Translate this page into:
Abstracts for the 59th Annual Scientific Meeting (November 2011) by American Society of Cytopathology (ASC) at Baltimore, MD, USA
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
These are peer-reviewed poster-platform submissions finalized by the Scientific Program Committee. A total of 153 abstracts (14 Platforms [PP1 through PP14] & 139 Posters [1 through 139]) were selected from 161 submissions to be considered for presentation during November 4 – 8, 2011, at the Hilton Baltimore Hotel, to pathologists, cytopathologists, cytotechnologists, residents, fellows, students, and other members of cytopathology-related medical and scientific fields.
Keywords
Abstracts
American society of cytopathology
ASC
cytopathology
cytology
Gastrointestinal Tract
25: Evaluating immunophenotypes of normal stomach, duodenum, ampulla, pancreas, and colon
Haiyan Liu, MD1, David Lucas, MD1, Yajue Huang, MD2, Fan Lin, MD1
1Laboratory Medicine, Geisinger Health System, Danville, Pennsylvania; 2Department of Pathology, Temple University Hospital, Philadelphia, Pennsylvania
Introduction: Distinction of mucin-producing neoplasms of the pancreas from gastrointestinal contaminants on fine needle aspiration biopsy specimens can be very challenging. Many biomarkers have been reported to be useful in this regard. However, a reliable diagnostic panel is still not available. To address this issue, the immunophenotypes of potential contaminants, including normal tissues of gastric antrum and fundus, duodenum, pancreas, ampulla, and less likely colon, are studied.
Materials and Methods: We immunohistochemically evaluated the expression of, (1) epithelial markers (CK7, CK20, CK17, CK19), (2) mucin gene products (MUC1, MUC2, MUC4, MUC5AC, MUC6), (3) tumor suppressor genes and transcription factors (p53, CDX2, pVHL), and (4) tumor-associated proteins (S100P, IMP3, maspin, MOC31, CEA, CA19-9, CD10, CD15, HepPar1, villin, and P504S), on 20 cases of each of the aforementioned normal tissues. The staining intensity and the distribution were recorded.
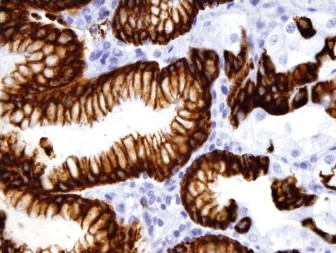
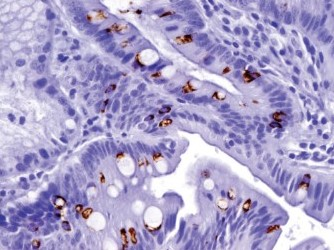
Results: The positive staining results are summarized in Table 1. CK7 was positive in the mucosa of the duodenum and ampulla, focally positive in about 40% of the cases of gastric mucosa (both antrum and fundus), and negative in the colonic mucosa. CK20 was positive in mucosa of the duodenum, ampulla, and colon, and only positive on the surface epithelium of the gastric mucosa. Gastric mucosa mainly expressed MUC5AC [Figure 1], while colonic and duodenal mucosa expressed MUC2 (mainly Goblet cells) [Figure 2], with the exception of Brunner's glands, which demonstrated strong reactivity to MUC6 [Figure 3]. EMA reactivity was seen in 100% of the stomach tissue, but rarely in colonic and duodenal tissue, with the exception of Brunner's glands. Cytoplasmic staining for HepPar1 was seen in the duodenal mucosa (except Brunner's glands) and it was non-reactive in the stomach and colon tissues. Vimentin was non-reactive in all the tissues tested.
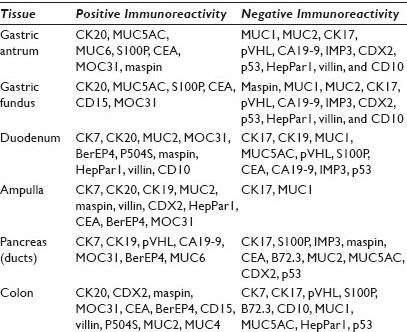
- MUC5AC
- MUC2
- MUC6
Conclusions: These data provide some important information in building an antibody panel for differentiating mucin-producing neoplasms of the pancreas from the gastrointestinal contaminants. Evaluation of the immunostain profile of MPN in a large series of cases is being undertaken.
26: CINtec® PLUS as a triage tool for anorectal Pap smears with atypical squamous cells of undetermined significance (ASC-US) / low- grade squamous intraepithelial lesions: A pilot study
Ann Walts, MD, Shikha Bose, MD
Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California
Introduction: CINtec® PLUS (CINtec), a dual immunostain for p16 and Ki67, has shown promise for a triage of women, with abnormal findings on a cervical Pap smear. In both anal and cervical cytology, atypical squamous cells of undetermined significance (ASC-US) / low- grade squamous intraepithelial lesion (LSIL) are the most frequent abnormal diagnoses rendered and require further triage for optimal patient management. This study was designed to assess the utility of CINtec for the detection of the underlying or subsequent (U / S) anal high-grade squamous intraepithelial lesions (AIN2 / 3) in individuals with ASC-US / LSIL, on anorectal Pap smear.
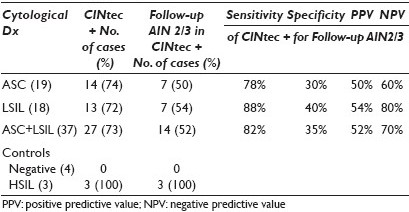
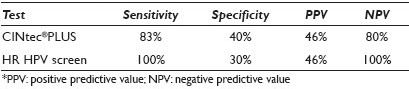
Materials and Methods: Forty-four anal SurePath® Pap smears (19 ASC-US, 18 LSIL, seven controls (four negative, three HSIL)) with histological and / or cytological follow-up (range, 1 to 72 mos; median, 5.5 mos) were retrieved from our departmental files. The Pap-stained slides were de-stained and then immunostained using the CINtec® PLUS Kit (mtm laboratories, Inc. Westborough, MA) that detected the overexpression of p16 as a brown / cytoplasmic and Ki67 as a red / nuclear reaction product. As recommended by the manufacturer, the presence of ≥ 1 dual stained cell was interpreted as a positive test result. P16 or Ki67 staining alone was recorded as a negative test result. Dual stain results were correlated with follow-up diagnoses. Sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) for detection of U / S AIN2 / 3 were calculated. Sixteen of the LSIL cases had also been tested for high-risk human papilloma virus (HR HPV) (Third Wave Technologies, Hologic Inc, Madison, WI). Results of the CINtec dual stain and the HR HPV screen were compared.
Results: Dual stain results and the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), of a positive CINtec test result in ASC-US / LSIL anal smears for detection of U / S AIN2 / 3 are shown in Table 1. Use of a minimum threshold of 1 CINtec+ cell has the highest sensitivity for the detection of U / S AIN2 / 3, and is therefore, the optimal number of cells for triage purposes. Increasing the minimum number of CINtec positive cells required for a positive test result dramatically increases the specificity of the test, while only minimally decreasing its negative predictive value. The sensitivity, specificity, PPV, and NPV of CINtec and the HR HPV screen in LSIL anal smears for detection of U / S AIN2 / 3 are shown in Table 2. The two test results were concordant and correctly detected the presence (or absence) of U / S AIN2 / 3 in seven of sixteen (43.5%) LSIL cases. The test results were concordant, but incorrect in three (18.8%) cases and discordant in the remaining six (37.5%) LSIL cases. Among the LSIL with discordant test results, the CINtec dual stain and HR HPV screen each accurately detected the AIN2-3 in three cases.
Conclusions: - CINtec® PLUS can be applied to routinely prepared and previously screened liquid-based anal Pap smears without loss of cytological detail.
-
CINtec is less expensive, less labor intensive, and offers a shorter turn-around time than HPV testing.
-
In a cohort of ASC-US / LSIL anal Pap smears, a positive CINtec test result has a sensitivity of 82%, a specificity of 35%, a PPV of 52%, and an NPV of 70% for detection of U / S AIN2 / 3, suggesting that CINtec could help stratify ASC-US / LSIL cases for clinical management.
-
In LSIL cases, a positive CINtec test result is less sensitive and slightly more specific than the HR HPV screen for detection of U / S AIN2 / 3.
-
A large prospective study is warranted to confirm our results.








