Translate this page into:
Efficacy of intraoperative imprint cytology of sentinel lymph node in breast cancer

-
Received: ,
Accepted: ,
How to cite this article: Yadav P, Ahuja S, Zaheer S, Singh M, Chintamani C. Efficacy of intraoperative imprint cytology of sentinel lymph node in breast cancer. CytoJournal. 2024;21:4. doi:10.25259/Cytojournal_37_2023
Abstract
Objective:
The most important determinant of patient outcome in cases of breast carcinoma is the regional lymph node status. Intraoperative assessment of sentinel lymph nodes (SLNs) allows the surgeon to perform axillary lymph node dissection in the same sitting if required. The commonly performed intraoperative methods for SLN evaluation are touch imprint cytology (TIC) and frozen section. The present study aimed to determine the sensitivity, specificity, and accuracy of TIC with histopathological diagnosis as gold standard.
Material and Methods:
The lymph nodes sent for intraoperative examination were bisected along the long axis and touched onto clean glass slides followed by Toluidine blue and rapid Papanicolaou staining. The imprints were reviewed and the interpretation was conveyed to the surgeon. Thereafter, the biopsy was fixed in 10% formalin followed by paraffin embedding with hematoxylin and eosin staining. The specificity, sensitivity, diagnostic accuracy, positive predictive value, and negative predictive value were evaluated with histopathological diagnosis as gold standard.
Results:
A total of 60 patients who underwent resection surgery were included in the study. Majority (36.7%) of patients were in the age group 41–50 years with a mean age of 48.1 ± 10.6 years. There were 54 cases (90%) and 6 cases (10%) of invasive carcinoma of no special type (ductal) and lobular carcinoma, respectively. According to modified Bloom–Richardson scoring, the cases were categorized as Grade 1–6 cases (10%), Grade 2–36 (60%), and Grade 3–18 (30%). The sensitivity and specificity of TIC were 87.5% and 100%, respectively. The diagnostic accuracy of TIC in the diagnosis of metastasis in SLN was 90%.
Conclusion:
TIC is an easy-to-perform, cost-effective, rapid, and accurate technique for axillary lymph node evaluation, which also overcomes the need for a cryostat.
Keywords
Touch imprint cytology
Sentinel lymph node
Breast cancer
Sensitivity
Specificity
INTRODUCTION
Breast cancer is the most common malignancy among females in the world, with about 2.3 million newly diagnosed cases and 6,85,000 deaths globally.[1] As a part of the staging procedure, axillary lymph node resection is an important part of the treatment of breast cancer. However, latest trends have now evolved from a radical approach of axillary lymph node dissection (ALND) to a conserving approach with sentinel lymph node biopsy (SLNB) in patients presenting with a clinically uninvolved axilla.[2]
Nodal status is a crucial prognostic factor in breast cancer as it also determines the selection of adjuvant therapy. The first lymph node to drain a particular area is defined as the sentinel lymph node (SLN). Intraoperative evaluation of SLN is useful in deciding the patients who need a complete lymph node dissection surgery in the same setting.[3]
The patients with a negative SLN status do not require any further surgical intervention, thus preventing the complications of ALND such as lymphedema and reduced shoulder movement.[3]
SLNB allows nodal staging and is the gold standard for evaluation of axillary nodal status in patients with clinically uninvolved axilla.[4]
Pre-operative clinical assessment of the axilla has high false-negative rates of around 45%. Radiological investigations such as ultrasonography, magnetic resonance imaging, and computed tomography have limited utility in clinically negative axilla staging. Thus, intraoperative evaluation is the gold standard for axillary assessment in these patients.[5]
Various techniques have been used to determine the intraoperative SLN status such as touch imprint cytology (TIC), frozen section, and infrared spectroscopy. Imprint cytology besides being rapid and easy to use provides excellent cellular details. It is a type of non-exfoliative cytology which not only preserves tissue but also avoids the freezing artifacts giving it an advantage over frozen section examination.[6] Imprint cytology has been previously used in the evaluation of brain tumors, vertebral lesions, lymphomas, ovarian tumors, and melanomas apart from breast cancers.[7]
MATERIAL AND METHODS
This was a cross-sectional study conducted in the Department of Pathology and General Surgery after obtaining patient consent and ethical clearance from the Institutional Ethics Committee. All biopsy-proven cases of breast carcinoma who underwent breast conservation surgery or modified radical mastectomy over a 1-year period from January 2020 to December 2020 were included and clinical details were retrieved from the requisition form. A total of sixty cases that conformed with the inclusion criteria were taken for study. Any breast sarcomas, premalignant, or metastatic lesions were excluded. SLNs were identified with the help of methylene blue dye injected in the periareolar region. They were dissected out and sent for intraoperative imprint cytology. The nodes were divided along their long axis and wet surface was touched on to a clean glass slide at least 3 times followed by toluidine blue and rapid Papanicolaou staining. The cytology impression was conveyed to the clinician within 15–20 min. Thereafter, the biopsy was fixed in 10% formalin followed by paraffin embedding with hematoxylin and eosin staining.
Statistical analysis
The data were analyzed using SPSS software (IBM, standard version 25). The specificity, sensitivity, diagnostic accuracy, positive predictive value, and negative predictive value of TIC were calculated taking histopathological diagnosis as the gold standard.
RESULTS
Majority (36.7%) of patients were of the age group 41–50 years with a mean age of 48.1 ± 10.6 years. The left breast (56.7%) was involved more often than the right breast. Based on histopathological diagnosis, there were 27 cases (90%) and 3 cases (10%) of invasive carcinoma of no special type (ductal) and lobular carcinoma, respectively. According to modified Bloom–Richardson scoring, the cases were categorized as Grade 1–6 cases (10%), Grade 2–36 (60%), and Grade 3–18 (30%).
Out of the 60 cases evaluated by intraoperative imprint cytology, 42 (70%) were positive while 18 (30%) were negative for malignancy. The imprint cytology for the positive cases showed clusters of atypical cells with enlarged hyperchromatic nuclei and nuclear overlapping against a lymphoid background. The remaining cases exhibited a polymorphous population of lymphoid cells with no tumor deposit [Figure 1].
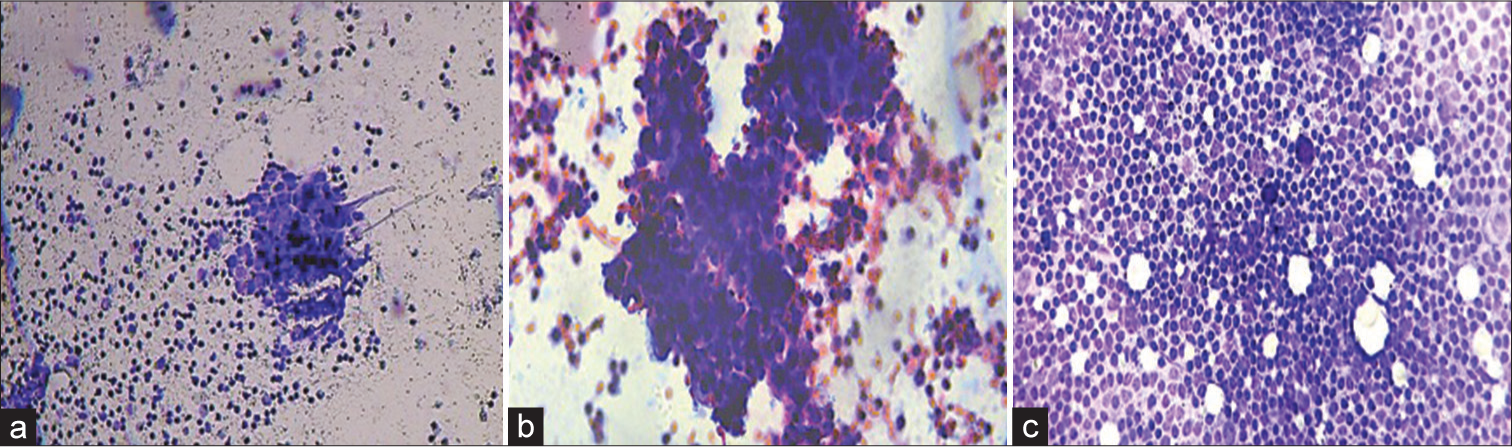
- Touch imprint cytology of sentinel lymph node in breast cancer. (a and b) Cellular smears from a case reported as positive for malignancy exhibiting clusters of atypical cells with enlarged hyperchromatic nuclei, nuclear overlapping against a lymphoid background. (Toluidine blue, Rapid Papanicolaou stain, 10×, 40×). (c) Cellular smears from a case reported as negative for malignancy exhibiting polymorphous population of lymphoid cells with no tumor deposit. (Toluidine blue, 10×).
The histopathological examination showed 48 (80%) to be positive and 12 (20%) to be negative for malignancy. The specificity, sensitivity, diagnostic accuracy, positive predictive value, and negative predictive value of TIC keeping histopathological diagnosis as the gold standard were 100%, 87.5%, 90%, 100%, and 66.7%, respectively.
DISCUSSION
Axillary lymph node status is a crucial prognostic parameter as it determines the need for systemic adjuvant therapy. SLN evaluation reduces the rate of axillary lymphadenectomy as only those cases with positive sentinel nodes will be subjected to axillary resection. In addition, it will also reduce the complications following axillary dissection such as lymphedema of the arm, restriction of shoulder movement, and numbness and pain in arm and chest wall.[3]
TIC is an economical, easy-to-use, and rapid technique with excellent cellular details. It can be utilized in remote or resource-limited areas in the absence of a cryostat machine and also prevents freezing artifacts.[6]
In contrast, frozen section examination despite being reliable is a time-consuming and expensive technique.[8]
Majority (36.7%) of patients were of the age group 41–50 years with a mean age of 48.1 ± 10.6 years which was similar to the distribution of previous studies.[8-11] Most (90%) of the cases in the present study were invasive carcinoma of no special type (ductal) which is concordance to previous data.[8-12] There were six cases of SLN which were reported as negative for malignancy on TIC and turned out to be malignant on histopathological sections. The possible reason behind this could be a low-grade tumor mimicking the background lymphocytes leading to a false-negative report.
The specificity, sensitivity, diagnostic accuracy, positive predictive value, and negative predictive value of TIC keeping histopathological diagnosis as gold standard were 100%, 87.5%, 90%, 100%, and 66.7%, respectively. This performance analysis of TIC in the present study is comparable to the findings of Khanna et al., Hashmi et al., Saeed et al., Chang and Tzen, Abad-Licham et al, Safai et al., and Petropoulou et al.[10-16] In addition, the specificity and sensitivity of imprint cytology of the current study were almost equivalent to that of frozen section in a few studies.[11,15-19] Table 1 shows a comparison of the performance analysis parameters of TIC in SLN evaluation of the previous studies with the present study.
| S. No. | Author name | No. of cases | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|---|
| 1. | Saeed et al.[12] (2021) | 25 | 88.9 | 93.7 | 92 | ||
| 2. | Chang and Tzen[13] (2022) | 4327 | 82.7 | 99.3 | 95.9 | ||
| 3. | Abad-Licham et al.[14] (2023) | 887 | 91.6 | 97.7 | 96 | 94.1 | 96.7 |
| 4. | Delgado-Bocanegra et al.[8] (2018) | 64 | 61.8 | 100 | 100 | 82.4 | 86.3 |
| 5. | Creager et al.[9] (2002) | 646 | 53 | 98 | 84 | ||
| 6. | Khanna et al.[10] (2011) | 108 | 88 | 98 | 94 | 97 | 92 |
| 7. | Safai et al.[15] (2012) | 49 | 90 | 100 | 100 | 93 | |
| 8. | Abe et al.[18] (2020) | 49 | 50 | 100 | 100 | 60 | |
| 9. | Mori et al.[17] (2006) | 138 | 47.1 | 98.3 | |||
| 10. | Hashmi et al.[11] (2021) | 114 | 83.7 | 98.5 | 92.1 | ||
| 11. | Aihara et al.[19] (2004) | 27 | 85 | ||||
| 12. | Petropoulou et al.[16] (2017) | 60 | 90 | 100 | 98 | 100 | 98 |
| 13. | Present study | 60 | 87.5 | 100 | 90 | 100 | 66.7 |
SUMMARY
TIC is an easy to perform, reliable, fast and accurate method for the evaluation of SLN and prevents unnecessary axillary nodal resection and its morbidities.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
ALND: Axillary lymph node resection
SLN: Sentinel lymph node
SLNB: Sentinel lymph node biopsy
TIC: Touch imprint cytology
AUTHOR CONTRIBUTIONS
PY- data curation, formal analysis; SA- data curation, formal analysis, writing- original draft ; SZ- conceptualization, methodology, validation, writing- review and editing; M S- resources, supervision; CC- resources, supervision. This material is the authors’ own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere. The paper reflects the authors’ own research and analysis in a truthful and complete manner. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Ethics Committee of Vardhman Mahavir Medical College and Safdarjung Hospital vide approval number IEC/VMMC/SJH/Thesis/2019-10/135.
Written informed consent was obtained from all the participants prior to the publication of this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of cytojournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
None
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol. 2017;13:289-95.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of axillary lymph node dissection and sentinel lymph node biopsy on upper limb morbidity in breast cancer patients: A systematic review and meta-analysis. Ann Surg. 2023;277:572-80.
- [CrossRef] [PubMed] [Google Scholar]
- Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927-33.
- [CrossRef] [PubMed] [Google Scholar]
- Sentinel lymph node biopsy in breast cancer: A clinical review and update. J Breast Cancer. 2017;20:217-27.
- [CrossRef] [PubMed] [Google Scholar]
- Touch imprint cytology and frozen-section analysis for intraoperative evaluation of sentinel nodes in early breast cancer. Anticancer Res. 2012;32:3523-6.
- [Google Scholar]
- Evaluation of intraoperative imprint cytology and frozen section in determination of tumor and tumor margins. Saudi J Pathol Microbiol. 2023;8:1-9.
- [CrossRef] [Google Scholar]
- Intraoperative imprint cytology versus histological diagnosis for the detection of sentinel lymph nodes in breast cancer treated with neoadjuvant chemotherapy. Clinics (Sao Paulo). 2018;73:e363.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative evaluation of sentinel lymph nodes for metastatic breast carcinoma by imprint cytology. Mod Pathol. 2002;15:1140-7.
- [CrossRef] [PubMed] [Google Scholar]
- Touch imprint cytology evaluation of sentinel lymph node in breast cancer. World J Surg. 2011;35:1254-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of intraoperative touch imprint cytology for the diagnosis of axillary sentinel lymph node metastasis of breast cancer: Comparison with intraoperative frozen section evaluation. Cureus. 2021;13:e12960.
- [CrossRef] [Google Scholar]
- evaluation of intraoperative touch imprint cytology of axillary sentinel lymph node accuracy in comparison to the permanent histology diagnosis. A prospective study of 25 invasive breast cancers. Gulf J Oncolog. 2021;1:70-8.
- [Google Scholar]
- Intraoperative sentinel lymph node imprint cytology diagnosis in breast cancer patients by general surgical pathologists: A single-institution experience of 4327 cases. J Cytol. 2022;39:20-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative cytology when cryostat is not available. A 7-year experience in a Peruvian cancer center. Diagn Cytopathol. 2023;51:E45-53.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing touch imprint cytology, frozen section analysis, and cytokeratin immunostaining for intraoperative evaluation of axillary sentinel lymph nodes in breast cancer. Indian J Pathol Microbiol. 2012;55:183-6.
- [CrossRef] [PubMed] [Google Scholar]
- Imprint cytology versus frozen section analysis for intraoperative assessment of sentinel lymph node in breast cancer. Breast Cancer (Dove Med Press). 2017;9:325-30.
- [CrossRef] [PubMed] [Google Scholar]
- Frozen section is superior to imprint cytology for the intra-operative assessment of sentinel lymph node metastasis in stage I breast cancer patients. World J Surg Oncol. 2006;4:26.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective comparison of intraoperative touch imprint cytology and frozen section histology on axillary sentinel lymph nodes in early breast cancer patients. Acta Cytol. 2020;64:492-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of frozen section and touch imprint cytology for evaluation of sentinel lymph node metastasis in breast cancer. Ann Surg Oncol. 2004;11:747-50.
- [CrossRef] [PubMed] [Google Scholar]








