiple myeloma. Curr Issues Mol Biol. 2022;44:1463-71.
- [CrossRef] [PubMed] [Google Scholar]
- Toxicities of chimeric antigen receptor T cell therapy in multiple myeloma: An overview of experience from clinical trials, pathophysiology, and management strategies. Front Immunol. 2020;11:620312.
- [CrossRef] [PubMed] [Google Scholar]
- A phase 1, multicenter study evaluating the safety and efficacy of KITE-585, an autologous anti-BCMA CAR T-cell therapy, in patients with relapsed/refractory multiple myeloma. Am J Cancer Res. 2021;11:3285-93.
- [Google Scholar]
- Anti-B-cell maturation antigen chimeric antigen receptor t cell function against multiple myeloma is enhanced in the presence of lenalidomide. Mol Cancer Ther. 2019;18:2246-57.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-CD19 and anti-BCMA CAR T cell therapy followed by lenalidomide maintenance after autologous stem-cell transplantation for high-risk newly diagnosed multiple myeloma. Am J Hematol. 2022;97:537-47.
- [CrossRef] [PubMed] [Google Scholar]
- BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020;13:125.
- [CrossRef] [PubMed] [Google Scholar]
- Co-stimulatory receptor signaling in CAR-T cells. Biomolecules. 2022;12:1303.
- [CrossRef] [PubMed] [Google Scholar]
- A novel lipidic peptide with potential to promote balanced effector-regulatory T cell responses. Sci Rep. 2022;12:11185.
- [CrossRef] [PubMed] [Google Scholar]
- Chimeric antigen receptors containing the OX40 signalling domain enhance the persistence of T cells even under repeated stimulation with MM target cells. J Hematol Oncol. 2022;15:39.
- [CrossRef] [PubMed] [Google Scholar]
- Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813-22.
- [CrossRef] [PubMed] [Google Scholar]
- A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma: Updated results from a phase 1 dose-climbing trial. Blood. 2019;134:930.
- [CrossRef] [Google Scholar]
- On the road to eliminating long-lived plasma cells-"are we there yet? " Immunol Rev. 2021;303:154-67.
- [CrossRef] [PubMed] [Google Scholar]
- Preclinical evaluation of CD8+ anti-BCMA mRNA CAR T cells for treatment of multiple myeloma. Leukemia. 2021;35:752-63.
- [CrossRef] [PubMed] [Google Scholar]
- Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34-48.
- [CrossRef] [PubMed] [Google Scholar]
- Chimeric antigen receptor T cell therapy during the COVID-19 pandemic. Biol Blood Marrow Transplant. 2020;26:1239-46.
- [CrossRef] [PubMed] [Google Scholar]








