Translate this page into:
A review of uncommon cytopathologic diagnoses of pleural effusions from a chest diseases center in Turkey
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
After pneumonia, cancer involving the pleura is the leading cause of exudative pleural effusion. Cytologic examination of pleural effusions is an important initial step in management of malignant effusions. The aim of this study is to evaluate the spectrum of uncommon malignant pleural effusions in a chest disease center in Turkey.
Materials and Methods:
A retrospective study of samples of pleural effusions submitted to Ataturk Chest Diseases and Chest Surgery Education and Research Hospital Department of Pathology between March 2005 and November 2008 was performed.
Results:
Out of a total of 4684 samples reviewed 364 (7.8%) were positive for cancer cells. Of the malignant pleural effusions 295 (81%) were classified as adenocarcinoma or carcinoma not otherwise specified (NOS). Pleural effusion specimens revealing a diagnosis other than adenocarcinoma/carcinoma NOS were: 32 (8.8%) malignant mesotheliomas, 14 (3.8%) small cell carcinomas, 13 (3.5%) hematolymphoid malignancies and 10 (2.7%) squamous cell carcinoma. Hematolymphoid malignancies included non- Hodgkin lymphoma (diffuse B large cell lymphoma, mantle cell lymphoma), multiple myeloma, chronic myeloid leukemia, and acute myeloid leukemia.
Conclusions:
Despite that adenocarcinoma is the most common cause of malignant pleural effusions, there is a significant number of hematological and non-hematological uncommon causes of such effusions. Cytopathologists and clinicians must keep in mind these uncommon entities in routine practice for an accurate diagnosis.
Keywords
Cytopathology
differential diagnosis
malignant
pleural effusion
uncommon causes
INTRODUCTION
Cancer is the second leading cause of exudative pleural effusion. Although most patients with a malignant effusion have a known history of cancer, a positive effusion may be the first sign of an unsuspected malignancy. Cytologic examination of a serous effusion may offer the possibility of an early and accurate diagnosis by using a minimal intervention.
Lung, breast, ovarian, and gastrointestinal cancers are most likely to cause malignant effusions. The histologic type of cancer most commonly seen in serous effusions is adenocarcinoma but a variety of other cancers can cause effusions.[1–5] Less common malignancies are squamous cell carcinoma, small cell carcinoma (SCC), hematopoietic malignancies, melanoma, germ cell tumours and sarcomas. Moreover, mesotheliomas often present with recurrent serous effusions.[2]
The aim of our study is to review the spectrum of pleural effusions over a 3 year period in a chest disease center and evaluate uncommon causes of malignant pleural effusions.
MATERIALS AND METHODS
For the present study we reviewed the cytologic specimens of pleural effusions submitted to Ataturk Chest Diseases and Chest Surgery Education and Research Hospital Department of Pathology between March 2005 and December 2008. The specimens were collected by thoracentesis, processed in a routine fashion and stained by hematoxylin- eosin (H and E) and Papanicolaou stain after wet fixation with 95% ethanol and Giemsa stain after air drying. Cell blocks were also prepared for all cases by fixing the sediment in 10% buffered formalin, processing and embedding in paraffin. Five- micrometer sections were cut and stained with hematoxylin- eosin. Immunohistochemistry was performed using available cell blocks and pleural biopsy specimens. Sections of 5 μm were cut from formalin- fixed, paraffin- embedded tissue specimens and mounted on poly- l- lysine- coated slides- paraffin sections and dewaxed by xylene, rehidrated, and finally washed in phosphate buffer (pH7.6) for 10 minute. Immunostaining was performed with the streptavidin- biotin complex kit. After incubation, the chromogen specimens were counterstained with Harris Hematoxylin and coverslipped. The antibodies ordered was chosen based on the differential diagnosis generated by the cytomorphologic findings and the clinical features. For the seperation of adenocarcinoma from benign or malignant mesothelial cells, a panel consisted of CEA (m), B72.3, TTF- 1, calretinin and CK5/6 were used. Additional antibodies; CD15, ber- EP4, HBME- 1, WT- 1 and thrombomodulin were included if the original panel had discordant results. For the malignant effusions of the unknown primary sites, many other antibodies were used such as CD20 and CD- X2 for gastrointestinal tract, thyroglobulin for thyroid, PSA for prostate, GCDFP- 15 for breast, RCC for renal cell carcinoma, neuroendocrine markers for SCC and CD45, B cell and T cell markers for lymphoma. Besides immunohistochemistry, histochemical studies such as mucin stains were used.
All effusions and associated pleural biopsy or VATS biopsy materials were reviewed. A total of 4684 specimens from 4516 patients analyzed, 56 of the patient's had multiple taps. In multiple taps, diagnosis as suspicious for cancer, was changed as positive for cancer, in 4 of 56 patients and diagnosis as positive for cancer, was changed as suspicious for cancer, in 5 of 56 patients.
RESULTS
Out of a total of 4684 specimens reviewed, 4171 (89%) were negative for cancer, 149 (3.2%) were suspicious for cancer and 364 specimens (7.8%) were positive for cancer. Of the 364 malignant pleural effusions the most common diagnosis was adenocarcinoma or carcinoma NOS, comprising 81% (295) of the malignant specimens. A total of 69 (19%) pleural fluid specimens have revealed diagnoses other than adenocarcinoma/carcinoma NOS. Among these rare cancers malignant mesothelioma was the most common, comprising 8.8% (32) of specimens [Figure 1]. Other uncommon malignant neoplasms were: 3.8% (14) SCC [Figure 2], 3.5% (13) hematologic malignancies and 2.7% (10) squamous cell carcinomas [Figure 3]. All the mesothelioma cases, had tissue confirmation by pleural biopsy or video- assisted thoracic surgery (VATS) biopsy materials. Immunohistochemistry was applied using available cell blocks and pleural biopsy specimens. An initial diagnosis of malignancy was made on 7 of 32 mesothelioma fluids.
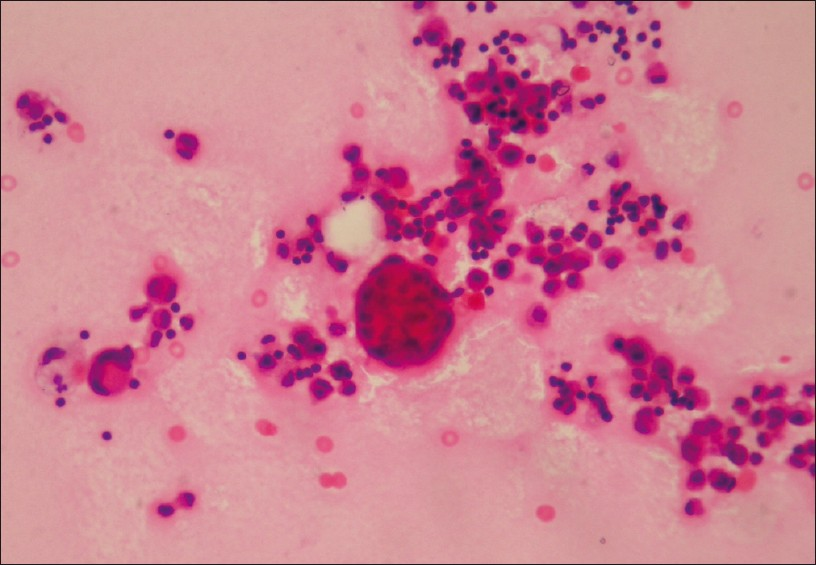
- Malignant mesothelioma showing giant atypical mesothelial cells and cell ball formation (H and E, ×400)
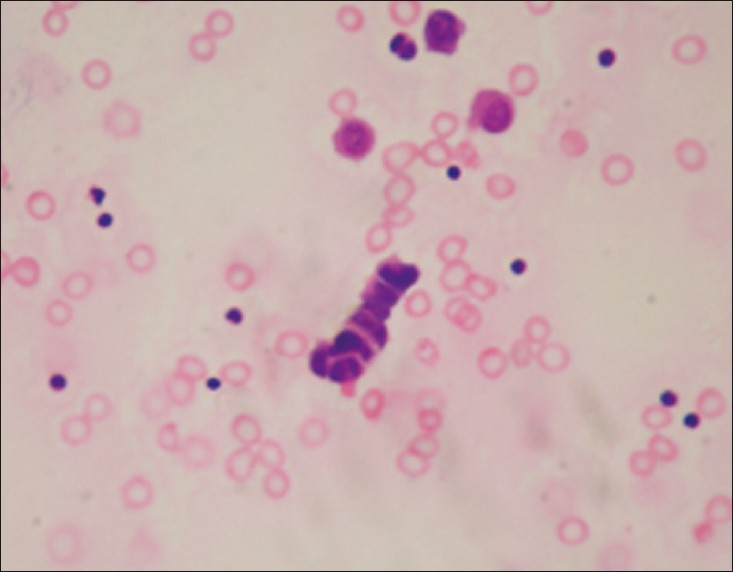
- Pleural effusion of small cell carcinoma characterized by a cord of small malignant cells showing prominent nuclear molding (H and E, ×400)
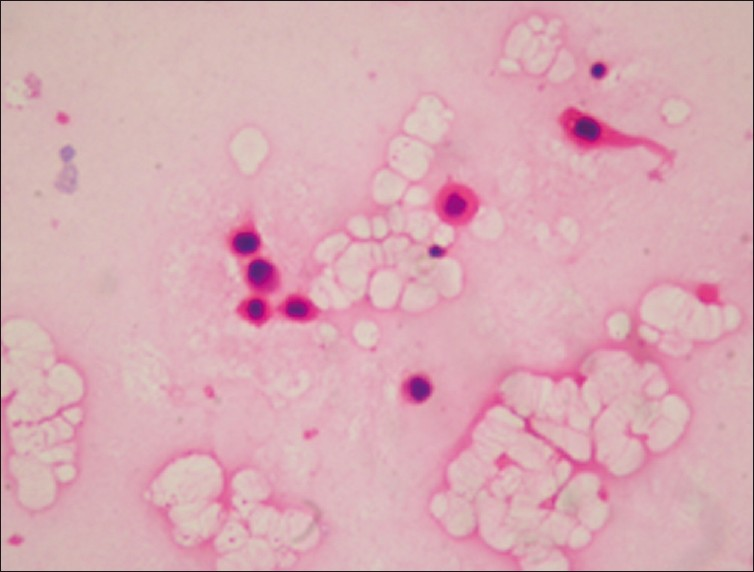
- Pleural effusion of squamous cell carcinoma showing atypical epithelial cells with keratinizing cytoplasm (H and E, ×400)
Hematolymphoid malignancies consisted of nine cases of non- Hodgkin lymphoma, one case of chronic myeloid leukemia, one case of acute myeloid leukemia [Figure 4] and two cases of multiple myeloma (MM) [Figure 5]. Of the nine non- Hodgkin lymphoma cases one has a diagnosis of diffuse B large cell lymphoma [Figure 6] and one of mantle cell lymphoma from lymph node biopsies. In four of the nine non- Hodgkin lymphomas and one MM the initial diagnoses were made by cytology of pleural fluid. Flow cytometry analysis was performed for the cases of leukemia. Immunohistochemistry was also applied by using available cell blocks and biopsy materials.
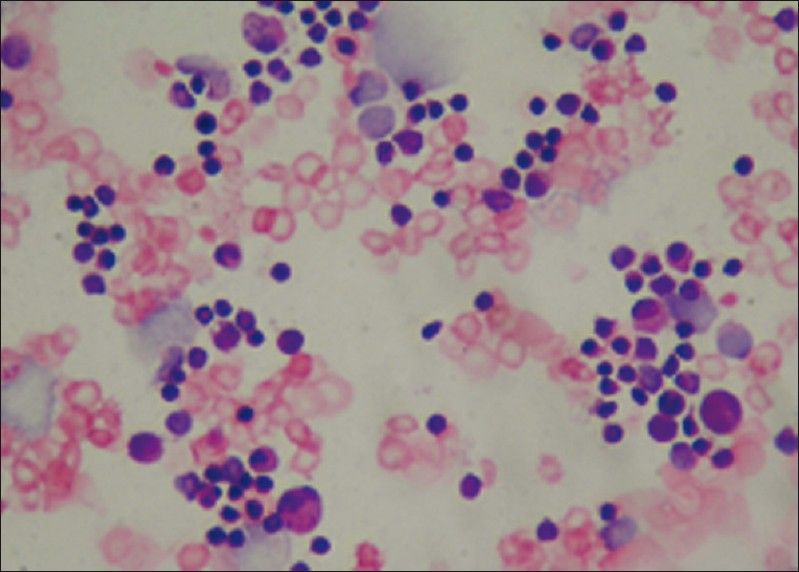
- Pleural effusion of acute myeloid leukemia showing blasts and karyorrhic cells (Giemsa stain, ×400)
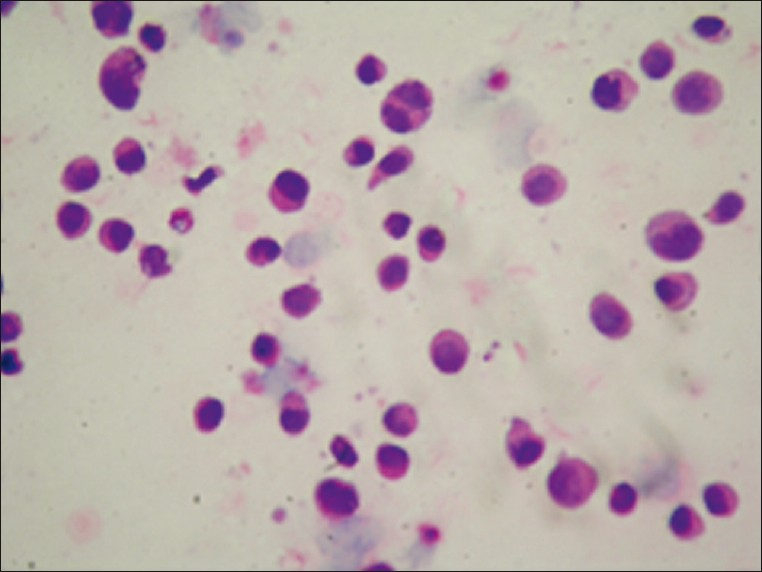
- Multiple myeloma characterized by immature plasma cells (H and E, ×400)
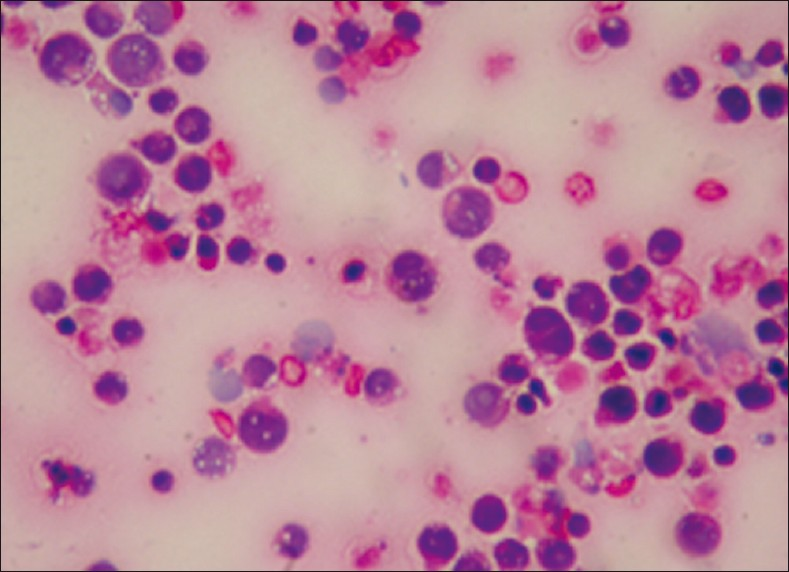
- Single atypical lymphoid cells showing karyorrhexis and mitosis in a case of diffuse B large cell lymphoma (Giemsa stain, ×400)
Out of a total of 149 specimens reported as suspicious for malignancy, 14 were reported as “suspicious of malignant mesothelioma” and four were reported as “suspicious of lymphoma”. Of the 14 cases reported as “suspicious of malignant mesothelioma”, three revealed pulmonary embolism in clinical follow up and two cases revealed atypical mesothelial proliferation in VATS material. No follow- up was available in nine cases. Of the four cases reported as “suspicious of lymphoma”, one revealed thymoma in VATS biopsy material.
DISCUSSION
Effusion is the accumulation of fluid in a body cavity, which has a variety of benign and malignant etiologies. It is generally not possible by cytologic examination of a benign effusion to determine the underlying cause. A marked neutrophilic effusion is associated with bacterial pneumonia in the majority of cases and less commonly with pulmonary malignancy or infarction. A pleural effusion with predominantly small mature lymphocytes and scant mesothelial cells has been associated with tuberculosis. An eosinophilic effusion, defined by the fluid containing at least 10% eosinophils, have been reported in association with a wide variety of conditions including trauma, toracotomy, repeat aspiration of pleural fluid, pneumothorax and hemothorax.[2678]
Most significant role of cytopathology is the examination of serous effusion for the presence of cancer cells. Malignant effusions are often the initial manifestation of cancer (particularly cancer of lung, ovary, and mesothelium). An early and accurate diagnosis may permit appropriate therapy and a better life expectation for these patients.[259]
The most common neoplasms causing malignant pleural effusions in males and females are those of lung, hematolymphoid system, breast, genitourinary tract, and gastrointestinal tract.[245] Although the histologic type most commonly seen in malignant effusions is adenocarcinoma a variety of other cancers can cause effusions. In the present study the most common but unusual cause of malignant effusions was mesothelioma. In some series, the rate of diagnosis of mesothelioma in effusions is extremely low. Recently Awasthi et al. reviewed 898 pleural effusions and reported cytopathologic spectrum of unusual malignant pleural effusions in north India. Of the 898 samples, 164 cases were positive for malignancy and 38 cases revealed malignancies other than adenocarcinoma. The 38 unusual malignancies included 29 hematological and 9 non- hematological malignancies but there were no cases of malignant mesothelioma.[1] Johnston et al. reviewed 5888 pleural effusions and of these, 9.9% diagnosed as malignant. Of the malignant effusions 75.7% were classified as carcinomatous in type. In their study, there were only 3 cases diagnosed as malignant mesothelioma.[5] The high rates of diagnosis of malignant mesothelioma in our study can be explained by the high incidence of mesothelioma in Turkey. Turkey is well known for its “mesothelioma villages” located in central Anatolia near Nevsehir. In central Anatolia, a natural fibrous zeolite, erionite has been documented as the cause of pleural malignancy; however, some studies of tremolite- and chrysolite- associated malignant mesothelioma series have also been reported. In addition, there is an increasing group of patients in whom a history of asbestos exposure can not be elicited (familial- genetic predisposition, role of SV40).[10–12]
The sensitivity of cytologic examination for the diagnosis of malignant mesothelioma ranges from 4% to 77%.[13] As experience with effusion cytology grows and with the help of ancillary techniques more cytopathologists become confident enough to make a diagnosis of mesothelioma on the basis of cytology alone. Differential diagnosis of malignant mesothelioma, mesothelial proliferation, and adenocarcinoma, in effusion samples is often a major diagnostic challenge.
Reactive mesothelial cells are the ubiquitous component of most serous effusions. They typically form smaller, less complex groups than those of malignant mesothelioma, which show larger, more complex agregats.[349] The nuclei of reactive mesothelial cells may have one or two large nucleoli as well as mitotic figures and binucleation. When binucleation and multinucleation are present, the nuclei tend to be uniform. Identification of very large or giant mesothelial cells displaying nuclear atypia, such as enlargement, irregularity of nuclear contours, is an important diagnostic tool for malignant mesothelioma.[29] The presence of many cell ball clusters admixed with many soliter giant cells with low nuclei/cytoplasmic ratio were the most helpful features in identifiying malignant mesothelioma in our case series. For the challenging distinction of reactive mesothelial cells versus mesothelioma, some immunostains such as PCNA, Ki- 67, p53, EMA have been reported as helpful but we did not use a specific panel for this distinction.[3414] The clinical setting, a good history of asbestos exposure, absence of other malignancy, radiographic findings, and detailed cytopathologic examination were used to distinguish malignant mesothelioma from reactive mesothelial proliferation.
Several cytomorphologic features have been described previously as favoring a diagnosis of mesothelioma and adenocarcinoma. However, overlapping features and unusual patterns of mesothelioma prevents definitive diagnosis in cytopathology alone. The cells of metastatic carcinoma are often but not necessarily more pleomorphic, and more hyperchromatic than mesothelial cells. Most commonly they are distinguished from benign mesothelial cells by their tendency to form large clusters. Some carcinomas however, exfoliate in a noncohesive way, in such cases cell size, increased nuclear to cytoplasmic ratio, irregular nuclear contours, prominent nucleoli or coarsely textured chromatine distinguish them from mesothelial cells. But in some instances, diagnosis based on morphologic features alone, the distinction of reactive mesothelial cells from adenocarcinoma can be difficult. Immunohistochemistry is an important ancillary test to differentiate adenocarcinoma from malignant mesothelioma. An immunopanel highlighting the mesothelial and inflammatory cells contribute to the understanding of the basic topography of different components in the effusion specimen. “second foreign population” in effusion is usually consistent with metastatic disease. Subtracting the immunoprofiles of various basic components of effusion specimen, highlights the existence of a ‘second foreign population’ if present. Dual color immunochemistry with reciprocally complimentary combinations of different immunomarkers would also facilitate identification of the foreign population of malignant cells in serous fluids.[15] There is a whole body of literature examining the role of immunocytochemical analysis and the value of individual and panels of markers in fluid cytology. However, there are great differences of opinion about which markers should be included in the panel for routine clinical settings. A diagnosis of metastatic adenocarcinoma is favored if the neoplastic cells demonstrate positive immunoreactivity for CEA, CD15, B72.3, or BerEP4, which are expected to be absent in mesothelial cells. Positive staining with at least two of the adenocarcinoma markers would favor a diagnosis of adenocarcinoma. Therefore, a panel of two or more antibodies often is used to confirm (or rule out) the possible diagnosis of metastatic adenocarcinoma. Calretinin and CK 5/6 may provide a valuable tool in the differential diagnosis of mesothelioma from adenocarcinoma.[1416]
For the malignant effusions of the unknown primary sites, some antibodies were used with the guidelines of clinical and radiological features; CD20 and CD- X2 for gastrointestinal tract, thyroglobulin for thyroid, PSA for prostate, GCDFP- 15 for breast, RCC and CD10 for renal cell carcinoma etc. Some morphologic clues, can point to the site of origin, of an adenocarcinoma. For example abundant hollow spheres (cannonballs) are a common pattern in metastatic breast cancer. Malignant signet ring cells in an effusion point to an origin from stomach cancer but other primary sites, such as the breast are also possible. Most colorectal cancers are composed of elongated cells with hyperchromatic nuclei arranged in acinar formations. Large cells with prominent nucleoli and abundant lacy vacuolated cytoplasm are typical of clear cell carcinomas of the kidney.[61617]
Patients with SCC infrequently develop malignant pleural effusions. Chhieng et al. mentioned that only 3% of patients with biopsy- proven SCC had a clinical significant pleural effusion that warranted thoracentesis. In our study 8 of 14 patients with SCC had their initial diagnosis by cytology of pleural fluid. Because SCC is amenable to chemotherapy and radiation therapy, it is important to recognize SCC in these patients to avoid unnecessary investigation and surgery. The cytologic features of SCC in malignant effusions include single or small clusters of neoplastic cells that are two to two and a half times the size of lymphocytes with scanty cytoplasm and hyperchromatic nuclei. Prominent molding and “indian files” are frequently present. In contrast to benign lymphocytes or lymphoma, SCC usually forms definite tissue aggregates. The use of immunohistochemistry may be helpful in the differential diagnosis. Neuroendocrine markers such as chromogranin and synaptophsin are often used to identify neoplastic cells of neuroendocrine origin. TTF- 1 is also a very sensitive marker for SCC; more than 90% of SCCs are reactive for TTF- 1.[141819]
Malignant lymphomas including Hodgkin and non- Hodgkin lymphomas as well as various leukemias can be diagnosed with exfoliative cytology.[4] The possible mechanisms for the formation of pleural effusions in hematological malignancies include; pleural infiltration by neoplasm, lymphatic obstruction due to infiltration of pulmonary and mediastinal lymph nodes and obstruction of the thoracic duct.[2021] Leukemic infiltration of the lungs may occur as a part of systemic relapse or rarely as an isolated leukemic infiltrate. Most cases reported in the literature concern acute lymphocytic leukemia and very few cases of acute non lymphocytic leukemia.[20] Blasts are round, two to three times the diameter of lymphocytes and dispersed as isolated cells. The nuclei is round or irregularly shaped. The chromatine is pale and the nucleoli are usually prominent. The cells of chronic lymphocytic leukemia are indistinguishable from small mature lymphocytes thus immunophenotyping is necessary.[6] The hand- mirror cell morphology had in the past been classified as a distinct morphologic variant of acute lymphoblastic leukemia.Hand- mirror cells have also been reported with many diagnostic entities including cutaneous natural killer cell lymphomas, Anaplastic Ki-1 lymphomas, acute lymphoblastic leukemia, Burkitt's lymphoma, other B-Cell lymphomas with surface IgM-lambda, and with granulocytic and acute myelomonocytic leukemia. These cells are characterized by an asymmetrical cytoplasmic elongation extending out from one pole of the nucleus (uropod). Both the nucleus and the elongated cytoplasmic “handle” together give the appearance of a hand-mirror.[622] In our study there was one case of acute myeloid leukemia and one case of chronic myeloid leukemia with positive pleural fluid. The diagnoses of these cases were confirmed by flow cytometry.
Pleural effusion is a relatively common finding in patients with non-Hodgkins lymphoma with a frequency of up to 20%.[1] Malignant lymphocytes in effusions are usually identical to the cells in the involved lymph nodes and in blood. A characteristic feature of these cells in serous effusions is that they are isolated from each other. If true tissue aggregates of cells are present lymphoma / leukemia can be excluded. The cells of diffuse B large cell lymphomas are the easiest to recognize because they are larger than histiocytes and their nucleoli are usually prominent. Nuclei are round or highly irregular and the chromatine is coarsely textured. Cytoplasm is often abundant, pale, and vacuolated. Those of follicular lymphomas have irregular and cleaved nuclei and scant cytoplasm. Small cell lymphomas and low- grade lymphomas can be difficult to distinguish from benign lymphocytes. However the monomorphic nature of the cell population is a clue of the diagnosis. Immunohistochemistry and flow cytometry can be helpful in the diagnosis and classification of these neoplasms. In general, benign lymphocytic effusions consist predominantly of T cells in contrast to well differentiated lymphocytic lymphoma / leukemia which is a B cell neoplasm. The cells of lymphoma / leukemia often have scant cytoplasm but some large cell lymphomas may have more abundant cytoplasm and needs to be distinguished from poorly differentiated carcinoma and melanoma. Karyorrhexis is particularly common in malignant lymphoma.[124] Marked chronic inflammation or tuberculosis and rarely thymoma may be difficult to distinguish from malignant lymphoma. In our study, of the four cases reported as suspicious of lymphoma one case revealed thymoma in VATS biopsy material. Immunohistochemically lymphocytes of thymoma will be of the T cell type. The cells of tuberculous effusions are also mostly of T cell type. A mixed pattern of chronic inflammatory cells in which small lymphocytes predominate and plasma cells are present favors a non- neoplastic process.[24]
In fact 10-15% of malignant effusions are caused by lymphoma, the proportion is significantly higher in pediatric population. Most represent secondary involvement of serosal surfaces, few are primary effusion lymphomas (PEL). PEL refers to a large- cell non- Hodgkin lymphoma localized in body cavities and presenting as pleural, peritoneal, or pericardial lymphomatous effusions. PEL is considered to be always associated with human herpes virus- 8 (HHV8) infection; therefore, this term is restricted to those lymphomatous effusions that are associated with HHV8. It has been shown that the presence of the virus in the neoplastic cells can be demonstrated either by immunohistochemical methods using the HHV8 LNA-1 latent protein antibody, or by molecular techniques such as PCR amplification or Southern blot analysis. Restricting the PEL terminology to the cases that are HHV8 positive is important to differentiate PEL from other lymphomas that can present as serous effusions and carry in general a more favorable prognosis.[23]
MM rarely causes an effusion but in some cases an effusion may be the presenting sign. Pleural effusions occur in approximately 6% of patients with myeloma. Large numbers of plasma cells suggest the diagnosis. A small number can be observed in a wide variety of diseases. Other etiologies of reactive pleural effusions like congestive heart failure, pneumonia, tuberculosis, collagen vascular disease, carcinomatosis, AIDS, other viral illness, and pulmonary thromboembolism should be excluded before a diagnosis of malignant myelomatous effusion is made. Cytologically these cases can have a predominantly lymphocytic infiltrate with scattered plasma cells showing atypical nuclear features. In the literature 80% of myelomatous pleural effusions are due to IgA MM. Malignant plasma cells are isolated, typical of lymphomas in effusions. A characteristic feature of these effusions is the presence of mature and particularly immature plasma cells. The classic cartwheel pattern of chromatin is infrequently observed in malignant plasma cells. Morphological features of malignant plasma cells are nuclear pleomorphism, prominent nucleoli, frequent mitosis, and asynchronous maturation of the nucleus in relation to the cytoplasm. Diagnostic criteria to confirm the myelomatous etiology are the demonstration of the monoclonal protein in pleural fluid electrophoresis, flow cytometry detection of typical plasma cells in pleural fluid cytology, immunocytochemically confirming the monoclonality of the plasma cells and histologic confirmation using a pleural biopsy specimens.[142024–27]
Squamous cell carcinoma rarely sheds diagnostic cells into an effusion. In the English literature less than 1% of pleural effusions contain cells of squamous cell carcinoma. In the present study 2.7% of malignant pleural effusions contained atypical squamous cells. Non-keratinizing squamous cell carcinoma cells are more commonly found in pleural fluids than keratinizing squamous cell carcinoma cells. The differential diagnosis may include benign squamous cells associated with fistula, extraneous contamination, and possibly squamous metaplasia of mesothelium.[14]
Adenocarcinoma is the most frequently diagnosed cancer in pleural fluids. In our study malignant mesothelioma was second to adenocarcinoma as a cause of malignant pleural effusion. There is significant number of hematological and non- hematological uncommon causes of malignant pleural effusions. Cytopathologists and clinicians must keep in mind these uncommon entities for an accurate diagnosis.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and reviewers are blinded for authors) through automatic online system as per Cytojournal's peer-review policy http://www.cytojournal.com/prp/asp.








