Translate this page into:
Scope and limitations of intraoperative cytological methods of diagnosis of ovarian tumors

*Corresponding author: Arvind Kumar, Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. drarvindkumar10@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ahsan E, Gautam SK, Singh A, Kumar A, Phulware RH. Scope and limitations of intraoperative cytological methods of diagnosis of ovarian tumors. CytoJournal. 2025;22:42. doi: 10.25259/Cytojournal_226_2024
Abstract
The mainstay of treatment for ovarian cancer is surgery. To prevent under-treatment and overtreatment and to choose the best surgical strategy for patients with ovarian tumors, intraoperative pathological assessment is essential. Frozen sections (FSs) have been historically used for intraoperative evaluation. In 1927, cytology was introduced by Dudgeon and Patrick as a new method of intraoperative pathological examination. Diagnosis can be made in minutes by making smears from the lesion, staining them quickly, and analyzing them under a microscope. Following a comprehensive search of the literature, using pertinent keywords in PubMed, and reviewing the data, it was discovered that intraoperative cytology (IOC) had been reported to have a diagnostic accuracy in ovarian lesions comparable to that of FSs. Few of the studies have confirmed that IOC has several benefits over FSs. There are drawbacks as well, which one should be mindful of. In this review, every aspect that is connected to IOC is covered in detail, along with the potential for raising the standard of IOC to make it more applicable in the present times.
Keywords
Frozen section
Imprint cytology
Intraoperative cytological methods
Ovarian neoplasm
Scrape cytology
INTRODUCTION
Ovarian tumors constitute a heterogeneous group of lesions, which include benign, borderline, and malignant tumors.[1] An increased risk of ovarian cancer has been linked to several modifiable and non-modifiable factors, such as aging, a family history of the disease, inherited genetic mutations (e.g., BRCA1/BRCA2 mutations), obesity, nulliparity, and the use of hormone replacement therapy.[2] The world’s developed and developing regions are both impacted; however, the incidence is twice as high in the former.[3]
Based on the Globocan 2018 Fact Sheet, ovarian cancer was found to be the eighth most prevalent cancer overall and the third most common among Indian women, accounting for 3.44% (36170) of all cancer cases.[4] Moreover, it is also a grave cause of cancer-related fatalities among Indian women, accounting for 3.34% (24015) of all cancer-related deaths in India in a given year.[5]
A physical examination, which includes a pelvic examination, a blood test for tumor markers like cancer antigen 125 (CA-125), and transvaginal ultrasonography, are the first steps in the diagnosis of ovarian cancer.
However, their usefulness in distinguishing among benign, borderline, and malignant categories is limited. CA-125, a serum tumor marker, lacks specificity. In the early stages of ovarian cancer, the CA-125 level may be normal; it may also be elevated in non-neoplastic diseases such as pelvic inflammatory disease and endometriosis.
In addition, imaging is not always reliable in accurately diagnosing ovarian masses, particularly when there are big, heterogeneous lesions. Moreover, on imaging, borderline ovarian tumors are frequently misinterpreted as benign.
Therefore, intraoperative pathological assessment is essential for determining the malignancy, stage, and course of treatment of ovarian tumors. The most widely used method for intraoperative diagnosis has traditionally been frozen sections (FSs). However, intraoperative cytology (IOC) has become the primary method of intraoperative pathological assessment for ovarian tumors at several centers in resource-poor settings with its benefits including quick results and ease of production of numerous high-quality preparations.
The goal of this study is to determine the potential benefits and drawbacks of intraoperative cytological diagnosis of ovarian tumors, as well as to evaluate the limitations and suggest corrective actions.
MATERIAL AND METHODS
A literature search was performed only in PubMed using the following terms: Ovarian tumors, intraoperative examination, FS, intraoperative cytological examination, limitations, improvement, history, and India. Relevance was assessed, and articles that discussed the advantages and disadvantages of IOC in comparison to FSs about ovarian tumors were included.
After searching for the keywords alone or in combination, without a time limit, 2048 source results were found. Three hundred one sources appeared in the results after the search parameters were adjusted. Fifty two of the 301 maximally applicable sources were used as references in this review article. For more pertinent information, the references to each article were reviewed. The information obtained was combined and included in this review. A flow chart depicting the selection of studies was included [Figure 1]. The software used was Microsoft® Word for Microsoft 365 MSO (Version 2501 Build 16.0.18429.20132) 64-bit. The manufacturer is Microsoft Corporation.
- Flow chart for the selection of studies.
HISTORICAL ASPECT
Dr. Louis B. Wilson, an American pathologist and the chief of pathology at Mayo Clinic, is widely acknowledged as having pioneered intraoperative FSs. Cytology was first used as an intraoperative pathological evaluation technique by Leonard S. Dudgeon and Vincent Patrick at the University of London in 1927.[6] This opened up new possibilities for the quick and accurate diagnosis of newly cut specimens. Following these preliminary attempts, traditional inspection of FSs was preferred for several decades, and the use of cytology smears during intraoperative consultation was overlooked. However, several recent investigations have shown that diagnostic effectiveness of the IOC is on par with FSs.
UTILITY OF FS IN OVARIAN TUMORS
FS is the gold standard in intraoperative diagnosis, and it can be used to evaluate the efficacy of IOC.[7] In a retrospective study, Sukumaran et al. compared the FS and permanent section diagnoses of 233 cases of ovarian tumors. The FSs total accuracy was 91.85%.[8]
From January 2011 to December 2018, Rukmangadha et al. conducted a retrospective study including 289 cases.[9] Thirty-five cases were found to be incompatible. The diagnostic accuracy of FSs was 87.89% overall. With a specificity of 91.8% and a sensitivity of 68.7%, borderline tumors showed the lowest results.[9] In a retrospective review of the FS shown by Kung et al., the diagnostic accuracy was 97.20%, with 100% specificity and 92.51% sensitivity considering borderline and malignant diagnoses together.[10]
Jena et al. compared FSs on 49 ovarian tumors that were radiologically and clinically diagnosed with the final histopathologic diagnosis. Five cases were false-negative, and there were no false-positive cases. With a mean diameter of 26 cm, the five discordant cases were all mucinous ovarian neoplasms.[11]
From January 2009 to December 2014, Hashmi et al. assessed 141 cases of ovarian tumors. Over 99% of FS diagnoses were made correctly. For benign and malignant tumors, the sensitivity and specificity were broadly similar, but for borderline tumors, they were 83% and 99%, respectively.[12]
A mucinous tumor of the ovary can lead to a discordant diagnosis between frozen and final.[13]
A study conducted by Sarma et al. over 2 years showed that the concordance rate of FSs with histopathological diagnosis was 91.67%. Diagnostic accuracy for benign, malignant, and borderline tumors was 88.64%, 72.63%, and 55.56%, respectively.[14] Table 1 and Figure 2 having various studies are given for the correlation of FS findings with corresponding final histopathology.
| S. No. | Study | Study period | Number of patients | Benign | Borderline | Malignant | |||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | ||||
| 1. | Palakkan et al. (2020)[7] | 2012–2013 | 60 | 95 | 100 | 75 | 94 | 90 | 97 |
| 2. | Sukumaran et al. (2014)[8] |
2009–2012 | 233 | 99.2 | 96.5 | 88.46 | 93.23 | 82.95 | 99.3 |
| 3. | Rukmangadha et al. (2022)[9] | 2011–2018 | 289 | 93 | 91.4 | 68.7 | 91.8 | 81.4 | 96.8 |
| 4. | Kung et al. (2019)[10] | 2006–2016 | 1143 | 100 | 92.51 | 92.51 (Borderline +Malignant) |
100 (Borderline+ Malignant) |
NA | NA |
| 5. | Jena et al. (2017)[11] | 2013–2016 | 49 | 93.5 | 72.7 | 58.3 | 90 | 100 | 100 |
| 6. | Hashmi et al. (2016)[12] | 2009–2014 | 141 | 100 | 97 | 96 | 100 | 83 | 99 |
| 7. | Suprasert et al. (2008)[13] | 2001–2005 | 112 | 100 | 92.7 | 84 | 97.9 | 92 | 100 |
| 8 | Sarma et al. (2023)[14] | 2020–2022 | 60 | 95.45 | 92.73 | 72.73 | 100 | 100 | 92.16 |
NA: Not available
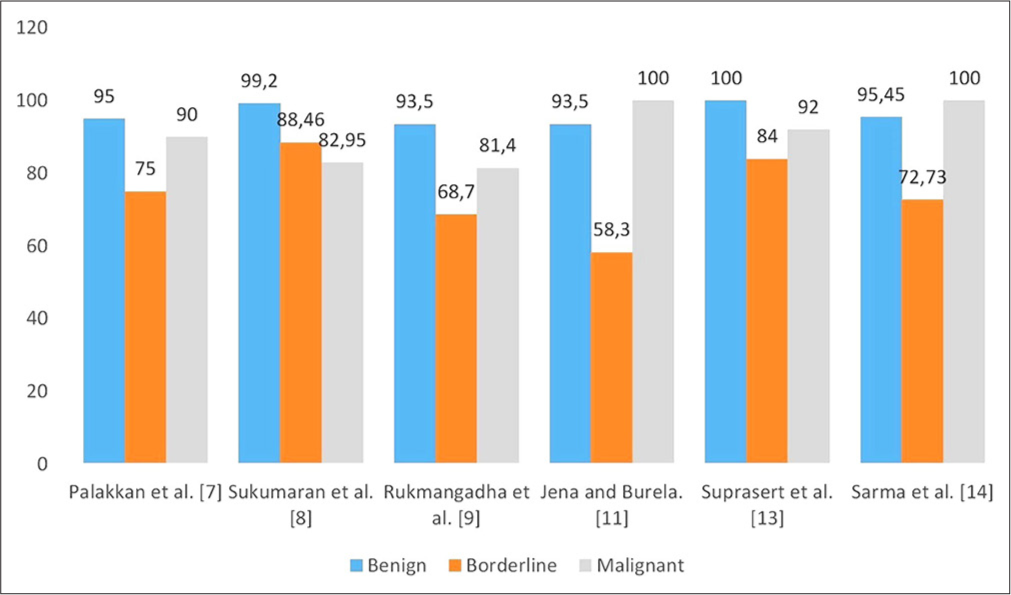
- Diagnostic accuracy (%) of the frozen section with final histopathological diagnosis.
LIMITATIONS OF FS
The accuracy of FS diagnosis can be affected by various factors such as the expertise of a pathologist, representative tissue, and solid or cystic gross specimen a histopathological type of tumor. In most research, sampling error was thought to be the primary cause of the diagnostic mismatch. This is especially true for certain tumor types, such as mucinous tumors and teratomas, where there is notable tumor heterogeneity within the same ovarian mass.[11,13] In Sukumaran et al.’s study, the diagnosis of a mixed tumor-mature teratoma and borderline mucinous tumor was made on an FS after noticing foci of atypical mucinous epithelial proliferation in a teratoma. Multiple paraffin-embedded sections of the specimen showed an adenocarcinoma component within a fully developed cystic teratoma.[8] In FS, it takes a while to search for immature components in ovarian teratomas. Therefore, until the tumor is sufficiently sampled later, a report of a benign teratoma does not completely rule out a malignant one. Unlike serous tumors, which are usually unilocular and homogenous, a single mucinous ovarian tumor might occasionally have benign, borderline, and malignant components.[15] An FS may miss sampling the most atypical area. Huang et al.’s meta-analysis confirms that unilateral tumors and mucinous histology are linked to the incorrect FS diagnosis of borderline ovarian cancers.[16]
Undersampling of borderline ovarian tumors typically results in underdiagnosis.[12] Overdiagnosis is rare, though there have been a few cases where tangential cutting gave the erroneous sense of invasion.[17,18]
Thick sections relative to permanent sections and freezing effects that mask the finer features are two other drawbacks of FSs. Significant harm is done to the FS’s tissue structure by freezing artifacts. Any water in the xylene solution used contributes to foggy portions, whereas inadequate xylene treatment and poor slide covering result in drying artifacts. Any of these can compromise the sections’ morphology.
The paraffin-embedded section’s cell morphology is superior to the majority of FS cases. When reviewing the tissue sample, the pathologist must take into account that FS frequently causes the cells to enlarge and seem bloated. For underdeveloped nations with limited resources, FSs are costly. Hence, the limitations of FS also make the IOC relevant in the present scenario.[19,20]
IMPRINT CYTOLOGY
Imprint cytology is a quick and easy method for intraoperative. Imprint is a touch preparation in which tissue is touched on the slide and leaves behind its impression in the shape of cells on the glass slide.[21]
The technique for imprint smear, as described by Tribe, includes appropriately labeling slides using a pencil and sectioning the tissue. The disease-suggestive areas are gently brushed with dry gauze to remove any blood. The slide is lightly pressed on the specimen’s freshly cut surface without gliding. The amount of pressure used to imprint depends on the consistency of the specimen.[22]
The advantages of imprint cytology are numerous. It can be performed with minimal training and underdeveloped infrastructure. It is economical and produces an instantaneous result with little or no artifacts. It is possible to swiftly prepare several slides in one sitting. However, imprint cytology cannot analyze the depth of penetration. Furthermore, this approach is not appropriate for tumors with dense fibrous stroma.[23]
IMPRINT VERSUS FROZEN
Ivan et al. used FS and imprint cytology to analyze 76 cases of ovarian tumors, with the histopathological report serving as the gold standard. Three out of nine cases of borderline tumors were correctly diagnosed with FSs; however, borderline tumors could not be diagnosed using an imprint smear. The diagnostic accuracy for benign tumors was determined to be slightly higher by the imprint technique, while it was equal for malignant tumors.[24]
Azami et al. used FS and imprint cytology to investigate 55 cases of ovarian tumors. Both FSs and imprint cytology provided appropriate diagnoses of benignity and malignancy. For imprint cytology, the diagnosis accuracy of benign tumors was 90.9% (50/55), whereas for frozen sections, it was 96.3% (53/55).[25]
Nomura et al. suggested using IOC for additional intraoperative evaluation of any tumors on FSs initially identified as borderline tumors. Seventy-eight percent of cases were compared by IOC, FS, and final histopathology in terms of both behavioral types that are benign, borderline, or malignant and histologic subtypes that are serous, mucinous, endometrioid, or clear cell tumors.[26]
Kediya et al. concluded that intraoperative imprint cytology has greater diagnostic accuracy (80%) than FSs (76%). For imprint cytology, the corresponding values for sensitivity, specificity, positive predictive value, and negative predictive value were 77.5%, 90.0%, 96.9%, and 50%; in contrast, for the FS, the corresponding values were 72.5%, 90.0%, 96.7%, and 45.0%.[27] Shiva Murthy et al. study included 53 cases of ovarian tumors with imprint smears, FS slides, and routine histopathology slides. The final histopathology diagnosis did not correspond with the results of imprint cytology in five cases. One case of endometrioid carcinoma was misdiagnosed as a cystadenocarcinoma; one case of mucinous carcinoma was misdiagnosed as a borderline mucinous tumor; two cases of borderline mucinous tumors were misdiagnosed as mucinous cystadenomas; and one case was a mixed germ cell tumor with a yolk sac component and an embryonal component. As a result, the sensitivity and specificity were, respectively, 93.75% and 97%.[28]
Abe et al. assessed the value of IOC in ovarian germ cell tumors. For immature teratoma, accuracy was 54.5% (6/11), but for other tumors, it was 91.7% (11/12). In both of the cases, cytologic testing allowed for an appropriate diagnosis; an intraoperative diagnosis by FS proved to be wrong.[29] In a study led by Melies et al., 100 patients with ovarian masses identified by radiographic, ultrasound, or clinical evaluation were included. The study’s results showed 84.85% sensitivity, 100% specificity, and 92.75% diagnostic accuracy. They found that because malignant and epithelial borderline neoplasms have comparable cytological characteristics, such as the presence of hyperchromatic nuclei, pleomorphism, and complicated branching architecture, it can be difficult to distinguish between the two types of neoplasms. The invasion of the stroma is the only distinction between them. This is the research restriction that Nagai et al. also encountered.[30]
In the study by Akbar et al., the corresponding values for sensitivity and specificity were 82.2% and 89.4%, respectively.[31] Their investigation was limited by inadequate cellularity and interpretation errors, particularly in cases of epithelial tumors. Due to overlapping papillary cell clusters displaying considerable nuclear pleomorphism, smears from two borderline serous tumors were classified as serous adenocarcinomas on the imprint. Therefore, histopathology is necessary to determine if stromal invasion has occurred or not. Ivan et al. proposed scrapping the slides to maximize cell production in response to a similar problem.[24]
Souka et al. evaluated to assess the combined use of cytological imprint and FS that led to the diagnostic pick up by 84% and 74% for frozen and imprint, respectively; however, combined cytologic and cryostat methods showed 90% accuracy of diagnosis.[32] Thirty-one cases out of the forty ovarian neoplasms evaluated in a study by Sireesha et al. showed a correlation with the histopathological findings. Four of the nine instances that did not correlate were carcinomas, and four were borderline cases. The reason could be that a huge tumor made it difficult to sample the representative area. A granulosa cell tumor had solid and cystic areas filled with serous material. Imprint smears were hypocellular and revealed cells with pleomorphism and slight nuclear atypia, which suggested that the tumor might be a borderline epithelial serous-type tumor. The study’s overall accuracy was 87.5%, with a sensitivity of 88.89%, a specificity of 86.36%, and a positive predictive value of 84.21%.[33]
Panicker et al. divided 194 cases into four categories: Surface epithelial tumors, sex cord stromal tumors, germ cell tumors, and tumor-like lesions. The diagnostic accuracy for each of the categories was 96.4%, 98.5%, 100%, and 97%, respectively.[34] . When cytologic smears were added to ovarian FSs, the total number of deferrals and discrepancies dropped from 13.75% to 7.85%. The majority of benefits were noted in cases of primary ovarian cancer.[35]
Of the 50 instances in Vijayakumar’s investigation, 45 showed diagnostic concordance. With 90% of the patients having results that matched the final diagnosis, the intraoperative imprint cytology demonstrated satisfactory overall diagnostic accuracy. Five cases where the intraoperative imprint cytology did not correspond with the final diagnosis included two endometrioid carcinomas misdiagnosed as cystadenocarcinomas, two mucinous carcinomas misdiagnosed as borderline mucinous tumors, and one borderline mucinous tumor misdiagnosed as mucinous cystadenoma, similar to the study done by Shivamurthy and Jaiprakash.[28,36]
Jennifer compared the imprint cytology and FS diagnostic accuracy with that of the permanent sections. The study comprised fifty-four individuals. While the FS had an accuracy rating of 90.7%, imprint cytology had a higher rate of 94.4%. Each was 100% specific.
Imprint sensitivity was 80%, whereas FS sensitivity was 66.7%.[37] When diagnosing ovarian neoplasms intraoperatively, imprint cytology is a highly useful diagnostic technique. In terms of accuracy, it is similar to the frozen portion and occasionally superior because imprint smears retain cytomorphology, whereas FSs retain tissue architecture but lack cytological features.[22] In FSs, freezing artifacts are frequently encountered due to ice crystal formation, resulting in the alteration of nuclear details and anisonucleosis.[38] Thicker sections result in the folding of the sections and removal from the slide surface due to insufficient adhesion during staining.[19] The imprint technique offers vivid cytological details. It makes it possible to analyze individual cells.[23] It is useful in identifying the malignant tumor’s histologic subtype.[26]
LIMITATION OF IMPRINT CYTOLOGY
One of the shortcomings of imprint cytology is the diagnosis of borderline ovarian tumors. This can be attributed to sampling inaccuracy and substantial tumor heterogeneity, which is an admixture of borderline and benign areas within the same ovarian mass.[24,25,28,30,37] It is also not possible to assess the depth of infiltration with imprint cytology.[23]
Therefore, a thorough gross examination is crucial. By obtaining several imprints from various representative areas, imprint cytology can be further refined for even greater precision.[21]
In conclusion, if a rapid diagnosis is needed or resources are limited, imprint cytology can be utilized independently to support intra-operative decision-making. In addition, it can be used in conjunction with an FS to provide a more precise intraoperative diagnosis.
SCRAPE CYTOLOGY
It is a variation of imprint cytology in which the tumor’s surface is gently scraped or brushed with a glass slide’s edge before being smeared over another clean glass slide.[39]
Samaddar and Talukdar conducted a study to evaluate the accuracy of scrape cytology in the diagnosis of ovarian tumors. Overall diagnostic accuracy was 90.91%; for benign and malignant tumors, it was 95.45% and 89.74%, respectively. With only 40% of cases correctly diagnosed, the results were dismal in the case of borderline tumors .[40]
Intraoperative scrape and FS procedures were used to examine 121 patients with ovarian masses who underwent surgery for ovarian neoplasms. The most frequent cases of diagnostic difficulties were corpus luteoma, mucinous cyst, and clear cell carcinoma. The overall accuracy percentages for scrape and FS were 91.86% and 92.68%, respectively. The final pathology report revealed the diagnosis of clear cell carcinoma, which was not made by scrape or FS. This could have happened because the tissues were not stained appropriately.[41]
The potential use of scrape cytology in intraoperative consultation for ovarian lesions was assessed by Khunamornpong and Siriaunkgul. In the benign group, scrape cytology accuracy was 95%; in the low malignant potential (LMP) group, it was 47%; and in the malignant group, it was 98%. In 78% of the instances, the histologic subtypes were accurately predicted. Scrape cytology has limits when it comes to diagnosing mucinous tumors and LMP, which require sufficient histologic sampling and an assessment of the histologic architecture.[42]
Ninety-two percent of instances correlated with the final diagnosis, according to observations made by Rao et al., scrape cytology had an overall satisfactory diagnostic accuracy. In mucinous tumors, the method was not very useful for differentiating between borderline cases and invasive carcinoma. Two endometrioid carcinomas were incorrectly identified as cystadenocarcinomas on scrape cytology, while two mucinous carcinomas were diagnosed as borderline mucinous tumors.[43]
These disparities were also seen in the imprint cytology study conducted by Shivamurthy and Jaiprakash.[28]
Stewart et al. conducted a study on 402 ovarian tumors that were submitted for intraoperative assessment. The performance of FSs and scrape cytology was also similar in the case of malignant tumors, with FSs correctly diagnosing 97% of malignant tumors and scrape cytology correctly diagnosing 93%. However, the diagnostic accuracy of scrape cytology was significantly lower (66%) as compared to that of frozen (86%) for borderline tumors.[44,45] The cytomorphology of clear cell carcinoma in the ovary was described by Vrdoljak-Mozetić et al. using intraoperative peritoneal fluid samples, imprints, and scrape smears of the cancer tissue. Apart from varying clear cell morphology, one or both of the following distinct cytological characteristics were observed in 33.3% of peritoneal fluid samples and 92.9% of imprint and scrape cytological samples: Eosinophilic, hyaline, extracellular, globular substance with or without formation of a “raspberry” body and eosinophilic, intracytoplasmic inclusions. Only the May-Grünwald-Giemsa-stained smears showed these structures clearly.[45] Tumor cells enveloping extracellular, magenta hyaline globules are known as raspberry bodies.[46] They are a distinct feature in ovarian clear cell carcinoma and are composed of deposits from the basement membrane.[47] When clear cell carcinoma is diagnosed intraoperatively, IOC is crucial because aggressive surgical care is required since optimum tumor debulking has been demonstrated to improve overall survival.[48]
Bhardwaj et al. study covered a total of 60 patients with clinically and radiologically suspected ovarian tumors. The final histopathological impression was compared with the intraoperative diagnosis in both cases. For scrape cytology, the diagnosis accuracy was 96%; for FSs, it was 100%. Scrape cytology allowed the tumor to be correctly classified as surface epithelial, germ-cell tumor, sex cord-stromal, or other in 93% of the instances.[49]
A summary in the form of a table and figure having a correlation of imprint cytology with FS is exhibited in Table 2 and Figure 3, respectively.
| S. No. | Study | Frozen (%) | Imprint (%) | Comments |
|---|---|---|---|---|
| 1. | Kediya et al. (2023)[27] | 76 | 80 | High diagnostic accuracy of imprint cytology with frozen section |
| 2. | Ivan et al. (2020)[24] | 84.6 (for malignant cases) |
84.0 (for malignant cases) | Borderline tumors could not be diagnosed by imprint. |
| 3. | Azami et al. (2017)[25] | 96.3 | 90.9 | Low diagnostic accuracy in the borderline group whereas higher in the malignant group |
| 4. | Nomura et al. (2021)[26] | 88 | 87 | Imprint had higher diagnostic accuracy for malignant tumors and histologic subtypes. |
| 5. | Shivamurthy and Jaiprakash (2021)[28] | 100 | 90.5 | One case of mucinous carcinoma was misdiagnosed as a borderline mucinous tumor, and two cases of borderline mucinous tumor were misdiagnosed as mucinous cystadenomas |
| 6. | Melies et al. (2018)[30] | NA | 92.75 | Difficulty in differentiating borderline and malignant tumors |
| 7. | Souka et al. (1990)[32] | 74 | 84 | Combined accuracy: 90 |
| 8. | Jennifer (1997)[37] | 90.7 | 94.4 | High diagnostic accuracy of imprint cytology with frozen section |
NA: Not available
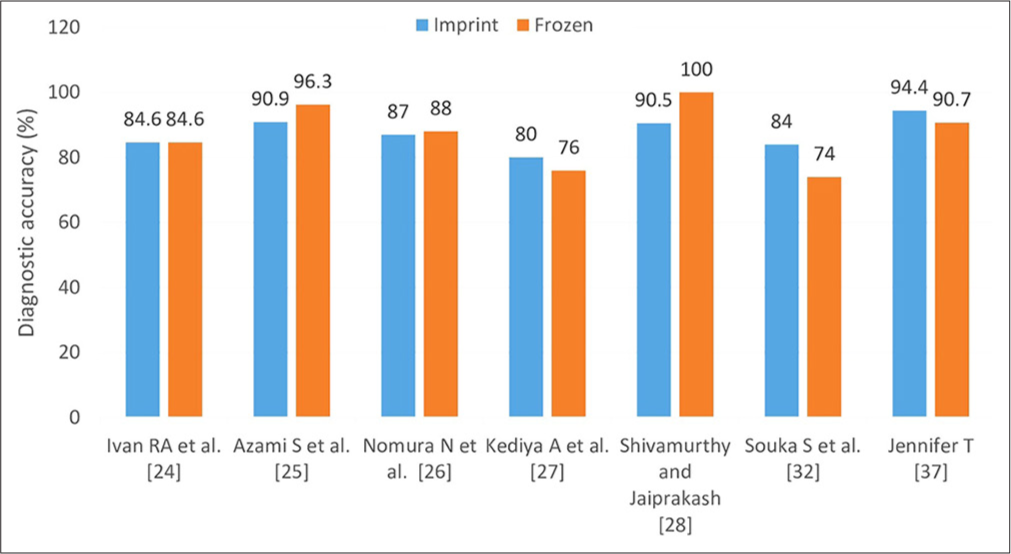
- Diagnostic accuracy (%) of frozen section and imprint cytology when compared with final histopathological diagnosis.
SCRAPE CYTOLOGY VERSUS IMPRINT CYTOLOGY
Scrape cytology carries similar merits and demerits as imprint, but the scrape technique provides better cell yield. The scrape approach was determined to be the most effective way of preparing the smears, according to a study done by Ivan et al.[24] In comparison to other cytological methods, there was a high level of cellularity and superior morphological preservation. The sensitivity of scrape cytology was found to be higher (92.1%) than that of imprint (73%), comparable with that of FS analysis (92.6%) in a study conducted by Mohammadnia Avval et al. on sentinel lymph nodes in patients with breast cancer.[50] Gautam et al. compared imprint and scrape cytology in the intraoperative diagnosis of soft tissue tumors and tumor-like lesions. The study found that the diagnostic yield of scrape smears was 100%, while that of imprint smears was only 24%.[51] Cellular yield is higher in scrape cytology in comparison to imprint. However, individual cellular details tend to be better appreciated in imprint cytology because of the monolayering of cells. It is particularly useful in low cellular tumors or lesions with cystic changes. A glimpse of three studies correlating diagnostic accuracy with scrape cytology is given in Table 3.
| S. No. | Study | Frozen (%) | Scrape (%) | Comments |
|---|---|---|---|---|
| 1. | Elaheh et al. (2015)[41] | 91.86 | 92.68 | The diagnostic accuracy of scrap cytology was slightly better than that of frozen section |
| 2. | Stewart et al. (2006)[44] | Malignant-97.0 Borderline-86.00 |
Malignant-93.0 Borderline-66.00 |
The diagnostic accuracy of scrape cytology was significantly lower for borderline tumors |
| 3. | Bhardwaj et al. (2019)[49] | 100 | 96.00 | The diagnostic accuracy of scrape cytology was equivalent to the frozen section |
CRUSH CYTOLOGY
Crush preparations are made by compressing the tissue between two slides, and the material obtained is smeared by another slide.[52] It is used for necrotic and friable tissues.
Fine-needle aspiration cytology in the pre-operative investigation of the ovarian tumor has been discouraged for the following reasons: Concern for tumor contents seeping into the peritoneal cavity and subsequent implantation; a ruptured capsule that causes the tumor to upstage. There is a need for repeated punctures in multi-loculated ovarian masses.[53]
LIMITATIONS OF IOC EVALUATION
FSs should be preferred over IOC in the following situations: To differentiate between in situ and invasive carcinoma, to estimate the depth of tumor penetration, to assess the surgical resection margins, and to verify whether the tumor has been eliminated or not. When there is a discrepancy between the tumor’s gross appearance and its intraoperative cytological features, FSs may be used. Scirrhous lesions are challenging to prepare for cytological analysis because they are dense and unyielding. IOC is not very effective in the diagnosis of borderline tumors. In such cases, FSs may be preferred over cytology for a more accurate diagnosis.[54-56]
ADVANTAGES OF IOC OVER FS
IOC, especially rapid Giemsa stain, can easily detect background mucin, intra-cellular mucin, cytoplasmic vacuolization, and background stromal fragments that help in the diagnosis of ovarian sex cord stromal tumor and serous and mucinous neoplasm. Cytology preparations do not require as much technical knowledge as FSs do, so they do not depend as much on the availability of qualified technical personnel. These cytology techniques are more widely available, particularly in settings with low resources. The cellular features have been preserved exceptionally well, and there has been no tissue loss, unlike cryostat sections. Tissue-freezing-related artifacts are absent. This decreases the minimum required quantity of diagnostic material. Moreover, preparations that may be infectious can be evaluated cytologically without contaminating cryostat facilities.
SUMMARY
IOC with rapid stain can yield a cost-effective and early diagnosis in resource-poor settings such as developing nations like ours, where FSs are frequently unavailable. Intraoperative cytological diagnosis can be used as an alternative to FSs since it can discriminate between benign and malignant tumors conveniently. When a clinician, radiologist, and pathologist work in close cooperation, IOC can be a very effective technique for the early diagnosis of ovarian masses.
ACKNOWLEDGMENT
Not applicable .
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
IOC: Intraoperative cytology
FS: Frozen Sections
NA: Not applicable
AUTHOR CONTRIBUTIONS
EA: Drafting the manuscript, literature search and final approval; SKG: Critical revision and final approval; AS: Editing, revision and did final approval; AK: Conceptualization and designed of the study, guarantor, and final approval; RHP: Editing, critical revision and final approval. All authors meet the authorship status of ICMJE.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This review was based on the previously published studies, thus ethical approval and patient consent are not required.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: Not applicable .
References
- Pathobiology of ovarian carcinomas. Chin J Cancer. 2015;34:50-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian cancer in the world: Epidemiology and risk factors. Int J Womens Health. 2019;11:287-99.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of ovarian cancer: A review. Cancer Biol Med. 2017;14:9-32.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of treatment and outcomes in epithelial ovarian cancer: A retrospective north Indian single-institution experience. JCO Glob Oncol. 2022;8:e2200032.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelial ovarian cancer 2024 May 6 In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025. Available from: https://pubmed.ncbi.nlm.nih.gov/33620837/
- [Google Scholar]
- Comparative study between intraoperative frozen section and scrape smear cytology in the diagnosis of ovarian neoplasm. Open J Obstet Gynecol. 2015;5:28-35.
- [CrossRef] [Google Scholar]
- Role of frozen section in the surgical management of ovarian neoplasm. Gynecol Minim Invasive Ther. 2020;9:13-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of frozen section in intraoperative assessment of ovarian masses: A tertiary oncology center experience. Indian J Surg Oncol. 2014;5:99-103.
- [CrossRef] [PubMed] [Google Scholar]
- Role of intraoperative frozen section in the diagnosis of ovarian neoplasms-a retrospective study in an oncology center. J Clin Diagn Res. 2022;16:EC11-4.
- [CrossRef] [Google Scholar]
- Intraoperative frozen section analysis of ovarian tumors: An 11-year review of accuracy with clinicopathological correlation in a Hong Kong regional hospital. Int J Gynecol Cancer. 2019;29:772-8.
- [CrossRef] [PubMed] [Google Scholar]
- Role of frozen section in the diagnosis of ovarian masses: An institutional experience. J Med Sci Health. 2017;3:12-8.
- [CrossRef] [Google Scholar]
- Accuracy of intraoperative frozen section for the evaluation of ovarian neoplasms: An institutional experience. World J Surg Oncol. 2016;14:91.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of intra-operative frozen sections in the diagnosis of ovarian masses. Asian Pac J Cancer Prev. 2008;9:737-40.
- [Google Scholar]
- Issues and challenges in diagnoses of borderline ovarian neoplasms by frozen section. Asian Pac J Cancer Biol. 2023;8:39-44.
- [CrossRef] [Google Scholar]
- Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist. 2012;17:1515-33.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of frozen section analysis of borderline ovarian tumors: A meta-analysis with emphasis on misdiagnosis factors. J Cancer. 2018;9:2817-24.
- [CrossRef] [PubMed] [Google Scholar]
- A review of artifacts in histopathology. J Oral Maxillofac Pathol. 2018;22:279.
- [CrossRef] [PubMed] [Google Scholar]
- Borderline ovarian tumors and the diagnostic dilemma of intraoperative diagnosis: Could preoperative He4 assay and ROMA score assessment increase the frozen section accuracy? A multicenter case-control study. Biomed Res Int. 2014;2014:e803598.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-operative frozen section consultation: Concepts, applications and limitations. Malays J Med Sci. 2006;13:4-12.
- [Google Scholar]
- Frozen section versus permanent section in cancer diagnosis: A single center study. Asian Pac J Cancer Care. 2022;7:247-51.
- [CrossRef] [Google Scholar]
- Imprint cytology: An invaluable technique to evaluate fresh specimens received in the pathology department for lymphoma workup. Cancer Cytopathol. 2021;129:759-71.
- [CrossRef] [Google Scholar]
- A comparison of rapid methods including imprint cytodiagnosis for the diagnosis of breast tumors. J Clin Pathol. 1973;26:273-7.
- [CrossRef] [Google Scholar]
- Comparison between imprint cytology and frozen sections in intraoperative consultation of ovarian tumors. Int J Res Med Sci. 2020;8:3315.
- [CrossRef] [Google Scholar]
- Useful aspects of diagnosis of imprint cytology in intraoperative consultation of ovarian tumors: Comparison between imprint cytology and frozen sections. Diagn Cytopathol. 2017;46:28-34.
- [CrossRef] [Google Scholar]
- Accuracy of imprint cytology and frozen section histology for intraoperative diagnosis of ovarian epithelial tumors: A comparative study and proposed algorithm. Diagn Cytopathol. 2021;49:682-90.
- [CrossRef] [Google Scholar]
- Evaluation of the accuracy of intraoperative frozen section and imprint cytology in gynecological neoplasms-a descriptive cross-sectional study of 50 cases in a tertiary care center. J Lab Physicians. 2023;15:552-7.
- [CrossRef] [Google Scholar]
- Role of imprint cytology in the diagnosis of ovarian neoplasms. Indian J Pathol Oncol. 2021;8:320-6.
- [CrossRef] [Google Scholar]
- Usefulness of intraoperative imprint cytology in ovarian germ cell tumors. Acta Cytol. 2013;57:171-6.
- [CrossRef] [Google Scholar]
- Evaluation of intraoperative imprint cytology in ovarian tumors. J Cytol Histol. 2018;9:2.
- [CrossRef] [Google Scholar]
- Efficacy of imprint cytology in the diagnosis of ovarian tumors. J Soc Obstet Gynaecol Pak. 2022;12:54-9.
- [Google Scholar]
- The combined use of cytological imprint and frozen section in the intraoperative diagnosis of ovarian tumors. Int J Gynecol Obstet. 1990;31:43-6.
- [CrossRef] [Google Scholar]
- Role of imprint cytology in rapid diagnosis of ovarian neoplasms with histopathology correlation. IAIM. 2018;5:56-62.
- [Google Scholar]
- Imprint cytology in the diagnosis of ovarian lesions. Int J Res Med Sci. 2017;3:3770-4.
- [Google Scholar]
- Value of cytology as an adjunctive intraoperative diagnostic method. An audit of 2,250 consecutive cases. Acta Cytol. 1997;41:1489-96.
- [CrossRef] [Google Scholar]
- The diagnostic utility of intraoperative cytology in the management of ovarian tumors. J Clin Diagn Res. 2013;7:1047-50.
- [CrossRef] [Google Scholar]
- Comparison between frozen section and imprint cytology in the intraoperative diagnosis of ovarian malignancy. Far Eastern Univ NRMF Med J. 1997;3:30-8.
- [Google Scholar]
- Diagnostic accuracy of cytology smear and frozen section in glioma. Asian Pac J Cancer Prev. 2019;20:321-5.
- [CrossRef] [Google Scholar]
- Role of scrape cytology smear preparation in the diagnosis of ovarian masses-utility and pitfalls. Diagn Cytopathol. 2023;51:639-45.
- [CrossRef] [Google Scholar]
- Utility of scrape cytology in the management of ovarian neoplasms: A cross-sectional Study in a tertiary care hospital of Eastern India. Indian J Surg Oncol. 2022;13:907-14.
- [CrossRef] [Google Scholar]
- Comparison of intraoperative cytology and frozen section with permanent pathologic results in ovarian masses. Int J Womens Health. 2015;3:99-102.
- [CrossRef] [Google Scholar]
- Scrape cytology of the ovaries: Potential role in intraoperative consultation of ovarian lesions. Diagn Cytopathol. 2003;28:250-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of scrape cytology in ovarian neoplasms. J Cytol. 2009;26:26.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative assessment of ovarian tumors: A 5-year review with assessment of discrepant diagnostic cases. Int J Gynecol Pathol. 2006;25:216-22.
- [Google Scholar]
- Intraoperative cytology of clear cell carcinoma of the ovary. Cytopathology. 2006;17:390-5.
- [CrossRef] [PubMed] [Google Scholar]
- The value of “raspberry bodies” in intraoperative cytologic evaluation of adnexal masses for the diagnosis of clear cell carcinoma of the ovary: A cytological-pathological correlation. Ann Diagn Pathol. 2022;59:151948.
- [CrossRef] [PubMed] [Google Scholar]
- Excessive formation of basement membrane substance in clear-cell carcinoma of the ovary: Diagnostic value of the “raspberry body” in ascites cytology. Diagn Cytopathol. 1997;16:500-4.
- [CrossRef] [Google Scholar]
- Debulking surgery for clear cell carcinoma of the ovary-a case report and literature review. Anticancer Res. 2017;37:5707-11.
- [CrossRef] [Google Scholar]
- Comparative diagnostic accuracy of frozen sections and scrape cytology in ovarian neoplasms. J Mid Life Health. 2019;10:89-92.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing scraping cytology with touch imprint cytology and frozen section analysis in the intraoperative diagnosis of sentinel lymph node metastasis in breast cancer. Diagn Cytopathol. 2021;49:475-9.
- [CrossRef] [PubMed] [Google Scholar]
- Role of intraoperative pathology consultation by imprint and scrape cytology in soft tissue tumors and tumor-like lesions. Sarcoma. 2021;2021:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- The role of intraoperative cytology in the diagnostic evaluation of ovarian neoplasms. Acta Cytol. 2012;56:467-73.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology's role in the diagnosis of ovarian tumor. J Mid Life Health. 2023;14:159-64.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the role of intraoperative cytology technique in diagnosis and management of cancer. J Cytol. 2020;37:126-30.
- [CrossRef] [PubMed] [Google Scholar]
- Role of scrape cytology in the intraoperative diagnosis of tumor. J Cytol. 2010;27:86-90.
- [CrossRef] [PubMed] [Google Scholar]









