Translate this page into:
Salivary duct carcinoma with striking neutrophil-tumor cell cannibalism
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cannibalism of neutrophils by tumor cells has previously been reported in certain carcinomas, lymphoma and melanoma. Tumor cannibalism is believed to serve as a tumor-immune escape mechanism, associated with high-grade aggressive cancers with a significantly increased metastatic potential. This interesting phenomenon has not been previously documented in association with salivary gland tumors. We report, for the first time, striking neutrophil-tumor cell cannibalism associated with a high grade, aggressive and metastatic salivary duct carcinoma of the parotid gland highlighted within cytological and surgical excision pathology specimens.
Keywords
Cannibalism
duct carcinoma
neutrophils
phagocytosis
salivary gland
INTRODUCTION
A wide variety of benign and malignant salivary gland tumors are recognized. These tumors exhibit an impressive range of morphological diversity, often exhibiting remarkable histopathological features. Tumor cell cannibalism of hematopoietic cells (neutrophils, lymphocytes and erythrocytes) has been reported in certain carcinomas, endometrial tumors and melanoma.[1–3] To the best of our knowledge, neutrophil-tumor cell phagocytosis (cannibalism) has not previously been reported in association with salivary gland tumors. We report, for the first time, striking neutrophil-tumor cell cannibalism in a salivary duct carcinoma of the parotid gland highlighted within both fine needle aspiration (FNA) and surgical excision pathology specimens.
CASE REPORT
Clinical features
A 55-year-old Caucasian man presented with left-sided facial pain, erythema and swelling of his left parotid gland. His past medical history was non-contributory. Magnetic resonance imaging (MRI) revealed an infiltrating mass centered in the tail of the left parotid gland involving both superficial and deep portions of the parotid, with probable invasion of the underlying left sternocleidomastoid muscle and overlying subcutaneous tissue. Imaging also identified a left posterior auricular lymph node suspicious for metastatic disease.
Cytopathological features
An ultrasound-guided FNA was performed of the left parotid mass from which Papanicolaou-stained direct smears and a ThinPrep slide were made. A cell block was also prepared and stained with H and E. The aspirate was cellular and showed crowded 3 -dimensional groups of poorly differentiated carcinoma cells that had enlarged pleomorphic nuclei with prominent nucleoli. Virtually all the tumor cells had vacuolated cytoplasm containing many (up to 50) intact as well as degenerated neutrophils [Figures 1 and 2]. The nucleus of some tumor cells was pushed to the periphery by the many ingested neutrophils. The background also showed numerous neutrophils.
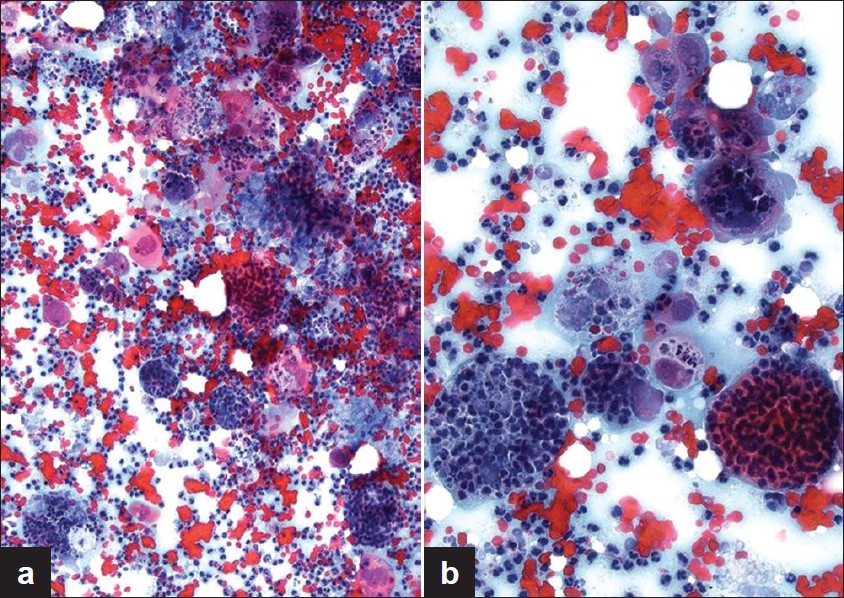
- Cytology smear showing salivary gland tumor cells packed with neutrophils at (a) intermediate and (b) high magnification. Note the abundance of neutrophils in the background (Pap stain, magnification (a) ×100 and (b) × 200)
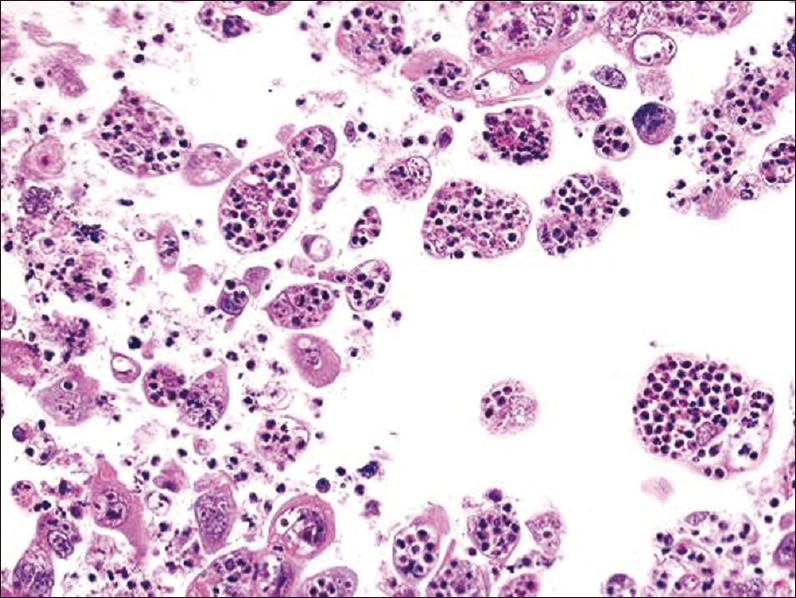
- Cell block of FNA material showing striking tumor cell cannibalism of neutrophils (H and E stain, magnification ×200)
Histopathological features
A left radical parotidectomy with facial nerve resection and accompanying left modified neck dissection was undertaken. Macroscopical evaluation revealed a solid, multifocal neoplasm almost 3 cm in greatest dimension. Light microscopic examination showed an infiltrative, high-grade salivary duct carcinoma (TNM pathologic stage pT2pN2BpMx) with ulceration of the overlying skin, extension into adjacent soft tissue, angiolymphatic invasion and focal necrosis. Similar to the FNA, there were many phagocytic tumor cells congested with neutrophils. Tumor cannibalism of leukocytes was identified both within the resected parotid gland tumor [Figure 3] and metastases to three lymph nodes. Immunohistochemical stains showed that the neoplastic cells were positive for cytokeratin-7 and androgen receptor, but negative for cytokeratin-20 and p63. ERG FISH studies were negative for translocations.
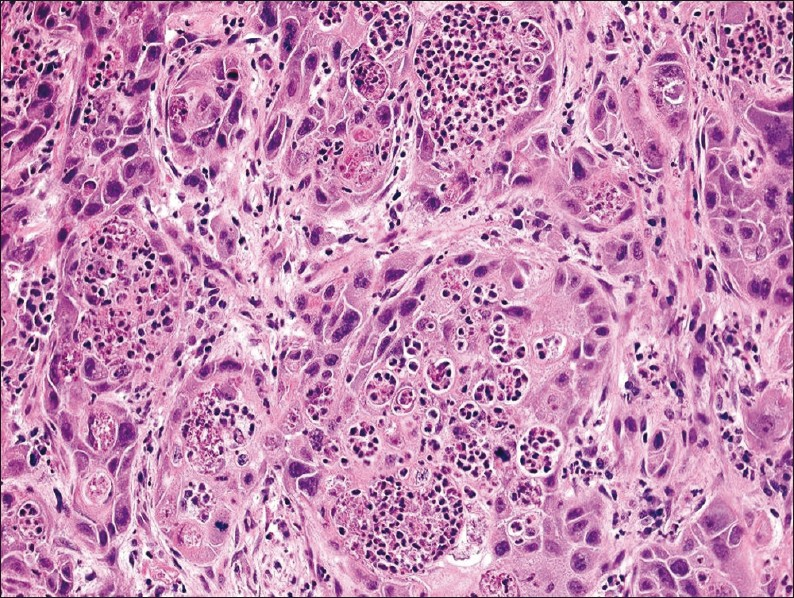
- Salivary duct carcinoma from the surgical excision showing marked neutrophil-tumor cell cannibalism (H and E stain, magnification ×200).
DISCUSSION
The phenomenon of cell cannibalism refers to the engulfment of cells within other cells. Until recently, cannibalism was recognized as a phenomenon seen mainly with tumor cells ingesting other tumor cells. Recent reports have shown tumor cell engulfment of other cells (xeno-cannibalism) as well, such as neutrophils, lymphocytes and erythrocytes.[245] With cannibalism, larger cells typically consume smaller cells. Cannibalism is a different entity from phagocytosis. Cannibalizing tumor cells exclusively ingest live cells, whereas macrophages exclusively phagocytose dead cells. The nucleus of the ingested cell typically remains unaltered while the nucleus of the cannibal cell becomes more semilunar in shape and pushed to the periphery. Eventually the cannibalized cell gets destroyed (“digested”).[4] Moreover, cannibalism appears to be a passive process, unlike phagocytosis where engulfment by pseudopods exists.[6] Cannibalism also differs from emperipolesis where cell engulfment (penetration) is temporary and the internalized cell is not destroyed.[7]
The exact mechanisms involved and factors controlling cannibalism are not completely resolved. It is believed that cannibalism occurs so that tumor cells can feed on ingested cells. In other words, the main factor regulating cannibalism may be related to tumor cell nutritional deficiencies.[2] A previous study showed that metastatic melanoma cells can engulf and digest live autologous melanoma-specific CD8+ T cells,[5] the immune cells programmed to kill cancer cells. These data suggest that by feeding on cytotoxic lymphocytes, tumor cells may use cannibalism as tumor-immune escape mechanism. Indeed, cannibalistic activity was shown to be significantly associated with increased metastatic melanoma cell survival, particularly under starvation conditions.
There is a paucity of literature on tumor cell cannibalism, particularly in the cytology literature. The largest published series to date reported 11 FNA cases with tumor cells showing neutrophil phagocytosis.[3] This series included carcinomas arising from the gallbladder (anaplastic carcinomas), small intestine (adenocarcinoma), pancreas (adenocarcinoma), breast infiltrating duct carcinoma), larynx (squamous cell carcinoma), lung (small cell carcinoma), and disseminated tumor of unknown origin (anaplastic carcinoma). Two cases in this series were non-Hodgkin lymphomas. As the infiltrate surrounding the tumor cells in these cases were found to be composed predominantly of neutrophils, similar to our salivary gland tumor, the authors of this series suggested that the tumor cells had possibly phagocytosed the neutrophils in an incidental fashion. Interestingly, all 11 cases reported in the aforementioned cytology series were high-grade (or poorly differentiated) tumors associated with metastasis at presentation. Giant cell carcinomas of the lung, that frequently also have neutrophils present within their tumor cells, are notoriously very aggressive sarcomatoid lung neoplasms that metastasize widely. These large cell undifferentiated carcinomas of the lung with tumor cell cannibalism are sometimes also associated with systemic neutrophilia, suggesting that the tumor cells may release a growth or trophic factor for neutrophils.[8] Other authors advocated that tumor cell cannibalism present in voided urine samples could be applied as a parameter to improve the diagnostic accuracy for urothelial carcinoma.[9] Another study in which a significant increase in the number of cannibalistic cells were demonstrated in higher grade breast carcinomas, the potential of using tumor cell cannibalism as an index in grading breast carcinomas was proposed.[10] In serous effusions, cannibalistic cells have been reported more commonly in malignant effusion cases, and not in benign conditions.[11] The ability for tumor cells to phagocytose other cells may be indicative of their cell surface features associated with malignancy and increased metastatic potential. We performed a 10-year retrospective review using a natural language search for “cannibalism” in salivary gland cytology cases in our laboratory information system database for any such cases in which cannibalism may have been described, but did not uncover any.
The case reported herein, with striking neutrophil-tumor cell cannibalism in a salivary duct carcinoma with nodal metastases is, to the best of our knowledge, the first such case reported from the salivary gland. Since cannibalism was identified in the initial FNA specimen, it is unlikely that the acute inflammation identified was the result of a complication of this invasive procedure. The presence of cannibalistic tumor cells is likely a feature associated with high-grade malignancy. A study evaluating this phenomenon in a series of salivary gland tumors in relation to diagnostic utility, frequency, tumor type, grade, stage and biology may be of value.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. All authors are responsible for the conception of this study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without patient identifiers, approval from Institutional Review Board (IRB) is not required at our institution.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model(authors are blinded for reviewers and reviewers are blinded for authors) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2011/8/1/15/84222
REFERENCES
- Polymorph phagocytosis by cancer cells in an endometrial adenoacanthoma. Cancer. 1980;45:2348-51.
- [Google Scholar]
- Neutrophil phagocytosis by tumor cells – A cytological study. Diagn Cytopathol. 2011;39:553-5.
- [Google Scholar]
- Morphological evidence of neutrophil-tumor cell phagocytosis (cannibalism) in human gastric adenocarcinomas. Ultrastruct Pathol. 2002;26:315-21.
- [Google Scholar]
- Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629-38.
- [Google Scholar]
- Serum dependent “cannibalism” and autodestruction in cultures of human small cell carcinoma of lung. Cancer Res. 1984;44:2947-51.
- [Google Scholar]
- Emeripolesis of hematopoietic cells in myelocytic leukaemia. Virchows Arch B Zellpathol. 1981;35:283-90.
- [Google Scholar]
- Production of granulocyte-macrophage colony-stimulating factor in two patients with lung cancer, leukocytosis, and eosinophilia. Cancer. 1992;69:1342-6.
- [Google Scholar]
- Cell cannibalism and nucleus-fragmented cells in voided urine: Useful parameters for cytologic diagnosis of low-grade urothelial carcinoma. Acta Cytol. 2007;51:547-51.
- [Google Scholar]








