Translate this page into:
Abstracts for the 59th Annual Scientific Meeting (November 2011) by American Society of Cytopathology (ASC) at Baltimore, MD, USA
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
These are peer-reviewed poster-platform submissions finalized by the Scientific Program Committee. A total of 153 abstracts (14 Platforms [PP1 through PP14] & 139 Posters [1 through 139]) were selected from 161 submissions to be considered for presentation during November 4 – 8, 2011, at the Hilton Baltimore Hotel, to pathologists, cytopathologists, cytotechnologists, residents, fellows, students, and other members of cytopathology-related medical and scientific fields.
Keywords
Abstracts
American society of cytopathology
ASC
cytopathology
cytology
Gynecological
38: Presentation of papillary serous carcinoma in Pap smears: A 15-year experience in a community hospital setting
Nyasha Bullock, MD, Vasavi Kaliki, MD, Srinivas Mandavilli, MD
Pathology and Laboratory Medicine, Hartford Hospital, Hartford, Connecticut
Introduction: Glandular lesions are often difficult to characterize in Pap smears and a significant portion of cervical glandular atypias are flagged as AGC (atypical glandular cells) with ‘adenocarcinoma’ (ACA) diagnosis, used usually with high-grade glandular atypia. We had the anecdotal experience of diagnosing ACA in a Pap smear and this represented the first diagnosis of ‘papillary serous carcinoma’ (PSC) for that patient. PSC are aggressive tumors, with early detection / treatment being the key in successful patient management. There is limited literature addressing this issue and the aim of this study is to evaluate whether the Pap smear diagnosis of ACA can be associated with PSC.
Materials and Methods: The pathology files were searched for the time period 1995 to present, for cases of ACA in Pap smear, for which there was tissue diagnosis available. The patient demographic information and pathological staging were also reviewed.
Results: There were a total of 75 cases of Pap smears with the diagnosis of ACA. Twenty-two cases (29.3%) were excluded (11 cases of metastatic carcinoma from a known extra-gynecological malignancy; 11 cases showing either primary cervical carcinoma or no available follow-up). Among the 53 cases included in this analysis, the follow-up showed 40 (75%) cases of PSC (31 cases pure PSC; 9 cases in which PSC was a component of MMMT or other mixed carcinoma) and 13 (25%) cases of non-serous endometrial adenocarcinomas. In 23 / 40 (58%) Pap smears, the diagnosis was the initial diagnosis of malignancy (i.e., no prior tissue or clinical diagnosis established). Ten of the 40 cases (25%) were in patients with a prior diagnosis of serous carcinoma. In 7 / 40 (17%) patients, there was a concurrent tissue biopsy at the time of Pap smear. Of the 40 cases, the endometrium was the primary site in the vast majority (29 cases, 73%), with the rest being ovarian (7 cases, 18%) and others (tubal-1, peritoneal-1, no clear primary source-2). The age range for patients with serous carcinoma was 40 to 92 years (mean age, 68 years).
Conclusions: This selected review of ‘ACA’ in Pap smears in this study shows that all women were > 40 years of age. PSC of the endometrium was the most common surgical pathology diagnosis on follow-up and in more than half the women, ACA in the Pap smear was the presenting pathological finding. This study suggests that the diagnosis of ACA in a Pap smear should raise the possibility of a ‘high-grade adenocarcinoma’ such as uterine and extra-uterine PSC and should warrant appropriate clinical follow-up.
39: Unsatisfactory ThinPrep® Pap tests in Mexico: Obscuring elements, formal cell counts, and reprocessing with glacial acetic acid
Kathryn Dyhdalo, MD1, Julie Shorie, CT(ASCP)1, Christine Booth, MD1, LucyBeth Nieves-Arriba, MD2, Jerome Belinson, MD3, Jennifer Brainard, MD1
1Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio;
2Gynecologic Oncology, Cleveland Clinic, Cleveland, Ohio;
3Preventive Oncology International, Inc, Cleveland Heights, Ohio
Introduction: The Michoacan Cervical Cancer Screening Study II (MECCS II) sought to determine the sensitivity and specificity of HPV tests compared to liquid-based cytology. In the cytology arm of MECCS II, a large number of Pap tests were unsatisfactory and these were studied in detail.
Materials and Methods: MECCS II was conducted in Michoacan, Mexico. Non-pregnant patients aged 30 to 50 years, with varied prior screening, and no prior hysterectomy or pelvic irradiation, participated. All patients had direct samples for HPV testing (Qiagen digene HC2, Gen-Probe Aptima) and a ThinPrep® Pap test (Hologic). Patients positive for any test were recalled. At the second visit, VIA was done to rule out large pre-invasive disease or cancer; colposcopy and biopsy followed using the POI biopsy protocol. All HC2 positive patients eligible by VIA triage were treated with cryotherapy. All unsat Pap tests were reviewed. Obscuring elements were recorded. Squamous cells were counted over 10 fields at 40X (adequate cellularity average, 3.8 cells / hpf). The presence of a transformation zone, endometrial cells, organisms and shift in vaginal flora was recorded. HPV results and biopsies were reviewed. After initial interpretation, ThinPrep® vials became available for reprocessing with glacial acetic acid (GAA).
Results: A total of 376 of 2503 Pap tests (15%) were called unsatisfatory. Fifty-nine Pap tests (2.3%) were reclassified as satisfactory after formal cell counts. Of the 374 Pap tests that were reprocessed, 125 (5.0%) remained unsatisfatory. Average cellularity increased from 2.51 to 7.61 cells / hpf after reprocessing. No squamous or glandular lesions were seen on review of the unsatisfatory cases, including those with biopsy-proven CIN. Thirty-eight patients were HPV positive and 15 had CIN on biopsy. Four patients with unsat Pap tests had CIN 2+ (12% of CIN 2+ in study). The remade Pap tests showed five ASCUS. None of these patients had CIN 2+. Transformation zone was present in 93.8%. Most Pap tests were unsatisfatory due to excess blood (69.4%); a significant subset showed marked acute inflammation (30.6%). Thirty-one Pap tests had endometrial cells, 17 a shift in vaginal flora, and seven had organisms.
Conclusions: Marked obscuring blood, inflammation, and low cellularity compromised Pap interpretations in our study. The transformation zone was sampled. A significant number of patients with an unsatisfatory Pap test and positive HPV test had CIN on cervical biopsy (39.4%). Reprocessing with GAA resulted in a marked decrease in unsatisfactory rates, although the average cellularity of those re-processed is still considered borderline. A 5% unsatisfactory rate after reprocessing remains increased.
40: Manual versus Imager: Comparison of full slide review using the ThinPrep® imaging system versus using a non-imager scope for full slide review
Carol Eisenhut, MD2, William Nelson, BS, CT(ASCP)1, Beth Eder, BS, CT(ASCP)1
1Cytology, and 2Pathology, DCL Medical Laboratories, a wholly owned subsidiary of Laboratory Corporation of America Holdings, Indianapolis, Indiana
Introduction: Experience, internet chat, and word-of-mouth activity suggests that laboratories using the ThinPrep® Imaging System differ in their approaches to performing full slide review (FSR). There is also confusion as to whether these approaches are in regulatory compliance or if only the FSR performed on imaging system scopes would reflect full compliance with the FDA-approved methodology. Practical reasons (efficiency, availability of scopes, space, auto-overlap) and more philosophical reasons (attention span, comfort, use of 4μ magnification) have created these disparate solutions. Off label use of any FDA-approved system would require additional validation to assure acceptable laboratory performance. This study attempts to demonstrate as a proof of concept that the two methods perform similarly in our laboratory.
Materials and Methods: An abnormal, enriched population of 102 previously diagnosed cases (17 HSIL, 46 LSIL, 18 WNL EC+, 12 WNL EC-, and 9 UNSAT) were divided equally among six slide sets that were subjected to 323 separate comparison events. Each of the 19 cytotechnologists (CTs) (with experience ranging from 2 years to 25+ years) reviewed one set of 17 comparison events representing a mixture of Bethesda categories. For the purpose of this study, ASCUS / ASC-H was removed from the population and the CTs were instructed to categorize any equivocal case into either SIL or Neg. They believed they were performing an efficiency study. The cases were pre-selected to avoid those with ‘memorable’ diagnoses or those with distinct cellular presentations. Cases that had few abnormal cells or that had poor representation in the 22 FOV were specifically included. Each CT reviewed their set of cases using 22 FOV. Those identified by the CT as needing FSR were first screened by their preferred method (either with a standard microscope or with the imager scope). The CTs were asked to perform the same task by using the other method of FSR, one-to-two weeks later. They were not informed that they would be receiving the same set of slides that were rearranged, relabeled, and re-imaged.
Results: The CT diagnoses were categorized numerically by increasing the clinical concern: 1 = WNL / EC+, 2 = WNL / EC-, 3 = Unsat, 4 = LSIL, 5 = HSIL. The differences of the outcomes were measured at three differing thresholds (detection of abnormality, differing degrees of abnormality, and the presence of a glandular component). The results showed that there was very little difference in the CT diagnoses, regardless of their method of FSR, at all three decision points. There were abnormal cases and EC+ cases identified by each method that were not detected by the other, with a slight bias toward the imager method for abnormal cases and a slight bias toward the manual method for a glandular component. None of the thresholds demonstrated a statistical significance [Tables 1–3].
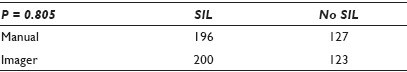
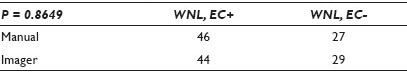

Conclusions: This study should be viewed as preliminary, as ASCUS (with its lack of reproducibility) was deliberately not included. The population was enriched with abnormal cases to amplify any differences between the methods. Still, the methods appear to perform so similarly that it is doubtful if the inclusion of ASCUS cases would cloud the issue enough to significantly change the outcomes. The data seem to suggest no differences between methods strongly enough, to be able to proceed with that study.
41: Quantitative profiling of p16 / Ki-67 immunoreactivity in epithelial nuclei for automated screening of Pap smear tests
Arkadiusz Gertych, PhD1, Harsha Rajendra Prasad, BS3, Anika Joseph, MS3, Shikha Bose, MD2
1Surgery, Cedars-Sinai Medical Center, Los Angeles, California; 2Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California; 3Biomedical Engineering, University of Southern California, Los Angeles, California
Introduction: The ambiguity of Pap test results, substantial manual burden, as well as lack of cytochemical reporters in Pap staining compounds has led to an ongoing effort in cytopathology to incorporate human papillomavirus (HPV)-specific biomarkers into the diagnostic and screening workflows. CINtec®PLUS, a dual immunostain that provides for simultaneous detection of p16 and Ki-67 overexpression in Pap smears has recently been shown to have comparable sensitivity, but significantly higher specificity, when compared to HPV testing, for identifying high-grade cervical intraepithelial neoplasia in women with mildly abnormal Pap smears. Overexpression of p16 in cervical cells has been validated as a biomarker for transforming HPV infections and marks the earliest stages of cervical disease, while Ki-67 is a marker for active cell proliferation. The color and intensity of the nuclei is crucial in the visual determination of immunoreactivity in epithelial cells. Although the assessment of p16 or Ki-67 overexpressions alone can be straightforward, the evaluation of the superimposed p16 and Ki-67 signals may be challenging for the human eye. This study aims to evaluate an automated quantitative profiling of mixed p16 / Ki-67-related signals in cell nuclei as a potential strategy for improved screening of liquid-based Pap smears.
Materials and Methods: Five cervical smears with atypical squamous cells in the Pap smear were destained and then restained utilizing a CINtec®PLUS (mtm laboratories, Westborough, MA) kit that marks the overexpression of p16 and Ki-67 as brown / cytoplasmic-nuclear and red / nuclear reaction products, respectively. The biochemical processing of smears was followed by the high-resolution imaging of slides, and selection and recording of fields of view, where abnormal cells were found by a cytopathologist. Our image analysis began with a nuclei localization algorithm that aimed to isolate objects of high optical density and roundness. Next, a training set of patterns was formed. It consisted of 60 nuclei manually assigned by a cytopathologist to four types of immunoreactivity: Ki-67 positive, p16 positive, dual p16 / K-i67 positive or negative. Then, quantitative profiling (QP), a technique for formation of immunoreactivity profiles in subsequent nuclear areas, was carried out, utilizing the training data. Finally, the profiles were classified to investigate the method's efficacy.
Results: Collected image data was successfully processed by automated QP-based algorithms returning profiles extracted from the cell nuclei. The classification of profiles reached a good agreement with cytopathologist evaluation: 98.7% concordance in non-immunoreactive (p16 / Ki-67 negative), 78.4% in dual p16 / Ki-67 positive, and overall 91.2%. Success rate in nuclei detection was nearly 95% as measured in n = 4174 cells found in the analyzed fields of view.
Conclusions: Owing to the drawbacks of the Pap test screening methods, the idea to evaluate epithelial cells using a quantification of p16 / Ki-67 immunoresponses in digitized smears has emerged. This study manifests QP-based potential as an enhancing screening tool that can boost automated techniques or reduce the manual burden, especially in the screening of negative slides. Future studies will validate the efficacy of the combined approach on a larger patient cohort.
42: Comparison of atypical glandular cells (AGC) on conventional versus SurePath® preparations
Stephanie Hamilton, EdD, SCT(ASCP), IAC, MB(ASCP)1, Marilyn Dawlett, CT(ASCP)2, Savitri Krishnamurthy, MD2
1School of Health Professions, The University of Texas MD Anderson Cancer Center, Houston, Texas; 2Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, Texas
Introduction: The improved sensitivity and specificity of liquid-based cytological preparations of gynecological specimens have been compared with conventional Pap smears for the detection of cervical intraepithelial lesions by several investigators. However, there are only rare reports that compared these two types of preparations for the detection of glandular lesions. We compared the utility of conventional Pap smears with SurePath® (SP) for the recognition of glandular lesions of the cervix. Even though the Pap smear was intended for the detection of cervical lesions and not for the detection of glandular lesions, which occur higher in the endocervical canal and uterus, the presence of atypical glandular cells (AGC) on cytological preparations warrants investigation as to the cause. Although there have been many studies comparing the sensitivity and specificity between conventional Pap smears and the ThinPrep® Pap Test, especially with respect to cervical intraepithelial lesions, there are scarce reports comparing the conventional with the SurePath® (SP) preparations for glandular lesions. In the present study, the cytological diagnosis of AGC on conventional Pap (CP) smears and SP tests was correlated with follow-up histological results.
Materials and Methods: The cytopathology records of MD Anderson Cancer Center were searched to identify all the gynecological specimens with a diagnosis of atypical glandular cells (AGC) on conventional Pap smears between January 1999 and December 2002 and on SP between January 2003 and December 2006. The cytological findings were compared with the histological diagnosis and / or clinical follow-up over a nine-month period. The finding of AGC in CP and SP was correlated with the subsequent significant findings, including squamous and glandular lesions (adenocarcinoma in-situ, glandular atypia / dysplasia), endometrial hyperplasia, fistula, polyp, Nabothian cyst, and leiomyoma / leiomyomata / adenomyosis statistically using the Chi-square test. This study has been submitted to the Institutional Review Board of The University of Texas MD Anderson Cancer Center (PA11-0403).
Results: AGC was reported in 180 CP smears and 120 SP preparations. Follow-up information was available in only 147 (82%) and 77 (64%) of these cases, respectively. A cytological diagnosis of AGC on CP was associated with significant lesions in 69 / 147 (47%) of the cases. Similar lesions were identified by SP in 45 / 77 cases (58%), however, the difference was not statistically significant (P < 0.1019). The sensitivity of SP for the recognition of significant glandular lesions was 41% in comparison to 36% for CP. The PPV of a diagnosis of AGC on SP for the recognition of glandular lesions was 50% in comparison to 41% with CP.
Conclusions: 1) A diagnosis of AGC on SP was associated more often with significant glandular lesions of the cervix in comparison to CP (58 versus 47%). 2) Sure path preparations were more sensitive than CP for the recognition of significant glandular lesions of the cervix.
43: Cytology of Pap tests in patients with benign endocervical polyps
Naima Kim, CT(ASCP), Kelisha Hendricks, CT(ASCP), Behzad Vakil, Cytology Supervisor, Andrew Schreiner, MD, Suzanne Brandt, MD, Rana Hoda, MD, FIAC
Department of Pathology and Laboratory Medicine, Papanicolaou Cytology Laboratory, New York Presbyterian Hospital, Weill Cornell Medical College, New York, New York
Introduction: Endocervical polyps (EC polyp) are the most common benign lesions of the uterine cervix. The mechanical pressure of the polyp may cause reactive and reparative changes in the EC epithelium. The cytology of the Pap tests in patients with benign EC polyp is not well-characterized.
Materials and Methods: Pap tests of a histologically proven benign EC polyp over a 7-month period (08 / 01 / 10 to 02 / 01 / 11) were retrospectively reviewed. All of them consisted of one Papanicolaou-stained ThinPrep® slide. Pap tests were either concurrent with the biopsy (bx) or up to six-months prior to the bx. Biopsies were performed for follow-up of an abnormal Pap or were clinically indicated for EC polyp removal. Results of human papillomavirus (HPV) tests were reviewed when pertinent.
Results: During the study period, there were 108 EC polyps from 108 women [age range, 21 to 75 years; mean age, 43.7 years]. Upon review of the Pap tests, no EC cells were seen in two cases. Significant findings were seen in 11 / 108 (10%) Pap tests and included: One case each of invasive squamous cell carcinoma and high grade squamous intraepithelial lesion (HSIL); Low grade SIL (LGSIL), four cases; and Atypical Squamous Cells of Undetermined Significance (ASCUS), five cases. Two of the five ASCUS cases were positive for HPV. Additional non-specific findings, in decreasing order of frequency, included: Acute inflammation (mostly present in clumps), reactive EC cells in flat sheets, squamous metaplasia, thick mucin (clumps and strands) associated with EC cells, reparative EC cells in flat streaming sheets, EC cells with cytoplasmic vacuoles with ingested neutrophils, altered blood, tubal metaplasia, EC atypia (enlarged vesicular nucleus with prominent nucleolus), and granulation tissue. Review diagnoses remained unchanged in 106 / 108 (98%) Pap tests. In the remaining two cases, that is, both the HPV negative ASCUS, the diagnosis was changed to EC atypia and reactive changes, respectively. Histologically, theEC polyps showed acute inflammation, squamous metaplasia, occasional repair, and rare ulceration only. Malignant and dysplastic abnormalities detected on the Pap tests were histologically present in the adjacent epithelium and not in the EC polyp. The ASCUS cases did not show any histological abnormality.
Conclusions: Significant squamous abnormalities were seen in 10% of the patients with benign endocervical polyp. HPV negative ASCUS may represent reactive changes in the EC polyp. No consistent combination of non-specific findings was noted in the Pap tests.
44: Identification of trichomonas vaginalis in different Pap test preparations: Trends over time in the college of American pathologist's educational inter-laboratory comparison program
Lydia Howell, MD1, Teresa Darragh, MD2, Rhona Souers, MS3, Nicole Thomas, CT(ASCP), MPH4, Ann Moriarty, MD5
1Pathology and Laboratory Medicine, University of California, Davis, Sacramento, California; 2Pathology, University of California, San Francisco, San Francisco, California; 3College of American Pathologists (CAP), Northfield, Illinois; 4College of American Pathologists (CAP), Northfield, Illinois; 5Ameripath, Indianapolis, Indiana
Introduction: Trichomonas is challenging to identify on Pap tests due to its small size, and the presence of distracting associated cellular changes resembling other inflammatory conditions or intraepithelial lesions. Clinically, the repercussions of misdiagnosis are discomfort, expense, and treatment complications. Artifacts of different slide preparations may amplify problems of Trichomonas detection. The College of American Pathologists Interlaboratory Comparison Program in Gynecological Cytology (CAP-PAP) has seen an increase in liquid-based challenges with a concomitant decrease in the enrollment for conventional Pap tests. This study evaluates CAP-PAP participant results for Trichomonas over 20 years, to ascertain if there is a change in performance with experience with different preparations types.
Materials and Methods: The concordance rates for the target diagnosis of Trichomonas vaginalis were evaluated for 167,956 responses on 3730 slides evaluated in the CAP-PAP, between 1990 and 2010. A nonlinear mixed model was fit with three factors participant type, preparation type, and a two-level program year (1990 – 1999, 2000 – 2010), and included interaction terms between these factors and a repeated measures component to model the slide factor correlation structure.
Results: The cytotechnologists (CT) showed higher overall concordance than pathologists (MD) (89.8% vs. 83.4%, P<.001). Both readers improved concordance in the second time interval, although the CTs showed higher concordance than the MDs (91.3 vs, 85.0%, P = 0.01). The lowest concordance rates were SurePath® preparations for MD and conventional smears for CT.
Conclusions: Detection of Trichomonas has improved with experience, with a liquid-based preparation. Awareness of differences between MD and CT and preparation types may help ensure accurate results in clinical practice.
45: Cervicovaginal cytology findings in uterine malignant mesodermal tumor (MMMT)
Kay Kasal, HT(ASCP), Hannah Krigman, MD, DengFeng Cao, MD, PhD
Pathology and Immunology, Washington University St. Louis, Missouri
Introduction: Mixed malignant mesodermal tumor (MMMT) is an aggressive uterine tumor that typically occurs in women aged 50 to 80 years. MMMT accounts for approximately 2% of the endometrial malignancies. The most common presentation is of a polypoid mass. These tumors are frequently necrotic, so biopsies are not informative. As this tumor is rare, the findings on cervicovaginal cytology are not well described. For this reason, we undertook a survey of preoperative cervicovaginal cytologies in an effort to identify features predictive of MMMT
Materials and Methods: Because MMMT is a relatively rare tumor, the database was searched for all patients with a diagnosis of MMMT from 1995 to 2010. The database was manually searched for cervicovaginal cytologies obtained within three months prior to resection. Pap tests were rescreened and examined for inflammation, tumor diathesis, and atypical cells, which were further classified as being of a glandular, stromal, undifferentiated or squamoid type.
Results: Sixteen monolayer cervicovaginal cytologies were identified in our files. Six were negative on rescreen, and one was considered unsatisfactory for evaluation due to paucicellularity. Nine cervicovaginal smears were abnormal. Four smears had glandular cells most typical of adenocarcinoma. Five had atypical endocervical type cells. We did not have samples with both atypical endocervical and endometrial type cells. Two samples with endometrial type groups and two smears with abnormal endocervical type epithelial cells had atypical squamoid cells as well. Of note, four samples had a population of small atypical, undifferentiated cells either singly or in groups. In two Pap tests, no other atypical cells were present. One sample had a striated muscle and one sample had fragments of stroma. Eleven samples had blood, inflammation or necrosis, including two samples without atypical epithelial cells.
Conclusions: Smears with abundant diathesis or inflammation should be carefully screened for small single or grouped poorly differentiated cells. In our series, that constellation seemed most predictive of MMMT. The admixture of two atypical epithelial cell types suggests a diagnosis of MMMT. The presence of striated muscle of abundant stroma also suggests a diagnosis of MMMT.
46: Cervista™ HPV HR, Cervista™ HPV 16 / 18 assays versus hybrid capture 2 assay: Comparative assay outcome evaluation in women with negative Pap smear cytology
Elizabeth Kurian, MD1, Mandi-Lee Caporelli1, Stephen Baker, MScPH2, Bruce Woda, MD1, Ediz Cosar, MD1, Lloyd Hutchinson, PhD1
1Department of Pathology, Memorial Medical Center, University of Massachusetts, Worcester, Massachusetts; 2Departments of Cell Biology, Quantitative Health Sciences and Information Services, University of Massachusetts, Worcester, Massachusetts
Introduction: A controversial commentary by Kinney et al. (AJCP 2010, 134:193-199) highlighted the poor clinical specificity of the Cervista™ assay. In response, we directly compared the Hybrid-Capture 2 (HC2) and Cervista™ assays.
Materials and Methods: The consecutive specimens (n = 601) were tested using HC2, then Cervista™ HR and Cervista™ HPV 16 / 18, with subsequent analysis of cytology negative cases (n = 533).
Results: Results indicated no significant difference (P = 0.458) in the prevalence rates between HC2 (7.5%) and Cervista™ HR (8.5%). Cervista™ 16 / 18 prevalence was 1.6%. Negative percent agreement was 96.5% (468 / 485) versus 70% (28 / 40) positive percent agreement. The internal Cervista™ DNA control had minimal impact for limiting the false negatives. Low genomic DNA limited evaluation in 4.1% (22 / 533) of the specimens with scant cellularity. In specimens with sufficient material, re-extraction and retesting yielded negative results (19 / 19). Indeterminate rates were as follows: Manual Cervista™ HPV HR 6.94% (37 / 533), Cervista™ HPV 16 / 18 6.80% (35 / 514) versus 1.13% for automated HC2 HPV HR (6 / 533).
Conclusions: Our data shows 29 discordant positive results (12 HC2 or 17 Cervista™ HR), suggesting that some women with negative cytology may be triaged for unnecessary follow-up with either assay. For clinical screening, both Cervista™ HR and HC2 are comparable, and by extension should provide an excellent negative predictive value for histologically relevant disease.
47: Comparing two methods of detection for chlamydia trachomatis using liquid-based specimens
Angelique Levi, MD, Danita Beckman, CT(ASCP), Kevin Schofield, CT(ASCP), Malini Harigopal, MD, David Chhieng, MD
Pathology, Yale University, New Haven, Connecticut
Introduction: Chlamydia trachomatis (CT) is a sexually transmitted infection that may remain asymptomatic in female patients. Because of the widespread use of liquid-based (LB) preparations, testing for CT as part of a Pap test examination is clinically useful, efficient, and convenient. This study compared the detection rate of CT in liquid-based gynecological specimens using the following two methods: The BD ProbeTec™ CT Qx-amplified DNA assay on the BD Viper™ System; and the Qiagen rapid capture system (RCS) CT DNA Test
Materials and Methods: A total of 1054 BD SurePath® and ThinPrep® specimens from female patients referred from multiple different referring practices in both an urban and suburban setting were tested for CT nucleic acids by the following two methods: The next generation assay BD ProbeTec™ CT Qx amplified DNA assay on the BD Viper™ System with XTR™ Technology (BD Diagnostic, Franklin Lakes NJ); and the Qiagen Rapid Capture System (RCS) CT DNA Test (Qiagen, Germantown MD). For all positive and any discrepant CT test results between the two methods, an additional PCR-based confirmatory test for CT was performed using TaqMan real-time PCR assay.
Results: Among the 1054 clinical LB Pap test samples, 13 (1.2%) specimens were positive for CT using the ProbeTec CT Qx assay on the Viper platform, while eight (0.76%) were positive for CT using the Qiagen RCS CT DNA Test. Twelve of the 13 CT-positive specimens using the ProbeTec CT Qx assay were confirmed by the real-time PCR assay. Therefore, among the 13 CT-positive cases using the Viper system methodology, there was one false positive case. There were no false positive cases using the Qiagen RCS methodology, but four of the 12 PCR-confirmed CT-positives cases were negative by the Qiagen RCS CT DNA Test.
Conclusions: In this study, the ProbeTec™ CT Qx assay detected more positive cases of CT from the LB Pap tests as compared to the Qiagen RCS CT DNA Test. Although the ProbeTec™ CT Qx assay on the Viper system may be more sensitive in the detection of CT from the LB Pap tests, confirmatory testing is suggested to exclude the possibility of a false positive test.
48: CINtec® PLUS for triage of women with low-grade squamous intraepithelial lesions on Pap smear
Sanam Loghavi, MD, Ann Walts, MD, Shikha Bose, MD
Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California
Introduction: Current practice guidelines recommend colposcopy, an invasive and expensive procedure, for women with low-grade squamous intraepithelial lesions (LSIL) reported on Pap smear. Colposcopic cervical biopsy has a sensitivity of 68 – 85% for detection of high-grade cervical intraepithelial neoplasia (HG CIN). CINtec® PLUS (CINtec) is a dual immunostain that provides for simultaneous detection of p16 and Ki67 overexpression in Pap smears. This study was designed to determine the utility of CINtec for detection of an underlying or subsequent HG CIN in women with LSIL on Pap smear.
Materials and Methods: Sixty-five cervical SurePath® Pap smears from women ranging from 17 to 75 years of age (median, 29.5 years) with LSIL and histological and / or cytological follow-up (range, 1 to 33 mos; median, 10 mos) were retrieved from our departmental files. The Pap-stained slides were destained and then immunostained utilizing the CINtec® PLUS Kit (mtm laboratories, Inc Westborough, MA) that showed p16 as brown / cytoplasmic and ki67 as red / nuclear reaction products. A ≥ 1 CINtec dual-stained cell was interpreted as a positive test result. Staining alone by p16 or Ki67 was recorded as negative. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for detecting an underlying or subsequent HG CIN were calculated.
Results: On follow-up, 51 cases had a negative or LSIL biopsy and / or Pap smear and 14 cases had HG CIN. Initial LSIL Pap smears were CINtec positive in 10 of these 14 women. CINtec had a sensitivity of 71%, a specificity of 47%, an NPV of 86%, and a PPV of 27% for detection of an underlying or subsequent HG CIN.
Conclusions: CINtec® PLUS is an inexpensive, noninvasive tool that can be applied to routinely prepared Pap smears and can contribute to the triage of women with LSIL on Pap smear.
49: CINtec® PLUS immunostain is better than p16 or ki67 for triage of women with abnormal Pap smears
Sanam Loghavi, MD, Ann Walts, MD, Shikha Bose, MD
Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California
Introduction: p16 and ki67 have each been shown to be good biomarkers for high-grade cervical intraepithelial neoplasia (HG CIN). Of late, CINtec® PLUS (CINtec), a dual immunostain for p16 and ki67, has been proposed as a tool for the triage of women with atypical squamous cells of undetermined significance (ASC) and / or low-grade squamous intraepithelial lesions (LSIL) on Pap smear. We compared the utility of CINtec with that of p16 and ki67 alone to detect the underlying or subsequent high-grade cervical intraepithelial neoplasia (HG CIN).
Materials and Methods: One hundred and fifty-two cervical SurePath® Pap smears (87 ASC and 65 LSIL) with histological and / or cytological follow-up (range 1 to 83 mos; median 10 mos) were retrieved from our departmental files. The Pap-stained slides were destained and then immunostained utilizing the CINtec® PLUS Kit (mtm laboratories, Inc , Westborough, MA) that detected the overexpression of p16 and ki67 as brown / cytoplasmic and red / nuclear reaction products, respectively. Each smear was evaluated with respect to CINtec, p16, and ki67 separately. A smear was considered CINtec positive if ≥ 1 dual stained cell was present and p16 or ki67 were positive if 10 immunostained cells were present. Sensitivity, specificity, and positive and negative predictive values for detecting an underlying or subsequent HG CIN were calculated for each stain.
Results: The results are shown in Table 1.

Conclusions: Compared to p16 or ki67 alone, CINtec® PLUS has a considerably higher specificity and comparable negative predictive value for detecting an underlying or subsequent HG CIN.
50: Significantly improved predictive value provided by ProExC immunostaining of Pap smears diagnosed as HSIL warrants leap to LEEP
Andrew Veyliotti, CT(ASCP)1, Sarah Wachs, CT(ASCP)1, Chris Rozelle, MD1, Robert Krum, MD2, Terry Morgan, MD1
1Pathology, OHSU, Portland, Oregon; 2Pathology, Kaiser NW, Portland, Oregon
Introduction: Management guidelines now allow for a ‘leap to loop electrosurgical excision procedure (LEEP)’ in women with a pap smear diagnosis of high grade dysplasia (HSIL). This change was due to an improved positive predictive value (PPV) of HSIL (70 – 80) provided by liquid-based paps sufficient to bypass inefficient colposcopy. However, our clinicians are hesitant to leap to LEEP because of the remaining significant false positive rates. HPV testing is not useful in these cases because the PPV is no better than cytology. Our objective was to test whether a specific marker of neoplastic transformation, such as ProExCTM, provides sufficient PPV to warrant the ‘leap to LEEP’.
Materials and Methods: SurePath® slides were prepared from the residual cell pellet from cervical Pap smears diagnosed as HSIL (n = 118) and immunostained for ProExC using a Ventana Benchmark XT. At least 5000 epithelial cells were required on each slide to be adequate for staining. Sufficient outcome data required either biopsy-proven high-grade dysplasia (CIN2+) or at least three years of negative follow-up. Biopsies were also stained for ProExC and a ‘Consensus Diagnosis’ between gynecological pathologists (TM and RK) provided the gold standard. Stained Pap smears were also independently scored by a cytopathologist (TM), pathology resident (CR), and cytotechnologists (AV and SW). Kappa statistics and predictive values were calculated and associations were tested by Chi-square analysis.
Results: We observed an excellent agreement between pathologists scoring ProExC (kappa statistic = 0.81) and between cytotechnologists (kappa = 0.82). Discordant scores were primarily in paps with fewer than 10 positive cells (52% discordance compared to 3 – 5% in negative or abundant cases). Surprisingly, cytotechnologists showed no additional improvement in their inter-rater reproducibility, also discordant in cases with few positive cells. The prevalence of CIN 2+ in HSIL was 81% with positive staining leading to a PPV of 95, NPV 78, and positive likelihood ratio of 4.5. Chi-square analysis revealed an association between positive ProExC staining and CIN2+ outcome (P < 0.0001).
Conclusions: We conclude by stating that ProExC staining may strengthen clinical confidence by significantly improving the positive predictive value in HSIL Pap smear diagnoses before leaping to LEEP. The weakness may be cases with only rare positive cells.
51: Proficiency testing of high-risk human papillomavirus DNA tests: The first three years of experience of the college of pathologists CHPV surveys
Ann Moriarty, MD1, Joel Bentz, MD2, Barbara Winkler, MD3, Andrew Fischer, MD4, Rodolfo Laucirica, MD5, Rhona Souers, MS6, Nicole Thomas, MPH, CT(ASCP)7, Chengquan Zhao, MD8
1Cytology, AmeriPath, Indianapolis, Indiana; 2Pathology, Lab Med Consultants Ltd, Las Vegas, Nevada; 3Director, Pathology, Mt Kisco Med Group PC, Mount Kisco, New York; 4Pathology, University of Massachusetts Medical Center, Worcester, Massachusetts; 5Pathology, Baylor College of Medicine, Houston, Texas; 6Biostatistics, College of American Pathologists, Northfield, Illinois; 7Surveys, College of American Pathologists, Northfield, Illinois; 8Pathology, Magee-Womens Hospital, Pittsburgh, Pennsylvania
Introduction: Laboratories that test for high-risk human papillomavirus (HRHPV) DNA must participate in annual proficiency testing (PT) for HRHPV as required by the Clinical Laboratory Improvement Amendment of 1988. The College of American Pathologists (CAP) Human Papillomavirus (High-Risk) Survey for Cytopathology and Other Laboratories (CHPV) meets this requirement, with five samples sent three times per year. It is the only national HRHPV proficiency test available in the United States adapted to all the commercially available transport / preservative media types, and it can be used for any HPV testing method. This study analyzes the performance of laboratories using various transport media and testing methods,
Materials and Methods: The analysis examines the CHPV survey results from 2008 through 2010. Each survey offered either Digene™, SurePath®, ThinPrep® transport media or a mixture of different media types. A correct response was defined as a challenge that achieved participant consensus at 80%. A nonlinear mixed model with a significance level of 0.05 was used to analyze the survey year, media, method, and challenge type, to identify factors associated with performance.
Results: There were 476 laboratories that submitted 14, 911 responses; 80% consensus was achieved for all challenges. Overall, 14,620 (98%) responses were correct. There were no differences in performance between positive and negative challenges, or the rate of correct responses from year to year. Significant differences in performance were identified for transport media and testing methods as shown in Table 1.

Conclusions: The CHPV surveys demonstrated consistently good laboratory performance of HR HPV DNA tests over the three years of the program. All challenges were concordant to the 80% threshold. Digene STM and ThinPrep® transport media performed better than SurePath® media. Digene, user developed test methods, and Cervista performed better than the other commercial kits and third wave invader assays. The CAP CHPV survey provided useful information for laboratories to assess their choices for HPV testing.
52: Analytic validation of the cervista HPV HR test using SurePath® cervical cytology specimens
Marilyn Nutter, BA, CT(ASCP), Brenda Sweeney, MS, SCT(ASCP) MB, David Wilbur, MD
Cytopathology, Massachusetts General Hospital, Boston, Massachusetts
Introduction: High Risk (HR) Human Papillomavirus (HPV) testing is important in the management of cervical cytological abnormalities and in assessing the risk in cytology-negative patients who are over 30 years of age. This study compared the prior validated Qiagen Hybrid Capture II HPV Test (HC II) with the Hologic Cervista HPV HR test in SurePath® specimens for the purposes of analytic validation prior to the initiation of the latter assay to routine use.
Materials and Methods: The tests were prospectively evaluated in a single center. DNA was extracted from the residual SurePath® cervical cytology specimens and assessed for the presence of HR HPV using the two tests. Results obtained were compared. Discordant results were adjudicated by PCR performed at an outside institution.
Results: One hundred and eighty-three cases had complete results for both tests (86 NILM, 87 LSIL, 5 HSIL, and 5 ASC-US). One hundred and sixty-six specimens showed concordant results between the two tests (91%). Of the 17 discordant cases, all were HC II positive and Cervista negative. PCR adjudication results on these cases showed that one case was positive for HR HPV (type 68), six were negative for HR HPV, and 10 were positive for low risk (LR) HPV. There was a single HC II positive / Cervista negative discrepancy in the HSIL group, which was found to be positive for LR HPV on PCR adjudication. Follow-up biopsy and endocervical curettage on this patient showed no pathology. Compared to HC II with PCR adjudication, Cervista HPV HR showed only one false negative and no false positive test, (1 / 183 = 0.6% error rate). The false negative case was in a specimen with ASC-US and follow-up showed no pathological abnormality.
Conclusions: The Cervista HPV HR Test could be used for the detection of HR HPV with residual cervical cytology specimens collected in the SurePath® media. The test did not show cross reactivity with LR HPV types as was noted in 10 of the 17 (59%) HC II specimens from the discordant test pairs.
53: Endometrial carcinoma: In need of a screening tool
Claudia Ormenisan1, Marina Mosunjac1, Bhagirath Majmudar1, Michael Koch1, Angie Earhart1, Victor Y Du1, Stefan Pambuccian2, Gabriela Oprea1
1Pathology, Emory University, Atlanta, Georgia; 2Pathology, University of Minnesota, Minneapolis, Minnesota
Introduction: Endometrial carcinoma (EC) is the most common invasive neoplasm of the female genital tract and the fourth most frequently diagnosed cancer in the United States. In 2010, there were an estimated 43,470 new cases of endometrial carcinoma and approximately 7,950 deaths caused by EC. Even as the Pap test represents the most successful screening tool for squamous cell cervical carcinoma and its precursors, it is of limited value in the detection of EC. A possible explanation for the low sensitivity of Pap test for EC includes the low shedding rate of EC cells. This study intends to determine the efficiency of the Pap test in detecting Endometrial Carcinoma in our patient population. We also plan to evaluate the presence in the Pap test of diagnostic cells for glandular neoplasia
Materials and Methods: A retrospective review for surgically removed uterine carcinomas between 1997 and 2009 was performed. Demographic data, Pap test results, pathological type and stage were entered in a Microsoft Excel spread sheet. Trends and Chi-squared statistical analyses were performed. The likelihood of preoperative diagnosis of EC by cytology via Pap test was correlated with the type and grade of the tumor. The age range for the types of EC (Endometrioid, Serous / Mixed and MMMT) was assessed.
Results: One hundred and seventy-three patients were treated surgically for EC. Table 1 details the type of endometrial carcinoma with age, stage, and relation to the cervical cytology.
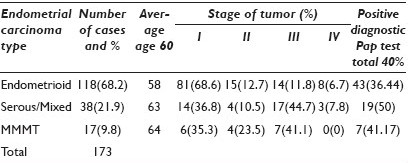
Conclusions: Serous carcinomas and malignant mixed mullerian tumors (MMMTs) were more likely diagnosed at an older age. Patients with serous carcinoma and MMMT were more likely to present with a higher stage. MMMTs were the least likely to have a preoperative positive Pap test. Our data shows that early diagnosis still remains a challenge for endometrial carcinoma.
54: Cytological features of malignant mixed mullerian tumor (MMMT): A report of twenty-three cases
Michelle Reid, MBBS1, John Crosby, MD2, Sravankumar Kavuri, MBBS3, Preetha Ramalingam, MBBS2
1Pathology, Emory University School of Medicine, Atlanta, Georgia; 2Pathology, Georgia Health Sciences University, Augusta, Georgia; 3Pathology, Charlie Norwood Veterans Affairs Medical Center, Augusta, Georgia
Introduction: Malignant mixed mullerian tumor (MMMT) is a biphasic malignant tumor of the female genital tract that is often seen in the elderly. It may arise in the vagina, cervix, endometrium, ovaries or fallopian tubes, as well as the peritoneum. Although its histological features are well-established , its cytological features are only depicted in isolated reports. We describe the cytological features of 23 MMMTs, the largest such series to date.
Materials and Methods: We identified 23 cytologically sampled MMMTs from 21 patients who ranged in age from 45 to 97 years (mean 70 years.). The site of origin, specimen-type and cytological features of each case were analyzed and tabulated.
Results: There were eight primary and fifteen metastatic tumors. Primary sites included cervix (Cx) (1), endometrium (13), ovary (3), and peritoneum (P) (4); 20 / 21 patients had biopsied (2) or resected (18) primary tumors confirmed histologically, and 14 / 20 of these had heterologous elements (HE) on histology. These HEs included rhabdomyoblasts (6) and chrondrosarcoma (8). The cytological specimens included Pap smears, ascitic fluid (AF), pleural fluid (PF), pelvic washings (PW), and fine needle aspirations (FNAs) from liver and lung, as well as inguinal and iliac lymph nodes (LNs). The specimen types and sites of sampling are summarized in Table 1. The cytologic findings included carcinoma (CA), sarcoma (SAR), and less commonly HE [Table 2]. The most common CA was adenocarcinoma, not otherwise specified (AD, NOS), with a few less common cytomorphological subtypes, including papillary serous, squamous, papillary, and plasmacytoid carcinoma.

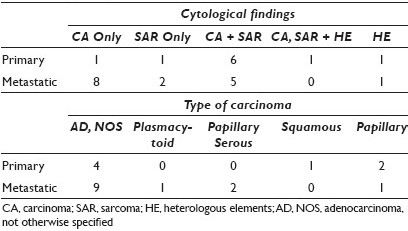
Conclusions: The majority of cytologically sampled MMMTs were metastatic and most represented pelvic washings and FNAs of peritoneal metastases. Primary tumors were most often endometrial and cervical MMMTs, sampled via Pap smear, and followed by FNAs of the primary MMMT of the peritoneum. Most cytologically diagnosed metastatic MMMTs had only CA cells and no SAR component, and in these cases, the cytological diagnosis was facilitated by the knowledge and review of the histology of the corresponding resection or biopsy specimen. The latter observation in our review represents a potential diagnostic pitfall that could lead to the erroneous misclassification of MMMT as CA, resulting in the under-staging and suboptimal management of patients. Not surprisingly, most primary MMMTs resembled their histological counterparts and had a mixture of both CA and SAR cells. Although HEs were identified frequently on histology, they were rarely identified on cytology in both the primary and metastatic MMMTs. As such, the identification of HEs is not helpful in the cytological diagnosis of MMMT, which relies instead on the identification of a mixture of sarcomatoid cells as well as malignant epithelial cells. The diagnosis of MMMT must be borne in mind by cytotechnologists and cytopathologists when evaluating cytology specimens, especially those from the gynecological tract and peritoneum.
55: Cytological parameters predicting neoplasia in atypical glandular cells with histological follow-up: A single institution experience
Jordan Reynolds, MD, Andrew Sciallis, MD, Jesse Voss, CT(ASCP), Ashley Johnson, CT(ASCP), Mohammad Dairi, CT(ASCP), Ocla Kigen, CT(ASCP), Jill Caudill, CT(ASCP), Brent Bedke, MD, Michael Henry, MD, Aziza Nassar, MD
Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota
Introduction: Studies investigating the histological follow-up of the 2001 Bethesda system diagnosis of ‘atypical glandular cells’ (AGC) have been performed focusing on various screening methods, patient populations, and Pap preparations. We report the histological follow-up of women receiving an AGC diagnosis using liquid-based screening (ThinPrep® Hologic, Inc., Bedford, MA). We also look for specific cytological features that would predict benign versus malignant follow-up results.
Materials and Methods: A retrospective medical record search was performed to identify liquid-based cervical cytology tests interpreted as AGC. The AGC diagnoses were stratified into five groups: Atypical endometrial cells (AGC-EM), atypical endocervical cells (AGC-EC), atypical glandular cells favor neoplastic (AGC-FN), atypical glandular cells not-otherwise-specified (AGC-NOS), and atypical glandular cells favor reactive (AGC-FR). Histological follow-up, either as a biopsy or resection specimen with a diagnosis of cancer, complex endometrial hyperplasia, or high-grade squamous dysplasia, was considered as an evidence of disease. Available slides were blindly reviewed by three cytotechnologists and a Fellow, and the specific, pre-determined cytological features were recorded for each case. The cytological parameters evaluated are shown in Table 1. Available cases were reviewed at a consensus session with a cytopathology Fellow and cytopathologist. Statistical analysis included a Pearson chi-square test, using a JMP 8.0 (SAS, Cary, NC), to compare the cytological factors, which would predict benign versus malignant follow-up.

Results: We analyzed 123,763 liquid-based cervical cytology specimens in our laboratory between 2005 and 2009. Of these, 264 (0.2%) samples were diagnosed as AGC. Patients without subsequent biopsy were excluded from the statistical analysis (n = 16). Of these remaining 248 patients, 60 (24%) were diagnosed with AGC-EM, 36 (15%) with AGC-EC, 28 (11%) with AGC-FN, 122 (49%) with AGC-NOS, and two (<1%) with AGC-FR. Of the 248 patients with AGC, 80 (32%) had a clinically significant lesion represented as: Severe squamous dysplasia (n = 9), endometrial hyperplasia (n = 4) , ADCA (n = 48), MMMT (n = 1), serous carcinoma (n = 7), endocervical ADCA (n = 3) , AIS (n = 3), metastatic carcinoma (n = 4), and endometrial stromal sarcoma (n = 1). Patients with evidence of neoplasia were significantly older (P < 0.001) than those without (59.6 and 49.2 year-olds, respectively). Two hundred and eighteen cases had available slides for review. Multiple parameters were assessed. Table 1 summarizes the cytological parameters that are significantly more or less frequently associated with malignant follow-up. Neoplastic cases showed significantly increased numbers of single cells, cells in three dimensional clusters, gland formation, engulfed neutrophils, nuclear enlargement, increased N : C ratio, irregular nuclear borders, reniform nuclei, loss of polarity, and macronucleoli.
Conclusions: These results are in accordance with the previous studies highlighting the clinical significance of a diagnosis of AGC on liquid-based screening. Cytological parameters can be used to predict benign from neoplastic follow-up conditions. Close clinical follow-up is warranted in the findings of AGC, especially in older women, by virtue of the frequency of significant neoplasia. Moreover, biopsy follow-up is necessary to correlate the cytological findings with the histology, and this should be addressed in the pathology reports.
56: Adequacy criteria for papanicolaou tests in postmenopausal women: Should we be content with less cells?
Melissa Rodgers, CT(ASCP), Ana Alvarez, MD, Swati Mehrotra, MD, Grazina Chatt, SCT(ASCP), Eva Wojcik, MD, Guliz Barkan, MD, FIAC
Cytopathology, Loyola University Medical Center, Maywood, Illinois
Introduction: The 2001 Bethesda System for reporting cervical cytology includes two categories for specimen adequacy, regardless of age or menopausal status: Satisfactory (SAT) and unsatisfactory (UNSAT). Liquid-based preparations require an estimated minimum of 5000 well-visualized / well-preserved squamous cells for the specimen to be considered adequate. Postmenopausal women frequently have hypocellular Pap tests (PAP) due to atrophy; therefore, the current criteria for cellular adequacy may be too stringent. The aim of this study is to evaluate the criterion of optimal cellularity in postmenopausal women.
Materials and Methods: A retrospective review from 2001 through 2008 showed that 170,094 ThinPrep® PAP tests were diagnosed at our institution; 68,821 of which were from women 45 years and older. Of these cases, 792 PAP (1.15%) were diagnosed as UNSAT. The following specimens were excluded from our study: Specimens that lacked follow-up, specimens from pre / perimenopausal patients, inadequate samples due to acellularity and inadequate samples due to obscuring blood / inflammation. This left 235 PAP (29.67%) UNSAT, due to low cellularity. These cases were evaluated for cellularity with follow-up frequency and diagnoses recorded. Follow-up was defined as a repeat PAP or biopsy performed within one year or more.
Results: Of the 235 UNSAT PAP, 189 (80.43%) had follow-up within one year (184 PAP and five biopsies). Of these, 163 (86.24%) were diagnosed as negative for intraepithelial lesion or malignancy (NILM), 21 (11.11%) as UNSAT, and five (2.65%) as atypical cytology or higher. Of the 21 repeated UNSAT PAP, further follow-up was 16 NILM (76.19%), one ASCUS (4.76%) (which reverted to NILM within one year), and four (19.05%) were lost to follow-up. The five UNSAT PAP that had atypical cytology or higher were diagnosed as one LGSIL on PAP, and one atypical endometrial cell, and three carcinomas (one endometrial adenocarcinoma and tow poorly differentiated carcinomas) on surgical biopsy. Both the LGSIL and atypical endometrial cells cases had NILM PAP within the following two years. Due to past medical history, all three carcinoma cases were clinically classified as high risk. Rescreen of the UNSAT PAP for these three cases revealed interpretive error (one case) and sampling error (two cases). In 46 cases, follow-up within two to three years was NILM. In total, 232 (98.72%) of the cases originally diagnosed as UNSAT were resolved to be NILM by follow-up over time. The mean squamous cell count per high power field for the cases was 1.5, leading to a mean cellularity of 2000 cells / PAP.
Conclusions: The knowledge of menopausal status is important in older women, for screening of their PAP. Postmenopausal women frequently have hypocellular samples due to atrophy. Upholding the same adequacy criteria in clinically low-risk postmenopausal women for PAP as pre / perimenopausal women, may lead to increased unnecessary medical procedures and increased healthcare costs. Our findings support lowering the cellularity criterion for adequacy in PAP of clinically low-risk postmenopausal women. However, if a postmenopausal woman is deemed to be clinically high risk, the current standard of cellularity should be observed and their cases should be targeted for a 10% prospective rescreen, to avoid / reduce interpretive error.
57: Clinical experience with Cervista™ HPV HR as a function of cytological classification: Comparison with retrospective Hybrid Capture (Hc2) data
Elizabeth Schroeder, Sandra Balzer, Lynn Kroeger, Jolanta Czarnecka, Judy Griep, Robert Amrhein, Connie Yauck, Kevin Ross, Erik Munson
Wheaton Franciscan Laboratory, Wauwatosa, Wisconsin
Introduction: Molecular high-risk human papillomavirus (HPV) detection has utility within the appropriate triage algorithms in the context of cervical cancer screening, particularly in women with a cytological diagnosis of atypical squamous cells of undetermined significance (ASCUS). Although hybrid capture technology (Hc2; Qiagen) has been a mainstay in molecular high-risk HPV-augmented triage, published laboratory validation and clinical trial data (e.g., Am. J. Clin. Pathol. 130: 401-408, 2008; J. Clin. Microbiol. 46: 1641-1646, 2008; Gynecol. Oncol. 118: 116-122, 2010) have suggested that the Invader® chemistry-based Cervista™ HPV HR (Hologic) possesses equivalent performance characteristics. However, one recent publication (Am. J. Clin. Pathol. 134: 193-199, 2010) raises caution over a purported increased high-risk HPV detection rate for Cervista™ HPV HR.
Materials and Methods: An audit of cytological diagnoses was conducted nine months prior to and following the in-house introduction of Cervista™ HPV HR for molecular HPV detection. The high-risk HPV molecular result (formally requisitioned by the clinician or indicated by cytology) was paired with cytological diagnosis. In a subset of patients with cytological diagnosis of ASCUS or absence of atypical cellularity, tandem results were stratified by patient age.
Results: In the nine months prior to the implementation of Cervista™ HPV HR, 2634 liquid-based cytological studies were subsequently forwarded for Hc2 performance; 72.6% of these studies yielded no significant cytological findings, while 15.1% were classified as ASCUS; 2539 cytological collections were forwarded for subsequent Cervista™ HPV HR within the first nine months of test implementation. Significantly more ASCUS cytological results (21.5%) were reported in this second testing interval (P < 0.0002), with fewer specimens yielding non-significant cytological findings (69.6%; P = 0.02). In spite of the increased ASCUS cytological findings during the Cervista™ HPV HR testing interval, the rate of molecular high-risk HPV detection (40.3%) did not exceed that observed in ASCUS-diagnosed collections subjected to Hc2 testing in the corresponding interval (43.0%; P = 0.41). In a similar fashion, the Hc2 high-risk HPV detection rate in 1913 cytological studies, without significant findings (7.7%), did not differ from the Cervista™ HPV HR detection rate (9.1%; P = 0.14) in 1752 analogous collections. A calendar-matched five-month subset of data, individualized to each molecular assay, revealed no difference in the non-significant finding — and ASCUS-based high-risk HPV detection rates with age delineations of < 30 years (P ≥ 0.24) and ≥ 30 years (P ≥ 0.10) were analyzed. Rejection rates on the basis of insufficient specimen volume were 9.4% for Hc2 and 5.5% for Cervista™ HPV HR (P < 0.0002).
Conclusions: Performance characteristics of Cervista™ HPV HR were largely unchanged from those of Hc2. When taken together with a decreased minimum specimen volume requirement and incorporation of a nucleic acid-based internal control, the Cervista™ HPV HR provided a viable nucleic acid hybridization alternative for high-risk HPV-augmented cervical cancer screening.
58: Evaluation of CINtec PLUS® testing as an adjunctive test in ASC-US diagnosed SurePath® preparations
Neil Edgerton, Cynthia Cohen, Momin Siddiqui
Department of Pathology and Laboratory Medicine, Emory University Hospital, Atlanta, Georgia
Introduction: The CINtec PLUS® system is an immunohistochemical cocktail composed of antibodies against p16INK4a and Ki-67, meant to improve the detection of high-grade dysplasia (HGD). Specifically, it has been examined as an adjunctive test that would serve to increase the overall specificity. In the presence of dysplasia, a red chromogen marks the Ki-67 expression in the nucleus and a brown chromogen marks the cytoplasmic p16INK4a expression. Only cells showing dual staining were interpreted as positive. This retrospective study examined the performance characteristics of CINtec PLUS® testing, when performed on ASC-US diagnosed samples, prepared by SurePath® preparations.
Materials and Methods: A total of 63 SurePath® Pap test slides diagnosed as ASC-US, with correlative colposcopic biopsy, were evaluated. HR-HPV testing was performed at the time of cytology diagnosis in all cases. Correlative colposcopic biopsy diagnoses were also derived from the medical records. In the event, multiple biopsies were taken, the most severe dysplasia (highest CIN) was recorded. Slides were analyzed by a single pathologist without adjudication. The pathologist knew the samples were composed of ASC-US-diagnosed cytology specimens, but was blinded to the HR-HPV results and surgical biopsy findings. The slides were interpreted as positive or negative for CINtec PLUS staining based on atlas and guidelines provided by the manufacturer. In the presence of dysplasia, there was red chromogen-marked Ki-67 expression in the nucleus and a brown chromogen-marked cytoplasmic p16INK4a expression. The testing was considered positive if a minimum of one cell showed simultaneous dual staining. The utility of CINtec staining on SurePath® slides was determined using the sensitivity and specificity calculations performed by the JavaSTAT online contingency table calculator.
Results: Within our sample set, 44% of atypical squamous cell diagnoses were found to have a CIN lesion (CIN I-III). Fourteen cases (22%) of our sample set of ASC-US cases had high-grade dysplasia (CIN II / III) at the time of cervical biopsy. No cases of cervical carcinoma were present. Staining with the CINtec PLUS® system showed modest sensitivity (64%) and specificity (53%) in identifying the presence of HGD at surgical biopsy. Positive and negative predictive values for HGD were found to be 28% and 84%, respectively. In contrast, HR-HPV DNA testing yielded a sensitivity of 100% and specificity of 21%.
Conclusions: Overall, the combined specificity gained by the performance of CINtec and HR-HPV testing was an improvement over current practice. The increased specificity of this test, besides simply creating another triage option, could conceivably lead to a reduction in colposcopic procedures. Cost effectiveness of this test remains to be demonstrated, as it would require the addition of both HR-HPV testing and immunohistochemical staining. The cost benefit lay in avoiding colposcopy, which carried both an evaluation and management fee along with the associated procedural cost. Although more difficult to calculate, the time and discomfort the patient is saved, is the biggest argument for increasing the specificity. Our study, although admittedly small, demonstrates an improvement in specificity above HR-HPV testing alone.
59: HPV positive / PAP negative diagnoses in women ages 30 and older: Looking to correlate morphological cues to determine QA / QC modifications
Rachel McMahon, BS1, Amber Schneider, BS1, David Tritle, MS1, Lynn Meyers, BS1, ChangHong Ye, BS, SCT(ASCP)1, Traci Arts, BS, SCT(ASCP)2, Michele Smith, MS, SCT(ASCP)1, Daniel F, Kurtycz, MD1
1Cytology Program, Wisconsin State Laboratory of Hygiene at UW-Madison, Madison, Wisconsin; 2Cytology Laboratory, Wisconsin State Laboratory of Hygiene At UW-Madison, Madison, Wisconsin
Introduction: The Wisconsin State Laboratory of hygiene (WSLH) provides gynecological services (Pap Tests, HPV, and limited biopsies) to under- and uninsured women in Wisconsin. The WSLH and its clinical partners modified a previous cost savings agreement to meet the 2006 ASCCP guidelines for women 30 years and older. The two-tiered model allowed liquid-based cytology (LBC) collection for all women of ages 30 years and older, to incorporate Pap and HPV testing. Women 29 years and younger were screened using conventional Pap (CP) tests with abnormal follow-up tests collected via LBC. During 2009, the WSLH performed 5530 HPV tests from a mix of 34,805 Pap tests. In this study we were interested in the cases where HPV was detected, but the cytology Pap test was diagnosed as Negative for Intraepithelial Lesion or Malignancy (NIL) (496 / 814, 60.93%). A retrospective case review was performed seeking potential morphological cues that might lead to a change in the quality assurance plan, in terms of modifications, for quality control through directed rescreen and / or routing cases directly, for a pathology review.
Materials and Methods: HPV testing was performed using Invader® / Cervista®. Cervista® was approved by the Food and Drug Administration in June 2009, and WSLH certified the method during the same year. WSLH had validated Invader as an Analyte Specific Reagent (ASR) in early 2007. HPV and Pap test data were obtained from the laboratory information system (LIS) that provided not only 2009 data, but also all the available follow-up of the gynecological tests (Pap, HPV, Biopsy / Colposcopy) in 2010 and 2011. Screening criteria was based on several text books and modeled from journal articles by Bollman et al., (2005) and Nijhawan et al., (2010). Third semester students evaluated all cases scoring them by the determined criteria as well as the established abnormal criteria. Instructors adjudicated case discrepancies. All abnormal cases were routed to the pathologist for final evaluation.
Results: Women, 30 years and older, comprised 4665 (84.36%) of the HPV testing results. Using Invader® / Cervista® (Hologic, Madison, WI) high-risk HPV (HRHPV) testing kits, 68.93% (3812 / 5530) reported as not detected, while 14.72% (814 / 5530) detected one of the 14 HRHPV types. General HPV and Pap results in women over 30 years are illustrated in Table 1. The retrospective review evaluated the HPV-detected and Pap NIL cases. Screening criteria are described in Table 2 and include selected cell morphology, inflammation, and infectious agents. The initial review of 319 cases found that a large majority met the multinucleated criteria (40%) as illustrated in Figure 1. Of the inflammatory and infectious agents, 40% of the cases also had coccobacilli as seen in Figure 2.



- Screening criteria
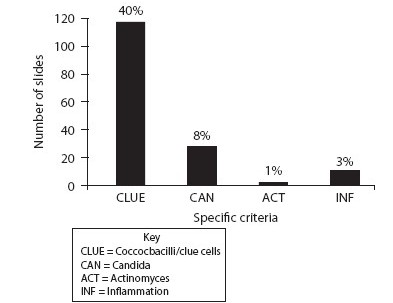
- Inflammation and inflammatory agents
Conclusions: The next steps in the review process include evaluating a random sample of cases from women of age 30 years and older with NILM Pap and no HPV detection, using the same criteria points. To complete the study, we will also evaluate the sample of cases diagnosed as ASC-US, regardless of the HPV status in the same population demographic. Initial findings show that 40% of the cases reviewed meet the criteria of bi- / multinucleation. Several authors have discussed these soft criteria of HPV. It is also interesting to note the increase in coccobacillus infection with this initial review sample. It is likely that these cases meet closer morphological changes to ASC-US rather than NILM, but do not provide clear diagnostic criteria.
60: Comparing the positive rates of the qiagen Hybrid Capture II and Hologic Cervista HR tests for high risk human papillomavirus detection in SurePath® cervical cytology specimens
Heather Smith, BS, CT(ASCP), Brenda Sweeney, MS, SCT(ASCP)MB, David Wilbur, MD
Cytopathology, Massachusetts General Hospital, Boston, Massachusetts
Introduction: HPV testing is an important component of the management of equivocal cervical cytology specimens and for the triage of women over the age of 30 years having negative cytological examinations. On the basis of the FDA labeling data showing a Cervista positive rate of 18.5% in the over 30 years negative cytology group, there has been concern in the literature that the Cervista HPV HR assay is too sensitive for use in these roles, and that the positive rates, particularly in the over-30-years population, will be too high compared to the current HC II standard, to yield appropriate clinical results (Kinney et al., Am J Clin Pathol 2010;134:193). In this study we compare the performance of the current standard Hybrid Capture II (HC II) to Cervista HPV HR in SurePath® specimens.
Materials and Methods: HPV test results were collected over a one-year period that used either HC II (six months) or Cervista HPV HR (six months) testing methods. The patient population used for each test was the same and was considered a low-risk screening population. Positive rates with each test were tabulated and the results in the ASC-US triage; and in the over-30-year, cytology-negative populations were compared.
Results: In the first six-month period, HC II testing was performed on 3823 specimens, and in the second six-month period, Cervista testing was performed on 3176 specimens. In the over-30-years negative cytology group a 5% positive HC II rate and a 3% positive Cervista rate were noted. In the ASC-US triage group, HC II yielded a 42% positive rate and Cervista yielded a 35% positive rate. Cases of ASC-H yielded a 71% positive rate with HC II and a 49% positive rate with Cervista. Cases of LSIL tested showed an 86% positive rate with HC II and a 72% positive rate with Cervista.
Conclusions: The data shows consistently lower rates of HPV test positivity in SurePath® samples in all patient groupings and interpretations. Linked with data (Nutter , 2011) showing that HC II with SurePath® specimens may show low-risk virus cross-reactivity leading to false positive HR results, the current data suggests that low-risk virus cross-reactivity may account for at least some of the discrepancies noted. In total, the results do not support the conjecture that the Cervista test is too sensitive for routine clinical use and suggest that the Cervista FDA labeling data represents a population-dependent prevalence effect.
61: The influence of previous Pap smear history on cervical cytology diagnoses
Andrea Snitchler, DO, FCAP1, Jan Silverman, MD1, Yulin Liu, MD1, Nathaniel Sherwood, DO1, Alok Mohanty, MD1, Uma Krishnamurti, MD2, Kathleen Bryer, BS2, Beth Ujevich, MBA, MS, SCT, HT(ASCP)1
1Pathology, Allegheny General Hospital, Pittsburgh, Pennsylvania; 2Pathology, Western Pennsylvania Hospital, Pittsburgh, Pennsylvania
Introduction: The Pap smear cytopathological interpretation may be influenced by each patient's prior history, especially when there is a prior Pap smear diagnosis, with the assumption that the cytological diagnosis may be upgraded in a patient with a prior abnormal Pap smear. The purpose of this study was to determine the influence of previous Pap smear history on cervical cytology diagnoses.
Materials and Methods: One hundred ThinPrep® (Hologic, Marlborough MA) Pap smears were retrospectively reviewed, wherein the original diagnosis was NILM (25%), ASCUS (50%) or LGSIL (25%). Four cytopathologists and two cytopathology Fellows examined the Pap smears with the submitted age and any history of prior Pap smear diagnosis. Then after approximately 14 days, the pathologists re-reviewed the same specimens blinded to case number, age, and previous Pap smear diagnosis. One cytopathologist was only able to review 80 of the blinded slides.
Results: In aggregate, 80 cases (13.8%) were upgraded with the submitted history, that is, NILM to ASCUS or ASCUS to LGSIL. Eighty-eight cases (15.2%) were downgraded with the submitted history. Two cytopathologists showed remarkably low intraobserver variability, with 90% or greater consistency. The other cytopathologists and Fellows showed 47 – 69% intraobserver consistency (31 – 53% variability in diagnosis). The most junior cytopathology Fellow showed the greatest variability. Nine cases (9%) were agreed on by all six evaluators both blinded and unblinded. Compared to the original diagnoses, two of the six cytopathologists tended to underdiagnose when no history was provided. Three of the six cytopathologists overdiagnosed and one showed no difference.
Conclusions: The Pap smear is a screening test with recognized imperfect intraoberserver and interobserver variability. This study demonstrates that there is only a slight (1.4%) difference between the diagnoses being made, if evaluated blinded to demographics and prior history, or with all accompanying information. To better account for test bias the original diagnosis was also compared with the Pap smear interpretation blinded to prior history and again demonstrated little impact on the cytological interpretation. Although there was some interobserver variability, these results substantiated the idea that Pap smear diagnoses were based on cytomorphological findings, with only a minimal impact by demographics and prior history.
62: Effects on Pap smear screening productivity by implementation of the BD FocalPoint™-guided screener imaging system (GS)
Brenda Sweeney, SCT(ASCP) MB, David Wilbur, MD
Cytopathology, Massachusetts General Hospital, Boston, Massachusetts
Introduction: Over two-thirds of the accredited Cytotechnology training programs have closed since 1976, leaving only 31 active programs in the United States and Puerto Rico. Additionally, only 164 candidates successfully passed the American Society for Clinical Pathology (ASCP) examination in Cytotechnology in the year 2010. In light of staffing shortages and potential changes in the available health care dollars, many laboratories are looking to automation as a partial solution.
Materials and Methods: In order to assess changes in Pap smear screening productivity due to automation we collected average daily screening productivity data from three time periods: 12 months pre-GS implementation, six months post-GS implementation, and seven to eighteen months post-GS implementation. Screening rates for five cytotechnologists were tabulated during each of the three time periods.
Results: Table 1.
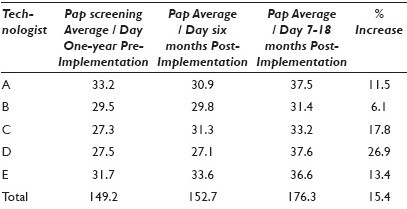
Conclusions: Conclusions: After the GS implementation there was a learning curve that negatively impacted some, but not all technologists. This screening volume downtrend was resolved within six months in all technologists, resulting in a 15.4% overall increase in daily screening volume (range 6.1 – 26.9%). As our laboratory represents a busy academic practice in which technologists perform many other duties outside of Pap smear screening, including HPV testing and an active rapid interpretation FNA service, the daily volumes do not reflect a typical eight-hour screening day. Laboratories that have more concentrated cervical screening environments may find greater levels of productivity resulting from the use of this device.
63: TelePAPology versus liquid-based ThinPrep® cervical cytology: A comparative study evaluating human papillomavirus testing by Hybrid Capture-2 and in-situ hybridization
Nevene Andraws, MD, Marilyn Davis, CT(ASCP), Susan Dillom, CT(ASCP), Fang Fan, Ossama Tawfik
Pathology and Laboratory Medicine, Kansas University Medical Center, Kansas City, Kansas
Introduction: Digital images are being used for telecytology, automated screening of Pap smears, training and education, as well as, proficiency testing. To date, the impact of digital imaging on routine day-to-day cytology remains far from perfect. Cellblock (CB) preparations from discarded / residual, conventional, and liquid-based GYN samples have been shown to be of diagnostic value. In a pilot study, we have demonstrated the feasibility of utilizing imaging technology to overcome current limitations by digitizing the cytological specimens from CB preparations. The current study was undertaken to evaluate the possibility of performing Human Papillomavirus (HPV) in-situ hybridization (ISH) on CB preparations and compare the results with Hybrid Capture-2 (HC-2) technique.
Materials and Methods: The Cellient system from Hologic (Marlborough, MA) was used to prepare CBs. Fifty-six H and E stained CB slides prepared from residual ThinPrep® (TP) samples were analyzed. These included ASCUS (42), LGSIL (7), normal (4), ASC-H (2), and HGSIL (1) cases. TelePAPology slides were obtained using the Aperio digital imaging system (Vista, CA). Virtual slides were reviewed by three cytopathologists and two cytotechnologists. HC-2 testing (QIAGEN, Inc, Valencia, CA) and HPV ISH testing (iVIEW Blue Detection Kit) (Ventana Medical Systems, Tuscon, AZ) were performed on all samples. Test performance characteristics of TP and TelePAPology samples were compared for diagnostic accuracy and HPV assay performance. Three CB samples / slides were included to reduce the cost and improve efficiency.
Results: TelePAPology virtual slides contained an optimal amount of material from all cases. Compared to TP diagnoses, fewer ASCUS cases were diagnosed by the TelePAPology method (33 vs. 42) and more normal cases were diagnosed (13 vs. 4). The diagnosis remained unchanged for LGSIL and HGSIL cases, except one case that was reclassified as LGSIL from ASCUS. One of the ASC-H cases diagnosed by TP remained as ASC-H by the TelePAPology method and the other was reclassified as ASCUS. A total of 46 of 56 cases were concordant by HC-2 and ISH testing including 24 positive and 22 negative cases. There were 30 positive and 26 negative HC-2 cases compared to 28 positive and 28 negative ISH cases. All discrepant cases were with ASCUS diagnosis. These included four positive by ISH / negative by HC-2 and six positive by HC-2 / negative by ISH. Both HC-2 and ISH reported similar ASCUS to HPV ratio positivity for TP and TelePAPology methods (48% for HC-2 and 45% for ISH).
Conclusions: CB preparations are a valuable source of material for additional ancillary testing such as HPV. In addition, combining the CB technology with digital techniques is a feasible method for widespread adoption, to achieve high quality specimen preparations. We have been successful in performing HPV ISH testing on all cases with an excellent concordance between ISH and HC-2 methods. ISH testing is feasible, cost-effective (three cases / slide), and practical. The concept of TelePAPology is suitable for routine cytology, ISH, and immunohistochemistry testing for HPV and other prognostic markers.
64: Determining implementation of ASCCP guidelines based on clinical evidence
Marla Taylor, BS, CT(ASCP)IAC, Lubna Sayage-Rabie, MD
Cytopathology, Scott and White Healthcare, Temple, Texas
Introduction: The American Society of Colposcopy and Cervical Pathology (ASCCP) developed practice guidelines for the management of women with an abnormal Pap Test. The guidelines changed the management of adolescent women (≤ 20 years) with a cytological diagnosis of Atypical Squamous Cells of Undetermined Significance (ASC-US) from reflex human papillomavirus (HPV) testing to repeat cytology in 12 months. Also included in the guideline was the recommendation of HPV testing for women ≥ 30 years with a diagnosis of negative for intraepithelial lesion (NIL) on cytology. Due to these changes the actual clinical practice of the guidelines varies among physicians. The aim of this study was to provide physicians with evidence from our patient population to determine if reflex HPV testing of adolescent women should be discontinued and if HPV testing of women ≥ 30 years should be implemented.
Materials and Methods: This study consists of two cohort-specific sub studies defined by cytological diagnosis and HPV testing. One cohort consisted of women ≤ 20 years, with a cytological diagnosis of ASC-US, with or without HPV testing, and biopsy correlation from January 2004 to December 2009. The other cohort consisted of women ≥,30 years with a cytological diagnosis of CIN2+ with biopsy correlation and HPV status for the year 2008. Data for the two cohorts was obtained through a computer search.
Results: There was a total of 1428 ASC-US diagnoses among adolescent women from January 2004 to December 2009; of these 1110 (78%) had HPV testing. High-risk HPV (hr-HPV) was detected in 708 (64%) of the cases by using Digene Hybrid Capture 2. Of these 167 (24%) had a follow-up biopsy. HPV testing was not performed on 318 (22%) of the ASC-US cases. Among those not tested, 89 (28%) had a biopsy. Table 1 shows the follow-up biopsy correlation of the cytological diagnosis of ASC-US with and without HPV testing among women ≤ 20 years from 2004 to 2009. Between January and December 2008, there was a total of 110 women ≥ 30 years with a diagnosis of CIN 2+. Of these 70 (64%) were confirmed by biopsy. A total of 27,794 women in this age group were screened for cervical cancer in 2008, resulting in a prevalence of 70 / 27,794 or 0.25%. Previous hr-HPV testing was performed on 17(15%) patients, of whom 13 (76%) were positive for hr-HPV with seven (54%) of them having biopsy confirmation of CIN2+. The HPV testing was due to either a diagnosis of ASC-US or ASC-H. The biopsy result of the seven hr-HPV cases are depicted in Table 2.


Conclusions: The results of the data collected among adolescent women with a cytological diagnosis of ACS-US with and without HPV testing shows no statistically significant difference in the follow-up biopsy diagnosis. This supports the ASCCP recommendation to not perform HPV testing on women 20 years of age or younger. The prevalence of CIN 2+ among women ≥ 30 years for 2008 was 0.25% or 2.5 per 1,000. This is slightly higher than the rate of 1.5 per 1,000 reported among women enrolled at Kaiser Permanente Northwest (Portland, Ore) in 1998. Previous HPV testing was performed on 17 cases, of which 13 were hr-HPV positive with only seven (54%) having a biopsy diagnosis of CIN2+. Even though the HPV testing was the result of an ASC-US or ASC-H diagnosis, this patient population had a higher prevalence of CIN 2+ as compared to other population groups. This would suggest that this institution should implement the ASCCP HPV testing guidelines for this patient population.
65: The yield of repeat biopsy following a second abnormal papanicolaou test after an initial negative or low-grade biopsy as follow-up for LSIL
Michael Thrall, MD
Department of Pathology, The Methodist Hospital, Houston, Texas
Introduction: The American Society for Colposcopy and Cervical Pathology (ASCCP) currently recommends that most women should undergo colposcopy and biopsy following a Pap test interpreted as LSIL (Wright et al. AJOG 2007;197:246). The exceptions are adolescents (aged < 21) for whom colposcopy is not recommended, and post-menopausal women, for whom colposcopy is one of several options. Many biopsies are negative or find only low-grade dysplasia. On the basis of the Advanced Trauma Life Support (ALTS) trial findings, repeat biopsy is recommended if a follow-up Pap test is abnormal or if high risk HPV is detected (Cox et al. AJOG 2003;188:1406). There is little data about the yield of these second biopsies in clinical practice.
Materials and Methods: The computer database of our institution was searched from the period from 5 / 1 / 2007 to 10 / 30 / 2008, to find women with a Pap test interpreted as LSIL (including LSIL-H). The previous and follow-up Pap test and biopsy results were then recorded for these women. The original slides were not reviewed. The total Pap test volume for this period was 67,223 (80% ThinPrep® and 20% SurePath®). Biopsy and Pap test follow-up was compiled up to 2 / 28 / 2011.
Results: Over the 18-month period, there were 2329 LSIL Pap tests in our laboratory (excluding a dysplasia clinic). Of these, 1140 were from non-adolescent women with no history of cervical dysplasia or prior abnormal Pap tests; 624 (54.7%) of these women had a first biopsy with the following findings: 221 negative, 351 HPV effect / CIN I, and 52 CIN II-III. Thus, the first biopsy high-grade dysplasia yield was 9.3%. Of the 572 women with no high-grade dysplasia found, 326 (57.0%) had a follow-up Pap test within two years, with the following findings: 126 NILM (HPV- or no HPV), six NILM HPV+, 49 ASC-US, 137 LSIL, and eight HSIL / AGC. Of the 200 women with positive results, 77 (38.5%) underwent repeat biopsy with the following findings: 33 negative, 36 HPV effect / CIN I, and eight, CIN II-III. Thus the second biopsy yield was 10.4% — similar to the first biopsy yield. Among the 137 women with repeat LSIL, five (9.8%) had CIN II-III found in the second biopsy.
Conclusions: Our follow-up data supports the current ASCCP guidelines regarding repeat colposcopy and biopsy for women who do not have high-grade dysplasia found in the first biopsy, but have persistently abnormal Pap tests. The yield of high-grade dysplasia found in second biopsies in this population is similar to the yield of the first biopsies for LSIL.
66: Use of p16 / ki67 cytology to detect cervical pre-cancer in a US referral population
Nicolas Wentzensen1, Joan Walker2, Rosemary Zuna2, Terence Dunn2, Michael Gold3, Mark Schiffman1
1Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, Maryland; 2University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; 3Vanderbilt University, Nashville, Tennessee
Introduction: Cytology-based screening for and treatment of cervical precancers has led to a substantial reduction of cervical cancer incidence and mortality worldwide. However, Pap cytology has limited sensitivity to detect prevalent cervical precancers. Here, we evaluated the performance of p16 / ki67 cytology to detect CIN2 and greater, in a US referral population.
Materials and Methods: Liquid-based cytology samples were collected from 551 women referred to colposcopy for abnormal cytology screening results. A ThinPrep® slide was produced from the vial and stained using the CINtec plus cytology assay (mtm Laboratories). Pap cytology and HPV genotyping were performed from the same vial. Women with CIN2 or greater identified in colposcopic biopsy were referred for LEEP. This analysis focused on the overall positivity, sensitivity, and specificity of p16 / ki67 cytology, to detect CIN2 or greater.
Results: In total, 136 CIN2+ were detected with colposcopic biopsy; 56.4% of women referred to the clinic were positive for p16 / ki67, while 86.6% were cytology-positive (at an ASCUS cutoff), and 77.7% were positive for carcinogenic HPV DNA. The sensitivity and specificity of p16 / ki67 to detect CIN2 or greater was 87.5% (95%-CI: 80.7 – 92.5%) and 53.8% (95%-CI: 46.3 – 61.2%), respectively. In comparison, the sensitivity and specificity of cytology at an ASCUS cutoff were 95.5% (95%-CI : 86.3 – 100%) and 17.5% (95%-CI: 13.1 – 22.7%); the sensitivity and specificity for carcinogenic HPV testing were 96.3% (95%-CI: 91.6 – 98.8%) and 29.3% (95%-CI: 22.8 – 36.5%).
Conclusions: In this high-risk referral population, p16 / ki67 had good sensitivity and specificity for detection of CIN2 or greater. If p16 / ki67 was used as a triage test, about half of the colposcopies could have been avoided, while most of the CIN2 or greater would have been detected. A detailed analysis of CIN2+ cases missed by p16 / ki67 to rule out non-dysplastic lesions overcalled in histology has been currently conducted.
67: Reflex HPV testing for ASCUS in a high-risk population
Vladislav Zakharov, MD, Diane Avery, CT(ASCP), Philip Valente, MD
Department of Pathology, University of Texas Health Sciences Center at San Antonio, San Antonio, Texas
Introduction: Reflex HPV testing following ASCUS cannot rule out LSIL. It is well-documented to increase the yield of CIN2+ on biopsy. However, recent guidelines have discouraged its use in women under 30 years of age. This retrospective study evaluates the relative sensitivity of Reflex HPV testing in younger and older patients in a high-risk population.
Materials and Methods: The computer database of the University Hospital in San Antonio, TX, was electronically searched for all ASCUS diagnoses from August 2000 to August 2010. These were further divided by age (Group A < 30 years; Group B ≥ 30 years) and high-risk HPV test status (Digene Hybrid® Capture II).
Results: A total of 6,125 ASCUS diagnoses (2,562 in Group A, 3563 in Group B) were identified. Unequivocal high-risk HPV test results were obtained in 3,036 (1,320 in Group A, 1,716 in Group B). The prevalence of high-risk HPV was 48.9% overall, 65.3% in Group A and 36.2% in Group B. Within a one-year interval, a total of 1,828 (29.8% of all ASCUS cases) had a histological follow-up, of which 908 (49.7%) had high-risk HPV testing with their original diagnoses (384 in Group A and 524 in Group B). A proportion of histological follow-ups differed significantly depending on the high-risk HPV test status. Among women of Group A, who tested negative for high risk HPV, 7.2% (33 out of 458) had a histological follow-up within a one-year interval compared to 40.7% (351 out of 862) in the same age group with a positive result for high-risk HPV. The same trend was observed in Group B , 14.0% (153 out of 1095) high-risk HPV negative versus 59.7% (371 out of 621) with a positive high-risk HPV test. Histological results are summarized in Table 1. Of note is that overall, Group A and Group B had similar positive biopsy rates among HPV positive patients (all CIN , 31.6% versus 28.6%; CIN2+ , 10.3% versus 10.8%). Among the small number of HPV negative biopsied patients, positive biopsies were 21.2% (7 out of 33) versus 6.5% (10 out of 153), with all cases in Group A being CIN 1. Five CIN2+ cases in Group B HPV negative patients had biopsies retrieved and reviewed. These were subjected to p16 immunostaining. Only one case was positive for p16. Two were reclassified as immature squamous metaplasia and two had features of CIN 2 despite negative p16 staining [Table 1].
Conclusions: In contrast to the ASCCP guidelines discouraging HPV Reflex testing, our findings suggest that this approach is equally useful in patients under 30 years of age, in a high-risk population. This may be due to a higher rate of HPV persistence in our older population (overall HPV prevalence in ASCUS was 65.3% in Group A, but still 36.2% in Group B). Our CIN 2 biopsies on HPV-negative patients have yielded two histological overcalls of immature squamous metaplasia. Two remaining CIN 2 cases may have been related to undetected low-risk HPV types. In our high-risk population, HPV Reflex testing for ASCUS appears to be useful even in women under 30 years of age.
68: Analysis of 70,123 cases with negative Pap test and high-risk HPV co-test results in a Large women's hospital
Chengquan Zhao, MD1, Xiangbai Chen, MD, PhD2, Agnieszka Onisko, PhD1, Baoying Weng, MD, PhD2, Marshall Austin, MD, PhD1
1Pathology, Magee Womens Hospital, UPMC, Pittsburgh, Pennsylvania; 2Pathology, Conemaugh Memorial Medical Center, Johnstown, Pennsylvania
Introduction: HrHPV-positive rates in women with cytology negative results vary widely in available reports. This variation has been attributed to differences in infection rates, patient ages, rates of HPV-associated cervical abnormalities, intensity of screening, and treatment programs to detect and ablate cervical lesions associated with persistent HPV infections, and false-negative cytology rates attributable to laboratory screening and interpretation.
Materials and Methods: A retrospective computer-based search of the Copath database of Magee Womens Hospital (MWH) of the University of Pittsburgh Medical Center was conducted over a six-year period, from 2005 to 2010, to retrieve data on Pap tests reported as negative for intraepithelial lesion or malignancy (NILM) from women who also tested for hrHPV DNA with the FDA-cleared Hybrid Capture 2 (HC2) method. The HPV-positive and HPV-negative rates for these NILM cases were assessed between younger and older age groups. Beginning in June 2005, the cytology-negative HPV-positive cases were routinely reviewed by a second quality control cytotechnologist and a cytopathologist. Long-term follow-up results of cytology-negative HPV-positive cases were analyzed.
Results: Among a total of 645,541 Pap tests performed over six years, 529,198 (81.98%) were reported as NILM (80.96 – 83.07%, varied yearly). Of these cytology-negative cases, 70,123 of 645,541 (13.25% (4.17 – 20.21%, varied yearly) had hrHPV co-testing. HPV testing of cytology-negative cases markedly increased in 2007. Cytology-negative HPV-positive results comprised of 1.92% (< 30, 7.22%; ≥ 30, 1.73%, P < 0.0001) of 70 and123 NILM cases co-tested for hrHPV. The HPV-positive rate in NILM cases decreased during the study period from 2.9% in 2005 to 1.0% in 2010. Eight hundred and sixty-nine women with NILM Pap and positive HC2 HPV testing results (average age 41.3 years) interpreted from July, 2005 to December, 2009 had repeat cytological or histopathological follow-up results. CIN1 / LSIL and more severe lesions were detected in 211 of 869 cases (24.3%) including 21 (2.4%) CIN2+ with average follow-up of 23.2 months. Kaplan-Meier projections of the cumulative incidence rates for CIN2+ in cytology-negative hrHPV-positive women, after 70 months, were 4.6% (95% CI: 2.4 – 6.8%). More LSIL / CIN1+ was identified with repeat positive HPV results than with repeat negative HPV results (P < 0.001). Since the last year women with negative Pap and positive HC2 HPV testing results underwent reflex HPV16 / 18 genotyping analysis. HPV16 / 18-positive cases accounted for 7.6% (8 / 105) of cytology-negative HC2 HPV positive cases [Table 1].

Conclusions: HPV positive rates in women with NILM Paps were significantly lower among women 30 years and older than among women less than 30 years, probably reflecting the increased transient HPV infection rates, known to occur in younger women. The decreasing HPV-positive rate in cytology negative cases observed in all age groups during the study period probably reflects the progressively increasing scrutiny of slides initially interpreted as negative, which were subjected to additional quality control reviews. As the CIN2+ detection rate was very low in these women and HPV16 / 18 positive cases accounted only for less than 10% of the cytology negative HC2 HPV positive cases, reflex HPV16 / 18 genotyping and triage may be the most cost-effective method for risk stratification of women with NILM Pap and positive HC2 HPV testing.








