Translate this page into:
Approach to Diagnostic Cytopathology of Serous Effusions

*Corresponding author: Lester J. Layfield. MD Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Drive, Columbia, Missouri, United States. layfieldl@health.missouri.edu
-
Received: ,
Accepted: ,
How to cite this article: Shidham VB, Layfield LJ, Approach to Diagnostic Cytopathology of Serous Effusions. CytoJournal 2021;18:32.
Abstract
Collection of most serous fluids from various effusions is a relatively simple procedure. Because of this, serous fluids are commonly submitted for pathologic examination including cytopathologic evaluation by various clinical institutions. As a consequence, even a general pathology laboratory which may not have expertise with highly trained cytopathologist would be confronted with serous fluids for cytologic evaluation.
However, cytopathologic evaluation of serous fluids is complex as compared to evaluation of fine needle aspiration cytology. This signifies the fact that all pathologists, irrespective of subspeciality cytopathology training and level of subspeciality expertise, should be conversant with the diagnostic challenges and pitfalls of effusion fluid cytology.
Although, majority of effusions are due to reactive and non-neoplastic etiologies, cancer is one of the causes of an effusion as a manifestation of advanced cancer. Detecting neoplastic cells in effusion specimens in most of clinical settings is related to the advanced status of the disease, which usually is equivalent to incurable stage. Thus, interpretation of cytopathology as positive for cancer cell is highly critical in planning the trajectory of the clinical management with an obvious negative impact of false positive interpretation.
Apart from cancer, effusions may be secondary to hemodynamic pathologies such as heart failure, hypoalbuminemia, cirrhosis etc. in addition to the different inflammatory conditions including parasitic infestations, bacterial, fungal, or viral infections, and other non-neoplastic etiologies including collagen diseases. Due to the cytomorphologic overlap of reactive mesothelial cells with malignant cells, general cytologic criteria for diagnosis of malignancy in single cells cannot be applied in most of the effusion specimens. This challenge is further amplified because of surface tension related phenomenon which ‘round up’ the cells after exfoliation in serous fluids. As a result, the native shapes of cancer cells cannot be a guiding feature. Thus the cytomorphologic features of cancer cells in serous fluids may not be same as seen in routine cytopathology of exfoliative, brushing, and fine-needle aspiration specimens.
The cancer cells may continue to proliferate after exfoliation in the nutrient rich effusion fluids and may form proliferation spheres. It is crucial to consider these factors when interpreting effusion cytology. Amongst malignant effusions, adenocarcinomas are the most common cause of metastatic cancers, but almost any type of malignancy including melanomas, hematopoietic neoplasms, sarcomas, and mesotheliomas may involve serous cavities. The interpreter must be aware of the wide range of the cytomorphologic appearances of reactive mesothelial cells in effusion fluids. It is essential to understand these and other nuances related to effusion fluid cytology. Understanding potential pitfalls during various stages from processing to application of ancillary studies would increase the diagnostic accuracy and minimize atypical interpretations and false positivity.
Keywords
Serous fluid
diagnostic cytopathology
effusion
tapping
paracentesis
immunohistochemistry
IHC
molecular pathology
SCIP approach
CellBlockistry
cell-block
cellblock
Diff-Quik stain
reactive mesothelial cells

GENERAL APPROACH
Aspiration of an effusion is a relatively simple procedure. Cancer is one of the frequent causes of effusions and it may be the first manifestation of advanced cancer. Consequently, any general pathology laboratory may receive effusion fluid for cytologic evaluation. As a consequence, all general pathologists and cytopathologists should be conversant with the diagnostic challenges and pitfalls of effusion fluid cytology. These facts emphasize the significance of understanding the approach to effusion fluid cytology by all practicing pathologists.
Finding neoplastic cells in effusion specimens not only reveals that a patient has cancer but also denotes the advanced nature of the disease, which, at this stage, is almost always incurable. Other than the detection of cancer cells in cerebrospinal fluid, no other exfoliative cytology specimen carries such an ominous prognostic significance for the detection of cancer cells. Apart from the finding of cancer cells, cytopathologic examination of serous effusions may reveal inflammatory conditions, parasitic infestations, bacterial, fungal, or viral infections, and certain other non-neoplastic conditions.
Other than high-grade neoplasms with pleomorphic cells, the standard cytologic criteria of malignancy based on evaluation of single cell morphology are not applicable for most of the effusion cytology specimens. Since cells in a fluid medium ‘round up’ because of surface tension, the native shapes of cancer cells cannot be a dependable guiding feature for deciding the primary origin of malignant cells. As effusion fluids are nutrient rich, the cancer cells have the potential to proliferate and may continue to divide and form proliferation spheres. It is crucial to consider these factors while interpreting effusion cytology.
Although adenocarcinomas are the most common neoplasm to spread to the peritoneum, pleura, and pericardium, almost any type of cancer may do so. Any neoplasm including carcinomas, melanomas, hematopoietic neoplasms, sarcomas, and mesotheliomas may involve serous cavities. Carcinomas are almost always adenocarcinomas. In general, sarcomas metastatic to serous cavities are rare. • The morphologic features of most of the cancer cells in effusion smears are different from those seen in exfoliative, brushing, and fine-needle aspiration cytology.
Reactive mesothelial cells are a consistent component of effusion fluids, and they may have significant morphologic overlap with malignant cells. Consequently, the interpreter must be aware of the wide range of cytomorphologic appearances of reactive mesothelial cells in effusion fluids.
To avoid various diagnostic pitfalls and increase the diagnostic accuracy, it is essential to understand multifactorial nuances in the setting of effusion fluid cytology. If one is familiar with the cytomorphologic features and the pitfalls associated with the effusion cytology, diagnostic interpretation may be facilitated, even without having to resort to the ancillary studies [Figures 1,2].
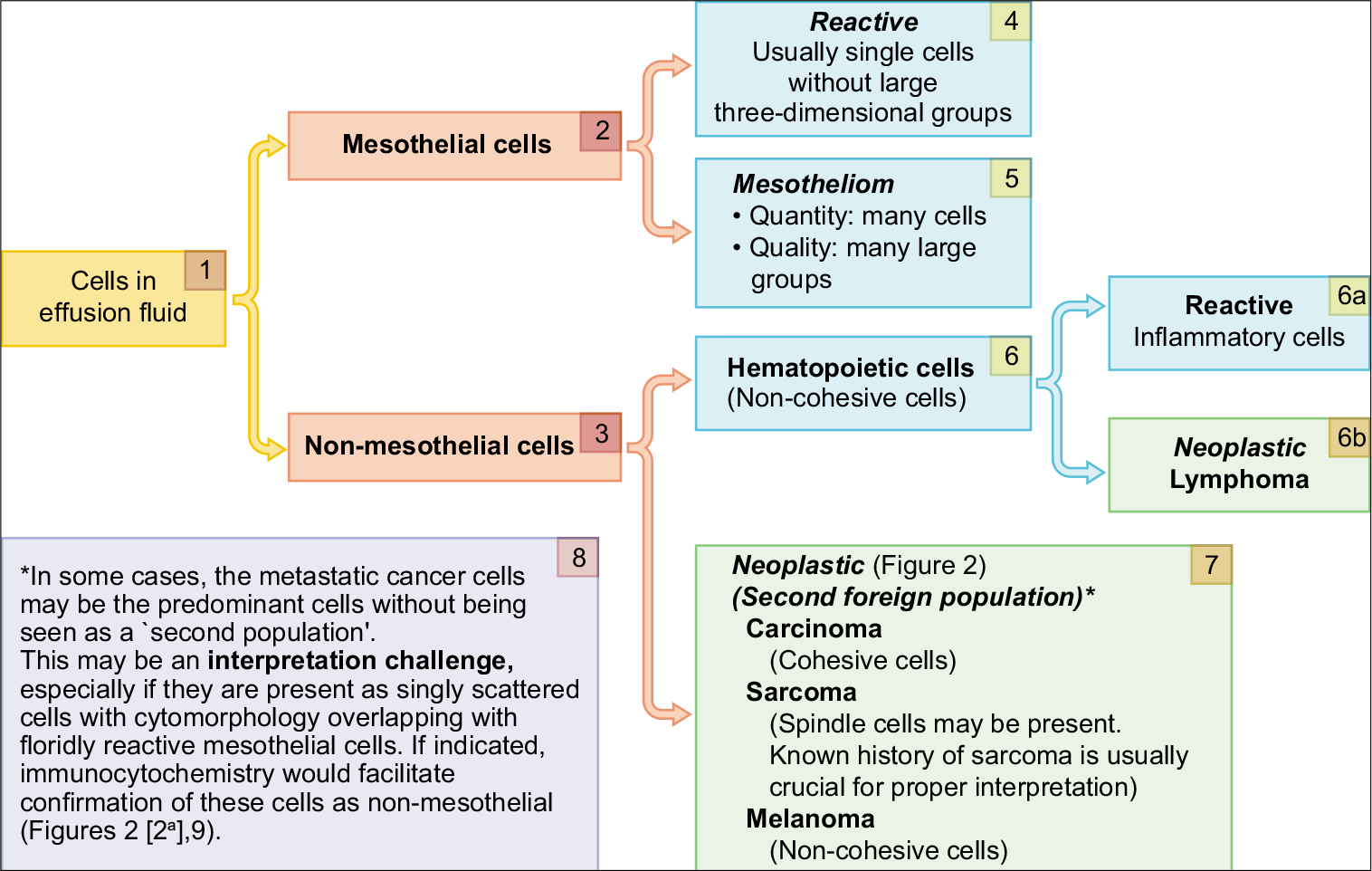
- Algorithm for evaluation of a ‘second foreign population’.
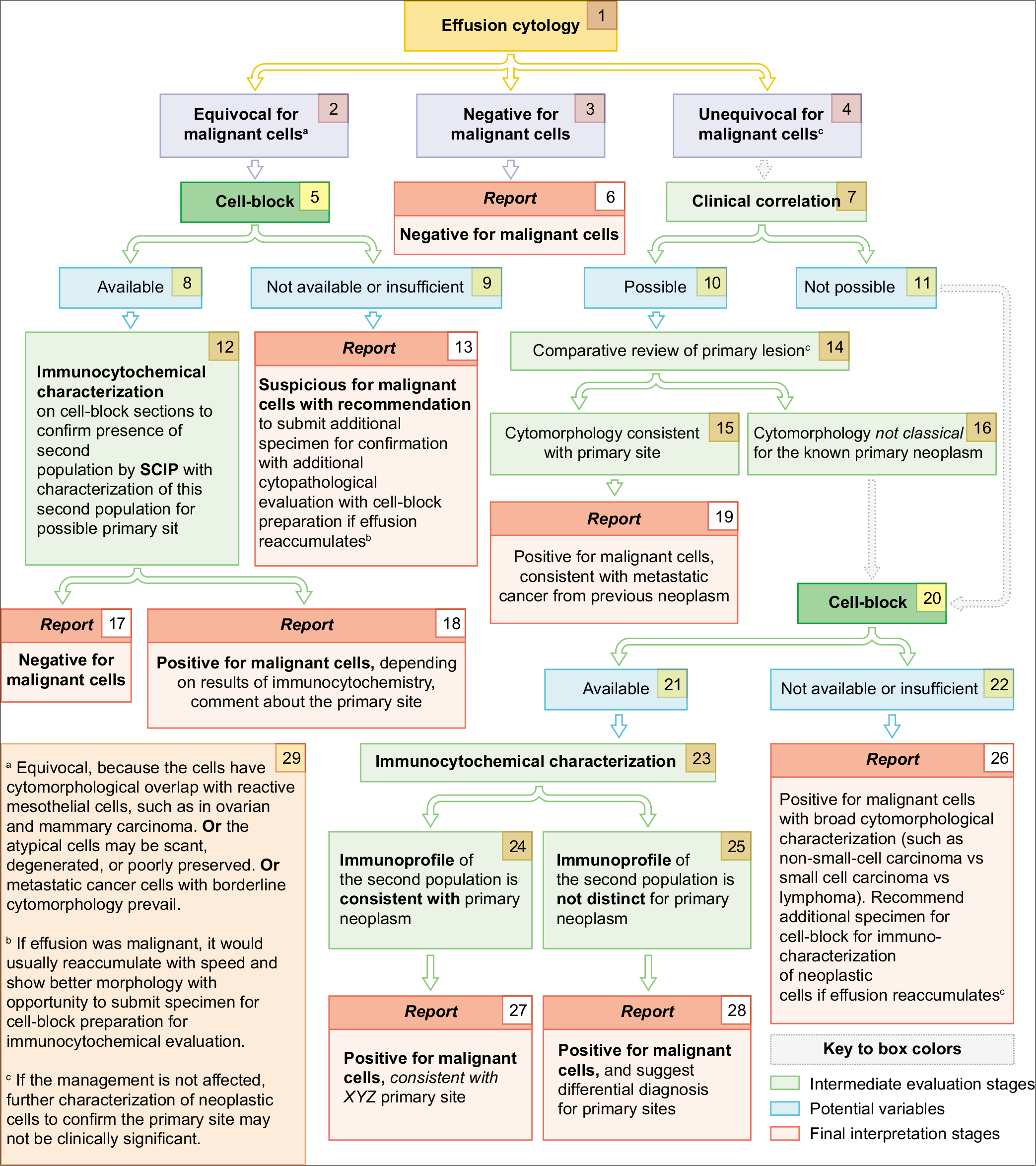
- Cytologic evaluation of effusion fluids for primary site.
Proper cytopathologic evaluation of effusion fluids thus needs a special approach at various stages from specimen processing to final interpretation level.
PROCESSING APPROACH
-
The backbone of effusion cytopathology is to organize the specimen processing in such a manner that it accomplishes the following objectives:
Detection of any ‘second population of foreign cells’ other than the mesothelial and inflammatory cells [Figure 8].
Studying the nuclear details of the ‘second population’.
Semiquantitative evaluation of individually scattered mesothelial cells and large groups of three-dimensional mesothelial cells, as in mesotheliomas [Figure 1[5]].
Objective confirmation of the ‘second foreign population’ and the differential diagnosis of their primary neoplasm.
Any approach that does not address these objectives may lead to misinterpretation and suboptimal results.
In our experience:
The 1st objective is best evaluated in Diff-Quik (DQ)-stained (Romanowsky-stained) air-dried Cytospin smears (or an air-dried direct smear prepared from the cell pellet).
The 2nd objective is best evaluated in Papanicolaou (PAP)-stained smears (such as wet fixed direct smears or rehydrated post-fixed Air-dried direct smears from the cell pellet, liquid-based cytology preps: SurePath, ThinPrep, Cytospin smears, or filters).
The 3rd objective is best evaluated in a DQ-stained direct smear prepared from the effusion fluid prior to concentration.
The 4th objective is evaluated better if a cell-block is prepared for elective ancillary tests, including immunostaining, as indicated.
- DQ-stained direct smear of effusion fluid prior to concentration.
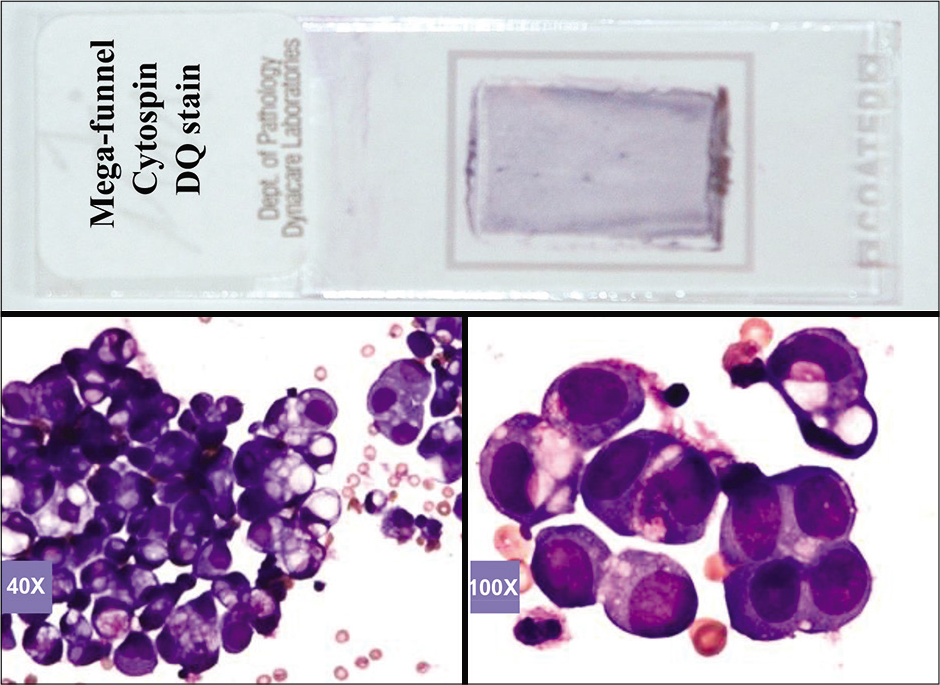
- DQ-stained Mega-funnel Cytospin smear of concentrated effusion fluid (metastatic ovarian carcinoma, peritoneal fluid).
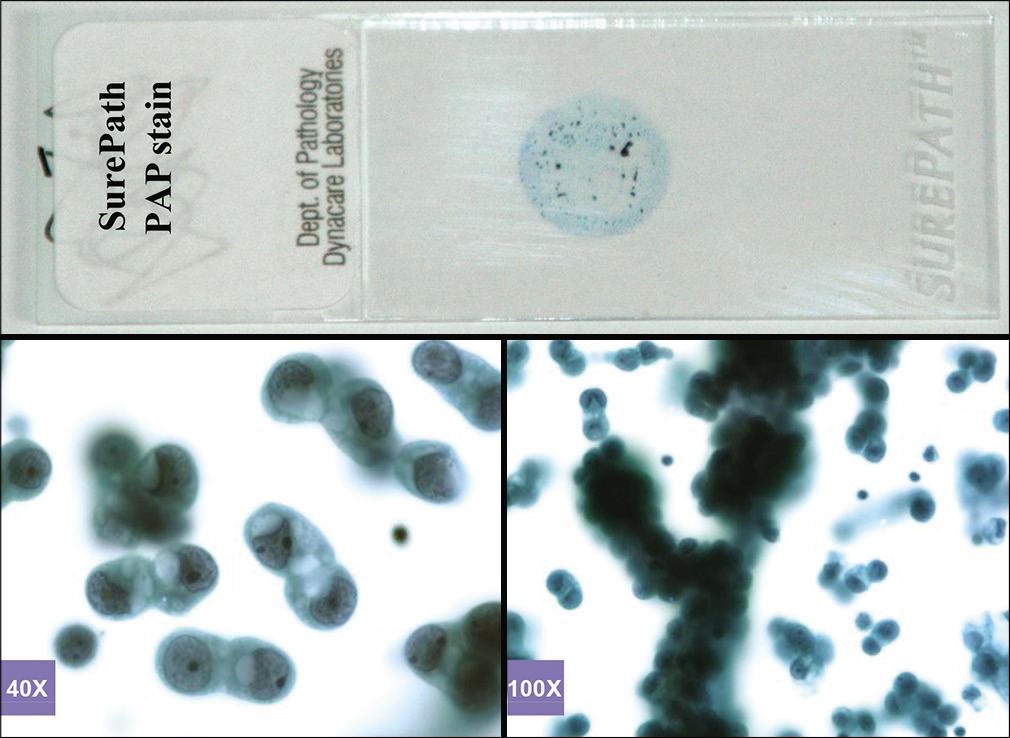
- PAP-stained SurePath (AutoCyte) smear (metastatic pulmonary carcinoma, pleural fluid).
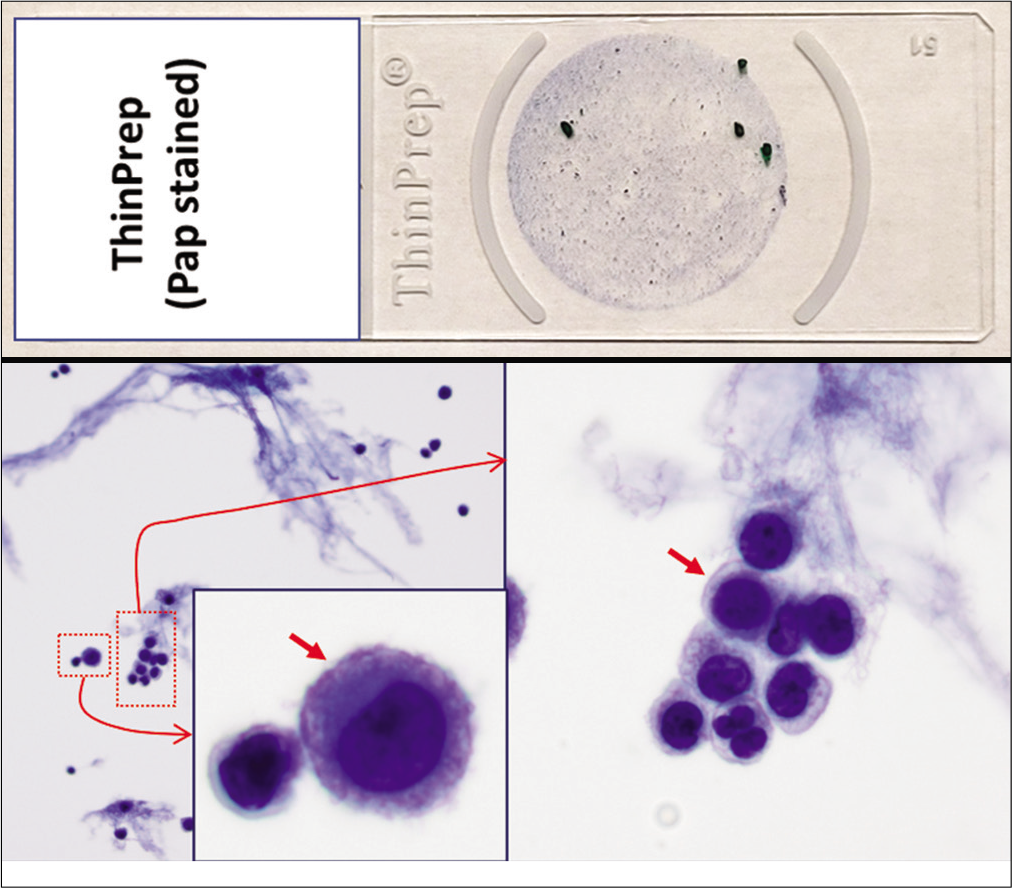
- PAP-stained ThinPrep (Reactive mesothelial cells (red arrows), pleural fluid).
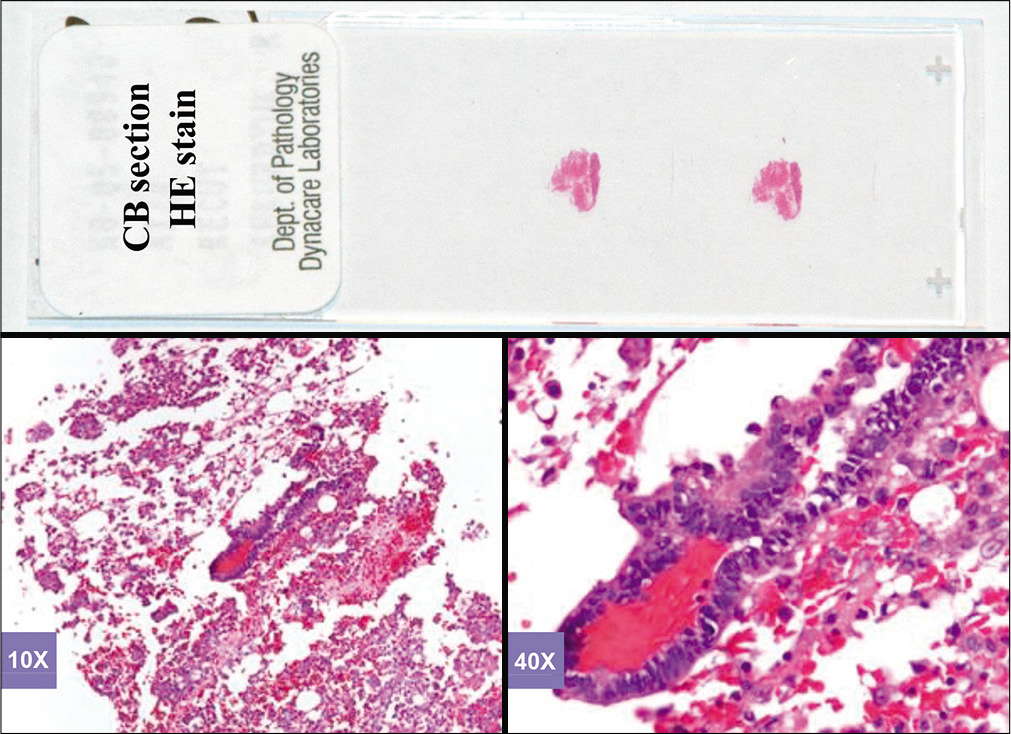
- Hematoxylin and eosinstained sections of cell-block of an effusion (metastatic colonic adenocarcinoma, pleural fluid).
![Metastatic adenocarcinoma (pleural fluid). An example with morphologically identifiable unequivocal ‘second foreign population’ (red arrow NC) other than mesothelial cells (blue arrow RM) and inflammatory cells (brown arrow IC) in DQ- and PAP-stained smears. This ‘second population’ of cells (red arrow NC) is easier to identify with the DQ stain (a) than with the PAP stain (b). IC, inflammatory cells; RM, reactive mesothelial cells; NC, neoplastic cells. [a, Diff-Quik-stained Cytospin smear; b, PAP-stained ThinPrep smear (a,b, 100X zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-32-g008.png)
- Metastatic adenocarcinoma (pleural fluid). An example with morphologically identifiable unequivocal ‘second foreign population’ (red arrow NC) other than mesothelial cells (blue arrow RM) and inflammatory cells (brown arrow IC) in DQ- and PAP-stained smears. This ‘second population’ of cells (red arrow NC) is easier to identify with the DQ stain (a) than with the PAP stain (b). IC, inflammatory cells; RM, reactive mesothelial cells; NC, neoplastic cells. [a, Diff-Quik-stained Cytospin smear; b, PAP-stained ThinPrep smear (a,b, 100X zoomed).]
Based on this approach, effusion fluids may be processed by preparing a DQ-stained direct smear of unconcentrated effusion specimen [Figure 3], DQ-stained Megafunnel Cytospin smear from the concentrated cell pellet [Figure 4], PAP-stained SurePath preparation [Figure 5], PAP-stained ThinPrep preparation [Figure 6], and PAP stained direct smears (Wet-fixed or Air-dried rehydrated postfixed).[1] In addition, the remaining effusion fluid may be processed for cell-block preparation for hematoxylin and eosin (HE)-stained sections [Figure 7], and for elective immunocytochemistry.[2]
It is recommended to process fluids contaminated with significant fresh blood after removing the erythrocytes. Such an approach allows optimum display of cells crucial for cytologic interpretation of corresponding effusion fluids by preventing interference from the contaminating erythrocytes, especially in DQ-stained Cytospin preparations. However, in our experience with Sure Path preparations, erythrocytes are lysed during the processing and are not a significant problem, unless the specimen is predominantly blood In ThinPrep, however, blood in the specimen may interfere with the final cellularity in the cytology preparation by occluding the pores of the ThinPrep filter. Another simple approach is removal of blood with ammonium chloride based lyzing reagent such as BloodLyz[3,4] used for flow cytometry. The erythrocyte depleted sediments can be used for preparing cytology preparations and for cell-block.
Both liquid based cytology (LBC) methodologies: SurePath [Figure 5] and ThinPrep [Figure 6] affect the thickness of individuals cells due to partial fixation in the LCB preservative/ fixative. This may compromise the cytomorphological evaluation of cellular/nuclear details. This introduces challenges related to optimal imaging of cellular details. However, both methods usually achieve concentration of cellular components in paucicellular specimens in a defined area on the slide.
Proper clinical details are a critical component for optimum interpretation of effusion fluid cytology. Availability of clinical information is important, even during the stage of specimen processing, so that the appropriate protocol is implemented. With a clinical history suggestive of cancer, preparation of cell-block for elective immunocytochemical evaluation is recommended as a routine protocol.
The initial evaluation of both DQ- and PAP-stained smears, even at low magnification, provides a general impression regarding the morphologic features of the cells. This further narrows the differential diagnosis. Examination at a higher magnification may assist in the final interpretation with or without tests such as histochemical stains, immunostains, flow cytometry, or other ancillary studies. With increased experience in cytomorphology, ancillary studies may not be required in straightforward cases without sacrificing accuracy of the final interpretation [Figure 1].
INTERPRETATION APPROACH [FIGURES 1, 2][5-26]
• In general, the standard diagnostic criteria of malignancy may not be applicable to single cells in serous fluids except in specimens with high-grade neoplasms [Figures 12, 13]. Final interpretation as to the cells in effusion fluids being a metastatic neoplasm has to be based on collective information deduced from various features mentioned below:[27]
The cytomorphology of reactive mesothelial cells overlaps significantly with neoplastic cells[28-30] [Tables 1]. Different clinical conditions [Table 2] may induce remarkably florid reactive changes in mesothelial cells, which may lead to the pitfall of misinterpreting them as malignant cells.[30]
A single population of cells with a wide spectrum of morphologic continuum between all cells may be considered as evidence of reactive mesothelial cells or mesothelioma cells. However, in rare cases, metastatic cancer cells may outnumber the mesothelial cells, resulting in a single population of neoplastic cells [Figure 9].
• Demonstration of a ‘second foreign population’ other than mesothelial and inflammatory cells in effusion fluids is generally consistent with metastatic neoplasm [Figure 8].
For neoplasms such as some sarcomas, this ‘second foreign population’ may be obvious and is usually easy to interpret. But sarcomas are rare in serous effusions; a previous history of sarcoma is almost always known.[28,29]
• The Romanowsky group of stains (e.g. DQ) highlight the ‘second population’ of cells with significant ease as compared to the PAP stain [Figure 8].
• Any ancillary test such as immunocytochemistry, with a properly organized immunopanel to distinguish and identify the ‘second population’ of cells, facilitates objective interpretation.
• Identical orientation of serial sections of cell-blocks on all slides facilitates precise evaluation of a ‘subtractive coordinate immunoreactivity pattern’ (SCIP)[35].
Most mesothelioma cells do not show marked atypia except for their increased size, which is easy to overlook. The important morphologic clues are quantitative (the presence of numerous isolated scattered cells of mesothelial lineage in a hypercellular smear) and qualitative (many large groups of three-dimensional cells of mesothelial lineage) [Figure 1[5]]. Both features are evaluated better in DQ-stained smears.
Apoptosis in unequivocally non-inflammatory cells, especially if associated with mitotic figures, correlates with malignancy. However, unequivocal interpretation of such apoptotic cells as non-inflammatory cells with apoptosis may not be possible in some specimens, especially if the cells are solitary, small in size, and scant in numbers.
![Pulmonary adenocarcinoma (pleural fluid). The non-cohesive metastatic cancer cell population is the predominant cell population without being seen as a ‘second population’ (a–d). Some apoptotic tumor cells (arrows in c and d) are present. If indicated, immunocytochemistry would facilitate confirmation of these cells as non-mesothelial. The predominant cell population shows immunoreactivity for thyroid transcription factor-1 (TTF-1), consistent with lung primary (e,f). Most of the cells without nuclear immunoreactivity for calretinin are carcinoma cells. A rare mesothelial cell (arrows in g,h) with nuclear (and cytoplasmic) immunoreactivity is seen as an internal positive control. [a–d: PAP-stained Cytospin smear, e–h: immunostained on cell-block sections (a, 10X; b, 40X; c,d, 100X; e,g, 40X; f,h, 100X).]](/content/105/2021/18/1/img/Cytojournal-18-32-g009.png)
- Pulmonary adenocarcinoma (pleural fluid). The non-cohesive metastatic cancer cell population is the predominant cell population without being seen as a ‘second population’ (a–d). Some apoptotic tumor cells (arrows in c and d) are present. If indicated, immunocytochemistry would facilitate confirmation of these cells as non-mesothelial. The predominant cell population shows immunoreactivity for thyroid transcription factor-1 (TTF-1), consistent with lung primary (e,f). Most of the cells without nuclear immunoreactivity for calretinin are carcinoma cells. A rare mesothelial cell (arrows in g,h) with nuclear (and cytoplasmic) immunoreactivity is seen as an internal positive control. [a–d: PAP-stained Cytospin smear, e–h: immunostained on cell-block sections (a, 10X; b, 40X; c,d, 100X; e,g, 40X; f,h, 100X).]
![Proliferation spheres (metastatic mammary carcinoma, pleural fluid). Cellular and nuclear details are better seen in a PAP-stained SurePath preparation (blue arrow in c), especially at the periphery under higher magnification (blue arrow in c), as compared to DQ-stained Cytospin smear (d, e, f). [a–c, PAP-stained Cytospin smear, d–f, DQ-stained cytospin smear (a, 20X; b, 100X; c, 100X zoomed; d, 20X; e, 100X; f, 100X zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-32-g010.png)
- Proliferation spheres (metastatic mammary carcinoma, pleural fluid). Cellular and nuclear details are better seen in a PAP-stained SurePath preparation (blue arrow in c), especially at the periphery under higher magnification (blue arrow in c), as compared to DQ-stained Cytospin smear (d, e, f). [a–c, PAP-stained Cytospin smear, d–f, DQ-stained cytospin smear (a, 20X; b, 100X; c, 100X zoomed; d, 20X; e, 100X; f, 100X zoomed).]
![Proliferation spheres (metastatic mammary carcinoma, pleural fluid). They may be round (a,d,e,f) or oblong (b,c). Conglomeration of more than one sphere (a) and oblong shape (b,c) of others may lead to irregular configurations, resembling papillary-like structures, especially in cytology preparations. They may be solid (a–f) or hollow (g–i). Some proliferation balls associated with metastatic adenocarcinoma may show formations of gland-like spaces (arrows) (j–l). [a–l, HE-stained cell block sections (a -c 40X; d -l, 100X).]](/content/105/2021/18/1/img/Cytojournal-18-32-g011.png)
- Proliferation spheres (metastatic mammary carcinoma, pleural fluid). They may be round (a,d,e,f) or oblong (b,c). Conglomeration of more than one sphere (a) and oblong shape (b,c) of others may lead to irregular configurations, resembling papillary-like structures, especially in cytology preparations. They may be solid (a–f) or hollow (g–i). Some proliferation balls associated with metastatic adenocarcinoma may show formations of gland-like spaces (arrows) (j–l). [a–l, HE-stained cell block sections (a -c 40X; d -l, 100X).]
![Metastatic poorly differentiated non-small-cell carcinoma of lung (pleural fluid). The specimen predominantly shows non-cohesive solitary cells (a–d). The neoplastic cells (NC inset d) are pleomorphic, with unequivocal features of malignancy. Mitotic figures (MF in c) are present in concert with apoptotic cells, which show apoptotic bodies (AP in d). Solitary cells (NC in d) may resemble high-grade lymphoma cells. DQ-stained preparation and immunocytochemistry on cell-block sections may help in cases with unknown primary. This patient had poorly differentiated adenocarcinoma of lung. AP, apoptotic cells; MF, mitotic figure; NC, neoplastic cells. [a–d, PAP-stained SurePath preparation (a, 10X; b, 40X; c,d, 100X; d inset, 100X zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-32-g012.png)
- Metastatic poorly differentiated non-small-cell carcinoma of lung (pleural fluid). The specimen predominantly shows non-cohesive solitary cells (a–d). The neoplastic cells (NC inset d) are pleomorphic, with unequivocal features of malignancy. Mitotic figures (MF in c) are present in concert with apoptotic cells, which show apoptotic bodies (AP in d). Solitary cells (NC in d) may resemble high-grade lymphoma cells. DQ-stained preparation and immunocytochemistry on cell-block sections may help in cases with unknown primary. This patient had poorly differentiated adenocarcinoma of lung. AP, apoptotic cells; MF, mitotic figure; NC, neoplastic cells. [a–d, PAP-stained SurePath preparation (a, 10X; b, 40X; c,d, 100X; d inset, 100X zoomed).]
![Metastatic poorly differentiated adenocarcinoma (peritoneal fluid). Specimen predominantly shows neoplastic cells (NC in insets c,d) with unequivocal features of malignancy. Mitotic figures (MF in c) are present in concert with apoptotic cells with apoptotic bodies (AP in d). Some cells show unequivocal cohesive pattern (arrowheads), consistent with carcinoma. Such specimens do not need ancillary help of DQ stain or cell-block sections for immunocytochemistry, unless a search for unknown primary is indicated. AP, apoptotic cells; MF, mitotic figure; NC, neoplastic cells. [a–d, PAP-stained SurePath preparation (a, 10X; b, 40X; c,d, 100X; insets of c,d, 100X zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-32-g013.png)
- Metastatic poorly differentiated adenocarcinoma (peritoneal fluid). Specimen predominantly shows neoplastic cells (NC in insets c,d) with unequivocal features of malignancy. Mitotic figures (MF in c) are present in concert with apoptotic cells with apoptotic bodies (AP in d). Some cells show unequivocal cohesive pattern (arrowheads), consistent with carcinoma. Such specimens do not need ancillary help of DQ stain or cell-block sections for immunocytochemistry, unless a search for unknown primary is indicated. AP, apoptotic cells; MF, mitotic figure; NC, neoplastic cells. [a–d, PAP-stained SurePath preparation (a, 10X; b, 40X; c,d, 100X; insets of c,d, 100X zoomed).]
| Individual cell morphology | Cell arrangements | Intercellular relationships |
|---|---|---|
| Enlargement | Solitary cells | Clasping (pinching) |
| Increased N/C ratio | Doublets | Windows |
| Hyperchromasia | Triplets | Side-by-side |
| Chromatin clearing | Flat groups | Molding |
| Multinucleation | Rosettes | Syncytia |
| Mitosis | Cells in a row (Indian-file) | |
| Signet rings | Tight cell balls | |
| Elongation | Cell-in-cell | |
| Blebbing | Pseudo-acini | |
| Granulation |
| Effusion fluid | Clinical association |
|---|---|
| Peritoneal fluid | Cirrhosis Renal failure with uremia Pancreatitis Bile peritonitis Huge intra-abdominal benign masses: e g ovarian fibroma (with florid reactive mesothelial hyperplasia of the overlying serosal surface)[31] |
| Pleural fluid | Pulmonary infarction[32] Infection/inflammation Pancreatitis[33] |
| Pericardial fluid | Pericarditis–viral Congestive heart failure |
| All fluids | Collagen vascular diseases Chemotherapy[34] Radiation therapy[34] |
CYTOLOGIC PARAMETERS[5-23]
Various cytomorphologic features applicable during interpretation of effusion cytology are summarized below:
Cell groups and intercellular cohesion
Cohesiveness of cells is a helpful feature in the differential diagnosis of effusion cytology [Figure 1[7,8]]. However, the pattern of cohesiveness of neoplastic cells may vary in preparations processed by different methods. • Non-cohesive, individual, solitary neoplastic cells, scattered throughout the smears or present as loose ill-defined clusters, are typical of malignant lymphomas and melanomas.
Most carcinoma cells demonstrate good intercellular cohesion, with the presence of cohesive clusters of neoplastic cells in the effusions. However, to avoid pitfalls, exceptions have to be kept in mind. Some carcinomas, which include linitis-plastica-type anaplastic gastric adenocarcinoma, non-cohesive cell type adenocarcinoma of the lung [Figure 12], pleomorphic giant cell carcinoma of the pancreas, keratinizing squamous cell carcinoma, giant cell carcinoma of the lung, and epithelioid mesotheliomas with a non-cohesive cell pattern, may show a predominance of scattered solitary cells in effusions [Figure 1[7,8]].
Neoplasms such as poorly differentiated small cell carcinoma of the lung show high proliferation activity with proliferation spheres in effusions with higher cohesiveness than that observed in smears of fine-needle aspirates. Prominence of this feature may depend on the time in relation to the course of the disease. • In newly developed malignant effusions, the cells of poorly differentiated small cell carcinoma of the lung are usually seen as solitary cells, but recurrent and longer duration effusions show cohesive clusters or proliferation spheres.
Proliferation spheres are also observed in other metastatic carcinomas with cohesive cells, especially in metastatic mammary carcinoma (ducal carcinoma) [Figure 10]. The cell morphology in such groups is difficult to study; however, the details are best observed in the cells along the periphery of such groups by adjusting the fine focus [see Figure 10], or in solitary neoplastic cells that are present in the background at least in a small number. In comparison to the cell groups in mesothelioma, the proliferation spheres (a term initially used by Dr Nathan Chandler Foot),[10] lack stromal cores and may be solid or hollow, round or irregular [Figure 11]. The neoplastic cells of adenocarcinoma in the proliferation spheres may show ill-defined gland formations [Figure 11].
Arrangement of neoplastic cells
Apart from proliferation spheres, groups of neoplastic cells may be seen in papillary configurations. In effusions, such papillary-like formations may be the result of specimen processing by a conglomeration of cell clusters or proliferation spheres. Consequently, cell groups with papillary arrangements in effusions may not represent true papillary tumors. Neoplastic cells in effusion are usually seen in three-dimensional groupings, while monolayers are rare. Some neoplasms, such as bronchioloalveolar cell carcinoma, appearing as monolayers in fine-needle aspirates, do not demonstrate similar features in the effusions. Benign papillary inclusions and müllerian inclusions, especially in peritoneal washings, should not be misinterpreted as malignant.[36-38]
Cytoplasm of neoplastic cells
Cytoplasmic features are extremely useful in identifying different types of neoplastic cells. DQ stain highlights cytoplasmic details in a much better manner than the PAP stain and is strongly recommended to be used routinely [Figure 8]. The cytoplasm of cells from mucin-producing tumors appears vacuolated and may show mucicarmine and PAS (periodic acid–Schiff) stain positivity. The non-cohesive solitary cells of mesotheliomas of epithelioid type may have an abundance of dense cytoplasm, which may be granular or vacuolated and overlap morphologically with different types of carcinoma such as renal cell carcinoma.
Special structures and cytologic features
Unique cytologic structures and formations, as characteristic morphologic features of certain primary neoplasms, may help identification of the primary site. Examples include a palisading arrangement of elongated nuclei in adenocarcinoma of the colon with apoptotic bodies and keratinization in squamous cell carcinoma. Psammoma bodies may be associated with papillary carcinoma of the thyroid, serous adenocarcinoma of the ovary, bronchioloalveolar cell carcinoma of the nonsecretory cell type, and the papillary-type mesothelioma of epithelial type. • However, caution is warranted to not overdiagnose free-floating psammoma bodies or those surrounded by mesothelial cells, when examining ascitic fluids, cul-de-sac aspirates, and pelvic washings in women. Psammoma bodies under these situations are non-specific, without diagnostic significance, and are not uncommon in patients with pelvic inflammatory disease.[39,40]
Other features
There are many ‘look-alikes’ in effusion cytology owing to the wide morphologic spectrum of reactive mesothelial cells.[33,41,42] As mentioned previously, the morphologic features of various neoplasms may be different in effusions than those in other types of specimens. However, some neoplasms commonly metastatic to serous cavities have a typical cytologic appearance that is recognizable with experience.[31,34]
Depending on the method of cell-block making, the cell-block sections of malignant effusion fluids show ‘lacunae’ surrounding individual cells or groups of cells in 75% of cases. Lacunae are very nonspecific and are observed in 32% of reactive effusions;[32] however, under low power they can be used to locate atypical cells (especially non-immunoreactive cells in immunostained sections) for further detailed scrutiny at higher magnification.
A combination of various morphologic features discussed above, with or without the help of ancillary tests, should be applied for the final interpretation of effusion specimens.
IMMUNOCYTOCHEMICAL APPROACH
If a ‘second foreign population’ is suggested in a DQ-stained preparation but cannot be confirmed with PAP stain, it may be analyzed further by immunostaining of cell-block sections [Figure 2[2a]]. This may be needed in effusions secondary to the well-differentiated adenocarcinomas, especially of the ovary and breast. In addition to the confirmation of the second population by evaluation with SCIP approach in cell-block sections[43], immunocytochemistry also helps in determining the primary site. Examples include prostate-specific antigen in prostatic adenocarcinoma, CDX2 in colonic adenocarcinoma, calcitonin in medullary carcinoma of the thyroid, GATA 3 in breast carcinoma, and thyroglobulin in thyroid carcinoma etc.
REPORTING SYSTEM
A five-tier cytopathology terminology system practiced in other organ system is also recommended for reporting serous fluid cytopathology findings as mentioned below:[43,44]
Non-diagnostic (ND)
Negative for malignancy (NM)
Atypia of undetermined significance (AUS)
Suspicious for malignancy (SFM)
Positive for malignancy (PM) – primary and metastatic
As discussed before, some cases may require ancillary testing. This is especially applicable to a significant number of specimens in atypia and suspicious ‘holding’ preliminary categories for objective confirmation or ruling out ‘second’ foreign population of metastatic cancer and narrow the grey-zone with minimum reporting in the atypical-suspicious categories. In the definitive malignant category, ancillary testing may be required for differential diagnosis of primary site including unknown primary and for evaluating prognostic markers such as Her2/Neu, Estrogen receptor, etc.
Most of the causes which lead to grey-zone reporting may be related to quantitatively and qualitatively sub-optimal specimens. Lower specimen volume and scant cellularity may lead to sub-optimal cell-blocks without the ability to perform effective ancillary testing such immunohistochemistry. In addition, in some cases the quality of the specimen may be sub-optimal due to in vivo degenerative changes associated with long-standing effusion, improper collection, transportation, or storage of the specimen leading to poor preservation related compromization.
Diagnostic criteria for atypical and suspicious categories are not methodically studied and show significant interinstitutional and interobserver variation. Because positive for metastatic disease implies late disease with decision not to plan for curative management but initiate palliative management, it is recommended to be conservative with efforts to avoid false positives. In addition, collection of most of the effusion fluids is relatively less complex and so repeating a properly collected specimen in optimum quantity may be performed for definitive interpretation as clinically indicated.
Most of the limitations related to this problem may be minimized by including an appropriate comment with such equivocal reports. The comment should explain the probable reason for the gray-zone interpretation along with a recommendation how to resolve it.
The freshly collected, unfixed effusion specimen should be transported to the laboratory without delay. If delay is expected, it should be submitted on ice without freezing. The fluid specimen may be refrigerated at 4°C until processing up to 24 hours period. The specimen is usually stable up to 7 days at 4°C for some testing including cell-block if required.[45,46]
Entire collected effusion fluid volume, up to 1000 ml, should be sent for cytopathological evaluation. Although, the minimum quantity is variable and depends on the cellularity of the specimen, it should be at least 50 ml so that cell-blocks can be prepared. However, 20–30 mL of cellular serous fluids may be processed but with relatively sub-optimal cytology preparation processing with potential to compromise the opportunity to make cell-blocks.
References
- Routine air drying of all the smears prepared during fine needle aspiration and intraoperative cytology studies: An opportunity to practice a unified protocol, offering the flexibility of choosing variety of staining methods. Acta Cytol. 2001;45:60-8.
- [CrossRef] [PubMed] [Google Scholar]
- Individual specimen triage of effusion samples: An improvement in the standard of practice, or a waste of resources? Diagn Cytopathol. 2000;22:7-10.
- [CrossRef] [Google Scholar]
- CellBlockistry: Chemistry and art of cell-block making-a detailed review of various historical options with recent advances. Cytojournal. 2019;16:12.
- [CrossRef] [PubMed] [Google Scholar]
- BloodLyz (AV BioInnovation) Available from: https://www.avbioinnovation.com/product/bloodlyz [Last accessed on 2021 May 11]
- [Google Scholar]
- Recognition of malignant cells in pleural and peritoneal effusions. Acta Cytol. 1974;18:118-121.
- [Google Scholar]
- Diagnostic Cytology and Its Histopathologic Bases (4th edn). Philadelphia: JB Lippincott; 1992.
- [Google Scholar]
- Pleural, peritoneal and pericardial fluids In: Bibbo M, ed. Comprehensive Cytopathology. Philadelphia: WB Saunders; 1991. p. :541-614.
- [Google Scholar]
- Benign mesothelial proliferation with collagen formation in pericardial fluid. Acta Cytol. 1979;23:428-430.
- [Google Scholar]
- The value of cells in the pleural fluid in the differential diagnosis. Mayo Clin Proc. 1975;50:571-572.
- [Google Scholar]
- Identification of types and primary sites of metastatic tumors from exfoliated cells in serous fluids. Am J Pathol. 1954;30:661-667.
- [Google Scholar]
- The comparative diagnostic accuracy, efficiency and specificity of cytologic technics used in the diagnosis of malignant neoplasm in serous effusions of the pleural and pericardial cavities. Acta Cytol. 1964;8:150-163.
- [Google Scholar]
- Benign papillary structures with psammoma bodies in culdocentesis fluid. Acta Cytol. 1969;13:178-180.
- [Google Scholar]
- Cells in pleural fluid: their value in differential diagnosis. Arch Intern Med. 1973;132:854-860.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of primary site by examination of cancer cells in body fluids. Am J Clin Pathol. 1972;58:479-488.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal cytology as an indicator of disease in patients with residual ovarian carcinoma. Obstet Gynecol. 1988;71:850-853.
- [Google Scholar]
- Identification of types and primary sites of malignant tumors by examination of exfoliated tumor cells in serous fluids. Acta Cytol. 1985;29:753-767.
- [Google Scholar]
- Cytopathology of Malignant Effusions In: American Society of Clinical Pathologists. Chicago: ASCP Press; 1996.
- [Google Scholar]
- Effusions In: Modern Cytopathology. Philadelphia: Churchill Livingstone; 2004. p. :257-309.
- [Google Scholar]
- Pleural, pericardial, and peritoneal fluids In: Cibas ES, Ducatman BS, eds. Cytology—Diagnostic Principles and Clinical Correlates (2nd edn). Philadelphia: WB Saunders; 2003. p. :119-144.
- [Google Scholar]
- Pleural and pericardial fluids In: Body Fluids (3rd edn). Chicago: American Society of Clinical Pathologists; 1993. p. :159-222.
- [Google Scholar]
- Peritoneal fluid In: Body Fluids (3rd edn). Chicago: American Society of Clinical Pathologists; 1993. p. :223-253.
- [Google Scholar]
- Fluids In: The Art and Science of Cytopathology— Exfoliative Cytology (1st edn). Chicago: ASCP Press; 1996. p. :257-325.
- [Google Scholar]
- Body cavity fluids In: Ramzy I, ed. Clinical Cytopathology and Aspiration Biopsy (2nd edn). New York: McGraw-Hill; 2001. p. :205-223.
- [Google Scholar]
- Serous effusions In: Kini SR, ed. Color Atlas of Differential Diagnosis in Exfoliative and Aspiration Cytopathology. Baltimore: Williams & Wilkins; 1999. p. :119-142.
- [Google Scholar]
- Cytologic identification of serous neoplasms in peritoneal fluids. Cancer. 2001;93:309-318.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal washing cytology findings of disseminated myxoid leiomyosarcoma of uterus: report of a case with emphasis on possible differential diagnosis. Diagn Cytopathol. 2002;27:47-52.
- [CrossRef] [PubMed] [Google Scholar]
- Effusion cytology of metastatic extraskeletal myxoid chondrosarcoma. Diagn Cytopathol. 2003;28:222-223.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic effects of photodynamic therapy in body fluids. Diagn Cytopathol. 1996;14:356-361.
- [CrossRef] [Google Scholar]
- Identification of types and primary sites of malignant tumors by examination of exfoliated tumor cells in serous fluids. Acta Cytol. 1985;29:753-774.
- [Google Scholar]
- Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991;4:320-324.
- [Google Scholar]
- Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20:350-357.
- [CrossRef] [Google Scholar]
- Determination of primary site by examination of cancer cells in body fluids. Am J Clin Pathol. 1972;58:479-488.
- [CrossRef] [PubMed] [Google Scholar]
- Serous effusions: Reactive, benign and malignant In: Gray W, Kocjan G, eds. Diagnostic Cytopathology Vol Ch. 3. (3rd ed). Amsterdam, Netherlands: Elsevier; 2010.
- [CrossRef] [Google Scholar]
- Atypical papillary proliferation in gynecologic patients: a study of 32 pelvic washes. Diagn Cytopathol. 2005;32:76-81.
- [CrossRef] [PubMed] [Google Scholar]
- Mullerian inclusions in peritoneal washings. Potential source of error in cytologic diagnosis. Acta Cytol. 1986;30:271-276.
- [Google Scholar]
- Peritoneal washings in ovarian tumors. Potential sources of error in cytologic diagnosis. Acta Cytol. 1985;29:310-316.
- [Google Scholar]
- Significance of psammoma bodies in serous cavity fluid: a cytopathologic analysis. Cancer. 2004;102:87-91.
- [CrossRef] [PubMed] [Google Scholar]
- Cytopathology of serous neoplasia of the ovary and the peritoneum: differential diagnosis from mesothelial proliferations. Diagn Cytopathol. 1996;15:292-295.
- [CrossRef] [Google Scholar]
- Malignant-appearing cells in pleural effusion due to pancreatitis: case report and literature review. Acta Cytol. 1981;25:412-416.
- [Google Scholar]
- Cytologic detection of malignancy in pleural effusion: a review of 5,255 samples from 3,811 patients. Diagn Cytopathol. 1987;3:8-12.
- [CrossRef] [PubMed] [Google Scholar]
- Announcement: The international system for reporting serous fluid cytopathology. Acta Cytol. 2019;63:349-51.
- [CrossRef] [PubMed] [Google Scholar]
- Indian academy of cytologists guidelines for collection, preparation, interpretation, and reporting of serous effusion fluid samples. J Cytol. 2020;37:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Cytopreparatory technique In: Bibbo M, ed. Comprehensive Cytopathology (3rd ed). Philadelphia, PA: WB Saunders Co; 2008. p. :977-1012.
- [Google Scholar]
- Serous cavity fluids: Momentum, molecules, markers… and more! Cancer Cytopathol. . 2020;128:381-3.
- [CrossRef] [Google Scholar]








