Translate this page into:
Cytomorphological spectrum of metastatic bone tumors: Experience at a tertiary care center

-
Received: ,
Accepted: ,
How to cite this article: Gupta S, Banerjee N, Gupta P, Rohilla M, Gupta N, Srinivasan R, Rajwanshi A, Dey P. Cytomorphological spectrum of metastatic bone tumors: Experience at a tertiary care center. CytoJournal 2022;19:1.
Abstract
Objectives:
Bone is a frequent site of metastases and typically indicates a short-term prognosis in cancer patients. The majority of skeletal metastases are due to breast and prostate cancer. Bone metastasis is actually much more common than primary bone cancers, especially in adults. Fine-needle aspiration cytology (FNAC) provides reasonably accurate pre-operative diagnosis in vast majority of cases. This study aims to elicit the cytomorphological detail of various metastatic bone tumors.
Material and Methods:
A total of 109 cases of tumors metastatic to bone have been included in this study. The details of the cases were available from the archives of the department of cytology. May Grunwald Giemsa and hematoxylin and eosin stained smears were studied and examined for the cytomorphological spectrum. Cell block and immunohistochemistry tests were done, wherever feasible.
Results:
Among 109 patients, the mean age was 54.52 years. There was male preponderance with 90 males and 19 females. The most common site of metastases was in the vertebra (82 cases), and 76 cases were in the dorsolumbar region. The most common type of tumor metastasizing was adenocarcinoma.
Conclusion:
FNAC is a very useful, economical procedure. There are characteristic cytological features of the metastatic lesions and the basic diagnostic categorization of the malignant tumors is possible on FNAC. Regarding the primary source clinical history, radiological features of the primary tumor, if any, and immunocytochemistry may be needed.
Keywords
Fine-needle aspiration cytology
Metastases
Bone
Cytology
INTRODUCTION
Metastases are considered to be the most common type of malignant tumor involving bone; the skeleton is the third most frequent site for metastatic carcinoma after the lung and liver.[1] Fine-needle aspiration cytology (FNAC) of bone tumor was first attempted by Coley, Sharp, and Ellis in 1931.[2] Fine-needle aspiration (FNA) is accurate, rapid management and conclusive in diagnosis of metastatic bone tumors, when aided with immunocytochemistry. Diagnosis by FNA can aid in identification of the primary and to provide early treatment. There are relatively less publications in this regard, therefore in our study, we aim to elicit the cytomorphological detail of 109 cases of tumors metastatic to bone and help in identification of primary.
MATERIAL AND METHODS
Ethical justification
This retrospective study was done on routine procedure with proper permission of the patient before the FNAC technique. No extra investigation was done on the patient’s material. Moreover, the identity of the patient was not disclosed. This study was approved by the departmental ethical committee and the study meets the conditions of the Helsinki Accord.
Records of all the patients who had got FNAC done during the past 3 years were retrieved from the archives. Demographics and relevant clinical details of the patients were documented along with the cytomorphological details. Aspirates were obtained with a 20–22 gauge needle attached to a 20 mL disposable plastic syringe fitted to the handle. Multiple passes were made, while maintaining negative vacuum. Majority of the cases were aspirated under computed tomography (CT) or ultrasound guidance. Air-dried smears were stained with May Grunwald Giemsa (MGG) stain and the smears fixed in 95% ethyl alcohol were stained with H and E and Pap stain. Immunocytochemical analysis was done on cell blocks in 21 cases to further categorize these lesions.
RESULTS
A total of 109 cases of FNAC of metastatic lesions of the bone were archived from the cytopathology department between January 2016 and December 2019. The demographic, clinical, and cytological data characteristics were analyzed. Table 1 shows the age-wise distribution of FNAC of metastatic lesions of the bone. Maximum patients of metastases to bone were in elderly age group with 60 patients in age range of 41–60 years. Of the 109 cases, 90 were male and 19 were female. Table 2 shows the site-wise distribution of these lesions. The most common site of metastases was vertebra, more commonly in the dorsolumbar region followed by iliac bone. Table 3 highlights the cytological typing of these lesions. The most common malignancy to metastasize to bone was found to be carcinomas, among which adenocarcinomas were the most common. Of these 109 cases, 43 cases were classified as adenocarcinomas, including seven mucinous adenocarcinomas. Thirty-one cases were classified as carcinoma and NOS while four cases were classified as poorly differentiated carcinoma and five cases as malignant neoplasms. Seven cases each were metastases from squamous cell carcinoma and renal cell carcinoma. Four cases were metastases from thyroid, three cases of follicular carcinoma thyroid, and one case of papillary thyroid carcinoma. One case each was diagnosed as metastatic sarcoma, melanoma, diffuse large B-cell lymphoma, and germ cell tumor. Three cases of metastatic small cell carcinoma and one case of neuroendocrine carcinoma were also included. Immunocytochemistry was done in 21 cases using appropriate markers and subject to availability of material in the cell blocks and available of the antibodies. Table 4 summarizes the clinical data, cytological diagnosis, and immunocytochemical findings with probable site of origin of these metastatic tumors. The immunocytochemical panel was devised and interpreted in the light of clinical inputs and imaging findings, wherever available.
| Age range (years) | No. of cases |
|---|---|
| 0–20 | 3 |
| 21–40 | 13 |
| 41–60 | 60 |
| >60 | 33 |
FNAC: Fine-needle aspiration cytology
| Site of metastatic involvement in bone | No. of cases |
|---|---|
| Dorsolumbar vertebra | 76 |
| Cervical vertebra | 2 |
| Thoracic vertebra | 2 |
| Sacral vertebra | 2 |
| Iliac bone | 7 |
| Clavicle | 2 |
| Skull | 1 |
| Mandible | 2 |
| Humerus | 1 |
| Pubic bone | 1 |
FNAC: Fine-needle aspiration cytology
| Cytological diagnosis | No. of cases |
|---|---|
| Adenocarcinoma | 39 |
| Carcinoma, NOS/poorly differentiated carcinoma | 35 |
| Renal cell carcinoma | 7 |
| Squamous cell carcinoma | 7 |
| Mucinous adenocarcinoma | 3 |
| Malignant neoplasm | 5 |
| Small cell carcinoma | 3 |
| Follicular carcinoma thyroid | 3 |
| Papillary carcinoma thyroid | 1 |
| Neuroendocrine carcinoma | 1 |
| Diffuse large B-cell lymphoma | 1 |
| Germ cell tumor | 1 |
| Sarcoma | 1 |
| Malignant melanoma | 1 |
| S. No. | Age | Sex | Site | Cytodiagnosis | Immunocytochemical profile | Probable site of origin |
|---|---|---|---|---|---|---|
| 1 | 38 | F | D6 | Metastatic carcinoma | CK20+, CK7- | Hindgut |
| 2 | 45 | M | FEMUR | Metastatic clear cell renal cell carcinoma | PAX-8, CD10 + | Renal |
| 3 | 48 | F | Iliac bone | Metastatic adenocarcinoma | CK7, TTF1+, CK20- | Lung |
| 4 | 49 | M | D11-12 | Metastatic adenocarcinoma | CK7, WT1, PAX-8 | Ovary |
| 5 | 50 | F | D5-6 | Metastatic neuroendocrine carcinoma | CG/SYN+ | Neuroendocrine |
| 6 | 50 | M | C7 | Metastatic carcinoma | TTF-1+, p63- | Lung |
| 7 | 50 | M | FEMUR | Metastatic follicular carcinoma thyroid | THYROGLOBULIN+ | Thyroid |
| 8 | 51 | M | D12 | Metastatic adenocarcinoma | CK7, Napsin A + | Lung |
| 9 | 53 | M | L2 | Metastatic carcinoma | CG/SYN+ | Neuroendocrine |
| 10 | 53 | M | D12 | Metastatic adenocarcinoma | CK7+, CK20- | Foregut |
| 11 | 56 | M | D12 | Metastatic renal cell carcinoma | panCK, CD10, VIMENTIN+ | Renal |
| 12 | 57 | M | L5-S3 | DLBCL | CD45/CD20+ | Lymphoma |
| 13 | 59 | M | D12 | Metastatic adenocarcinoma | TTF-1+, p63- | Lung |
| 14 | 59 | M | L5 | Metastatic carcinoma | CK7+, CK20- | Foregut |
| 15 | 59 | M | L4 | Small cell carcinoma | NSE, SYN+ | Neuroendocrine |
| 16 | 60 | M | Iliac | Metastatic mucinous adenocarcinoma | CK7+, CK20-, CDX2-, TTF1-, PSA-, S100- | Foregut |
| 17 | 62 | M | D5-6 | Metastatic carcinoma | CK7, CK19+ | Pancreas |
| 18 | 63 | M | D3-4 | Metastatic adenocarcinoma | CK7, TTF-1+ | Lung |
| 19 | 68 | M | L2 | Metastatic adenocarcinoma | CK7+, CK20- | Foregut |
| 20 | 70 | M | D1-2 | Metastatic adenocarcinoma | CK7+, CK20- | Foregut |
| 21 | 78 | M | L3 | Metastatic adenocarcinoma | CK7+, CK20- | Foregut |
FNAC: Fine-needle aspiration cytology
In the present study, the most common site of metastasis was dorsolumbar vertebra followed by cervical vertebrae and iliac bone. Majority cases are metastatic adenocarcinoma which is evident by the frequent glandular pattern. FNAC of metastatic adenocarcinoma showed papillary clusters of tumor cells, which on immunocytochemistry showed CK7 and TTF-1 positivity and diagnosis of metastases from lung primary was favored [Figure 1]. FNAC of metastatic renal cell carcinoma had abundant clear cytoplasm with central large nuclei [Figure 2a]. Frequent follicular pattern was characteristic of the metastatic follicular carcinoma of the thyroid [Figure 2b and c]. Immunocytochemistry showed strong thyroglobulin positivity [Figure 2d]. Papillae with fibrovascular cores, mildly pleomorphic cells with dense cytoplasm, PAX-8, and CD10 immunopositivity favor primary from papillary renal cell carcinoma [Figure 3]. A 24-year-old male presented with lower back pain. His magnetic resonance imaging and CT scan of the lower back showed an ill-defined sclerotic and lytic lesion in S1 and S2 vertebral region. The radiological picture suggested the possibility of an infective lesion or a giant cell tumor of bone. The patient had a history of swelling in the right knee region which was operated 4 years back. The histopathology was done that confirmed the diagnosis of fibrosarcoma from our institution. FNAC of spine showed cellular smears comprising of loosely cohesive clusters of spindled to plump oval cells showing moderate nuclear pleomorphism and eosinophilic cytoplasm [Figure 4]. The case was diagnosed as metastatic sarcoma in view of many clusters and discrete spindle cells. However, the history of already diagnosed fibrosarcoma was available at the time of diagnosis and so we reported sarcoma favoring metastatic fibrosarcoma.
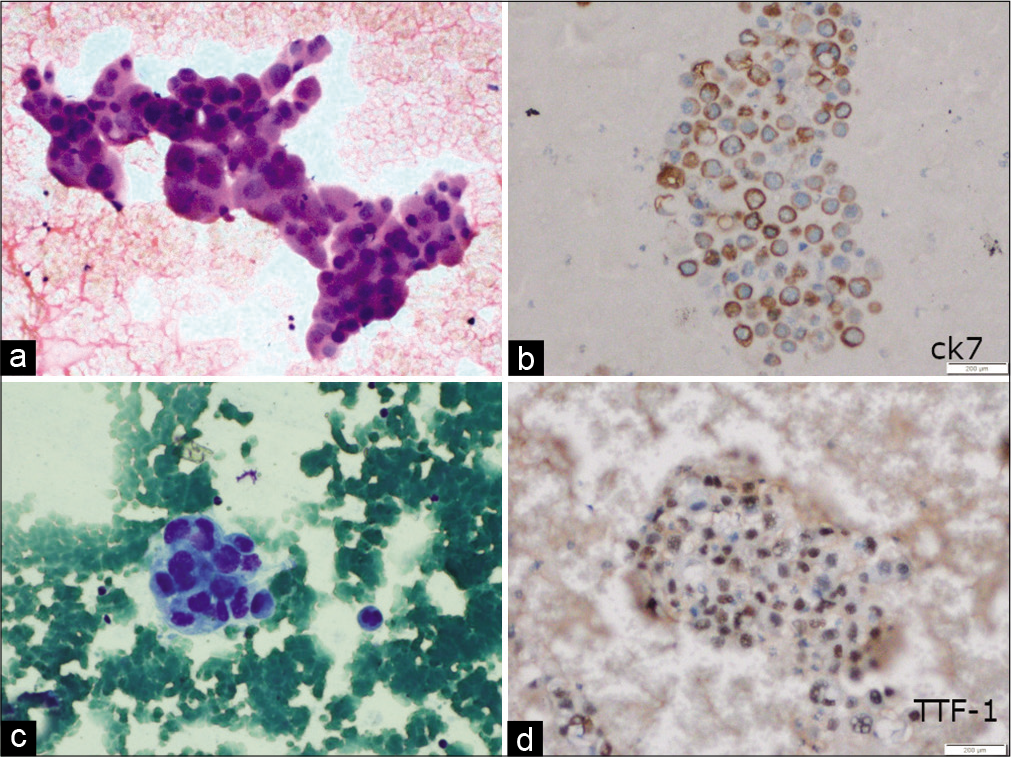
- (a) Fine-needle aspiration smears show a tumor cells arranged in tight clusters with moderate pleomorphism, coarse chromatin, and moderate cytoplasm (H and E, ×200). (c) Three-dimensional cluster with moderate pleomorphism, coarse chromatin, and moderate cytoplasm (May Grunwald Giemsa ×200); (b) tumor cells showing positivity for CK7 (immunoperoxidase ×200) and (d) for TTF-1 (immunoperoxidase ×200) suggestive of metastatic adenocarcinoma with lung primary.
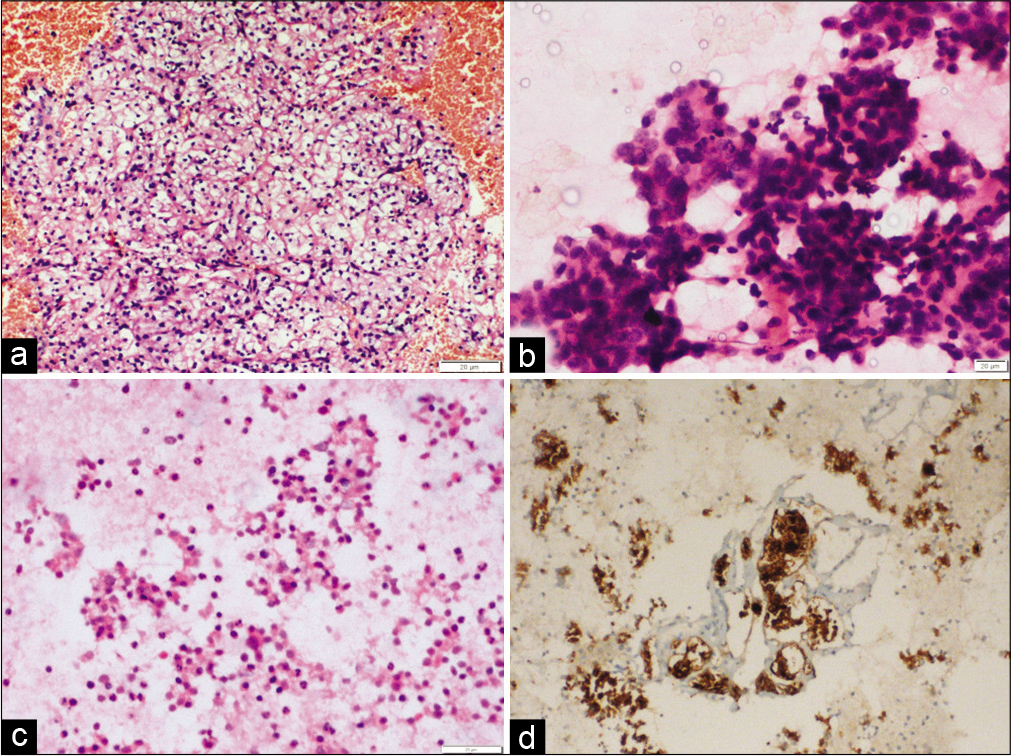
- (a) Fine-needle aspiration cytology smear showing large sheet of cells with abundant clear cytoplasm and central large nuclei, favoring metastases from clear cell renal cell carcinoma (H and E, ×200). (b and c) Fine-needle aspiration smear showing frequent follicular pattern of tumor cells. (d) Cell block section with immunocytochemistry showed strong thyroglobulin positivity (immunoperoxidase ×200), suggesting, and metastatic follicular carcinoma thyroid.
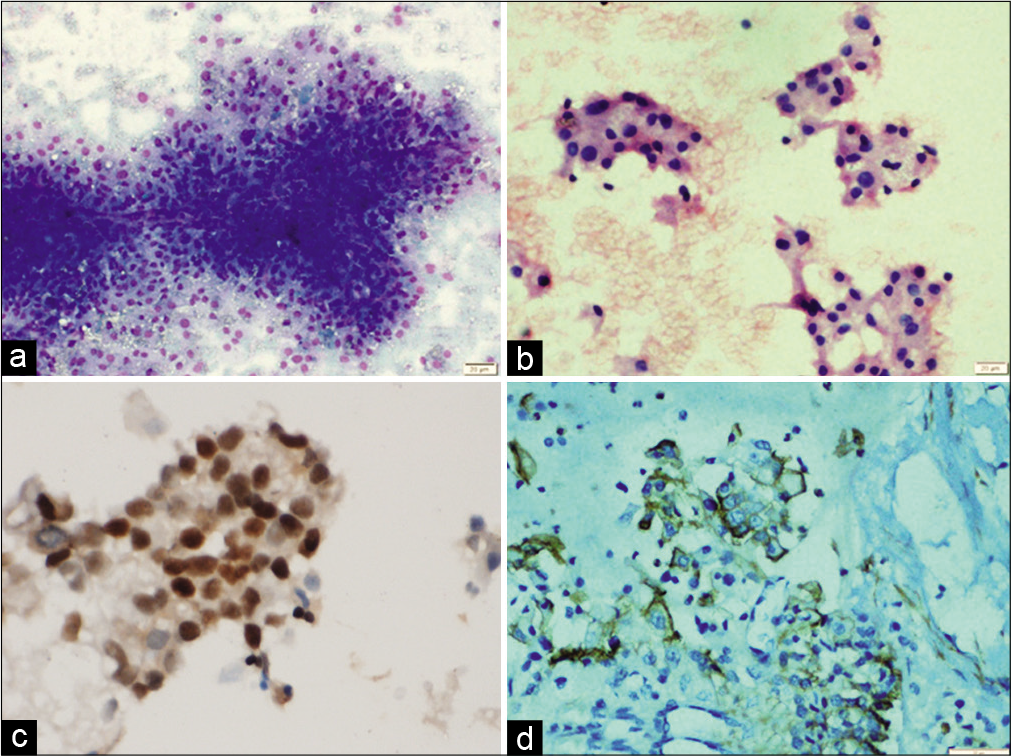
- (a) Fine-needle aspiration cytology smears showing papillae with fibrovascular cores (May Grunwald Giemsa ×200), (b) clusters of cells with mild nuclear pleomorphism and dense cytoplasm (May Grunwald Giemsa ×200), (c) tumor cells showing immunopositivity for PAX-8, (d) and for CD10 favoring primary from papillary renal cell carcinoma.
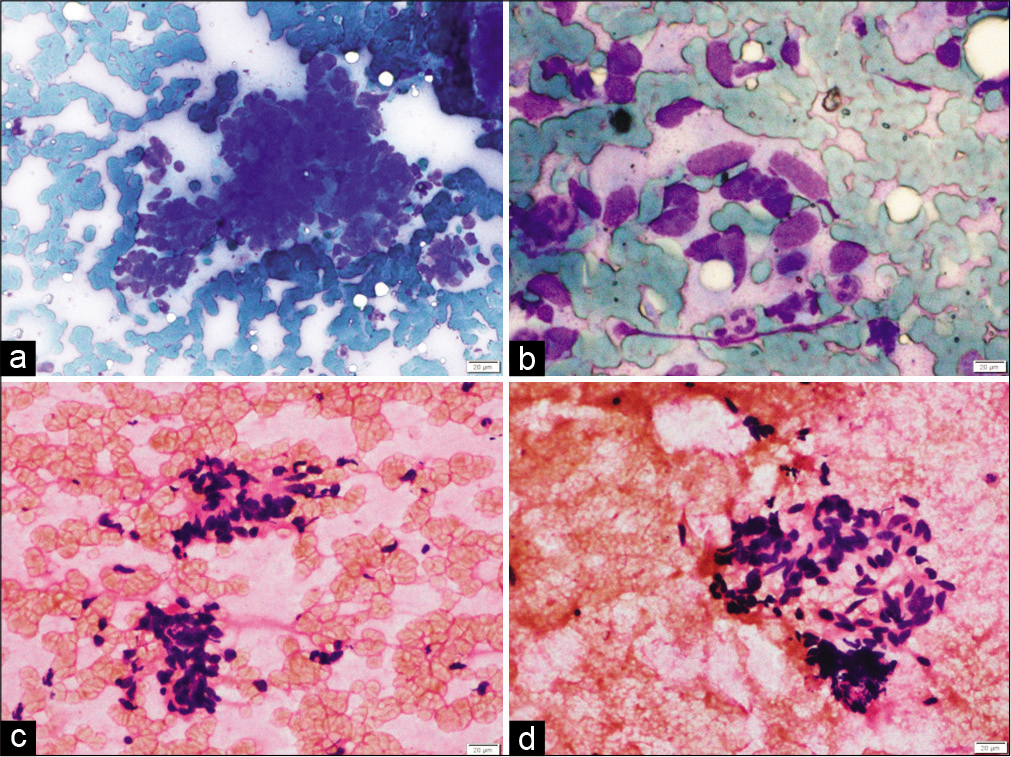
- (a) Fine-needle aspiration cytology smears from spine in a known case of fibrosarcoma show loosely cohesive clusters of spindled to plump oval cells (May Grunwald Giemsa ×200), (b) tumor cells showing moderate nuclear pleomorphism and indistinct cytoplasm (May Grunwald Giemsa ×400), (c and d) loosely cohesive cluster of spindled cells embedded in indistinct eosinophilic matrix, (H and E, ×200), favoring metastatic fibrosarcoma.
In few cases with limited material on cell block or limited availability of immunocytochemical markers and absence of imaging details, the exact site of origin could not be determined, however, we could still indicate the probable site of origin and facilitate further investigations to determine the unknown primary.
DISCUSSION
Bone, or bone marrow, evidently provides a fertile “soil” for disseminated tumor cells and is a major target organ for metastasis.[3] Skeletal lesions can be the first manifestation of malignancy in 25–30% of cases.[1] The true incidence of malignant tumors metastasizing to bone is not known. Approximately 50% of patients with malignant tumors develop metastases and half of them occur in the skeleton.[4] Practically, all malignant neoplasms can metastasize in bone, since the skeleton is a common metastatic site for several visceral carcinomas. Metastatic disease in these patients usually occurs late in the disease process, long after the primary disease has been identified. However, 3–4% of patients with metastatic carcinoma have an unknown primary site at the time of presentation.[5] Bone is the third most common site for metastasis of carcinomas than sarcomas. The axial skeleton due to the presence of red marrow has a predilection for metastasis than the appendicular skeleton. The secondary bone tumors with primary tumors in lungs, kidney, thyroid, breast, and gastrointestinal tract produce lytic lesion.[6] The areas commonly involved are the ribs, pelvis, and spine. This is partly due to Batson’s vertebral venous plexus, which bypasses the lung circulation. A cadaver study by Sundaresan et al. has demonstrated that 30–90% of patients with terminal cancer had spinal metastasis.[7]
Aspiration cytology has proven in recent years to be a rapid and reliable method for the rapid diagnosis of bony lesions.[8]
Most bony lesions are detected on plain radiographs and areas of cortical destruction can be identified before approaching the patient for FNAC. However, aspiration in cases of small deep seated lesions yields best results under imaging guidance. In our study, all vertebral lesions were accessed under CT guidance. The usage of guided FNA increases the possibility of targeting the lesion accurately, ensures multiple passes from radiologically different appearing areas, and also improves the diagnostic yield.
There are individual case reports of FNA diagnosis of metastatic bone tumors, however, studies on evaluation of FNAC of metastatic lesions of the bone are relatively sparse. The review of available literature did not reveal any individual case series which focusses the FNAC of metastatic bone lesions. Table 5 summarizes the various studies of FNAC of bone tumors and secondaries in the bone.
| S. No. | Year | Author | No. of cases | Most common cytological diagnosis | Most common metastatic site |
|---|---|---|---|---|---|
| 1 | 2000 | Jorda et al.[9] |
95 | Adenocarcinoma | - |
| 2 | 2006 | Obiagelli et al.[11] |
27 | Carcinoma | Femur |
| 3 | 2007 | Wahane et al.[12] |
17 | Adenocarcinoma | Vertebra |
| 4 | 2017 | Shergill et al.[10] |
124 | Adenocarcinoma | Pelvic bones |
| 5 | 2020 | Present study | 109 | Adenocarcinoma | Vertebra |
In a study by Jorda et al., 217 cases of FNAC diagnoses of bone tumors were studied, of which 37% of cases were classified as metastatic bone tumors.[9]
In a study by Shergill et al. of 158 cases of FNA of bone lesions, 124 cases were metastatic, most of which were originating in the breast. About 15.3% of cases were designated as metastasis from poorly differentiated or undifferentiated tumors followed by lung (12.9%) and prostate (11.3%). Among the site-wise distribution, most frequent site of metastases were the pelvic bones and the vertebral column, followed by long bones, bones of the shoulder girdle, and skull.[10]
In a study by Obiageli et al. of 47 malignant bone lesions, 27 cases were metastatic bone tumors, out of which 16 were diagnosed as metastatic carcinomas. Most common site of metastases were found to be femur followed by pelvis.[11]
In a study by Wahane et al., 17 cases of metastatic bone tumors were described among 122 cases of bone tumors. Among these 17 cases, eight were male and nine were female. Most common site of metastases was vertebra followed by femur.[12]
In our study, the most common morphology is adenocarcinoma, concordant with these large series. The most common site of metastases was vertebra, concordant with series by Wahane et al.[12] In the present series, we did not encounter any metastatic prostatic carcinoma. The main reason of such odd finding may be the prior diagnosis of metastatic prostatic carcinoma due to the presence of high prostate-specific antigen and characteristic radiological features.
CONCLUSION
FNAC is a very useful and economical procedure. There are characteristic cytological features of the metastatic lesions and the basic diagnostic categorization of the malignant tumors is possible on FNAC. Regarding the primary source, clinical history, radiological features of the primary tumor, if any, and immunocytochemistry can be a useful adjunct.
Data sharing
Only the published data are shared with proper references.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
No conflicts of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Shruti Gupta: Data collection, analysis, and drafting of the manuscript, Nirmalya Banerjee: Data collection and analysis, Manish Rohilla; Parikshaa Gupta; Nalini Gupta; Radhika Srinivasan; and Arvind Rajwanshi: Reporting the cases and analysis, and Pranab Dey: Concept of the work, execution, and manuscript writing.
ETHICS STATEMENT BY ALL AUTHORS
This is a retrospective study, and proper permission was taken from the patient before performing fine needle aspiration. The departmental Ethical committee approved the study. The study followed the Helsinki accord.
LIST OF ABBREVIATIONS (IN ALPHABETIC ORDER)
CD – Cluster differentiation
CK – Cytokeratin
CT – Computerised tomography
FNA – Fine needle aspiration
FNAC – Fine needle aspiration cytology
H and E – Haematoxylin and Eosin
MGG – May Grunwald Giemsa
PAP – Papanicolaou
TTF – Thyroid transcription factor
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
References
- Bone metastases of unknown origin: Epidemiology and principles of management. J Orthopaed Traumatol. 2015;16:81-6.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Rep. 2015;4:689.
- [CrossRef] [PubMed] [Google Scholar]
- Fine Needle Aspiration of Bone Tumours. In: Orell SR, ed. The Clinical Radiological Cytological Approach. Vol Ch. 13. Baselo: Karger; 2010. p. :75-80.
- [CrossRef] [Google Scholar]
- Primary bone metastasis as first manifestation of an unknown primary tumour. BMJ Case Rep. 2015;2015:bcr2015211302.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological study of bone lesions-a review of 102 cases. IAIM. 2016;3:27-36.
- [CrossRef] [Google Scholar]
- Spondylectomy for malignant tumors of the spine. J Clin Oncol. 1989;7:1485-91.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of bone: Accuracy and pitfalls of cytodiagnosis. Cancer. 2000;90:47-54.
- [CrossRef] [Google Scholar]
- Fine-needle aspiration biopsy of lytic bone lesions: An institution's experience. Diagn Cytopathol. 2017;45:989-97.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology of bone tumours-the experience from the National Orthopaedic and Lagos University Teaching Hospitals, Lagos, Nigeria. Cytojournal. 2006;3:16.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology of bone tumors. Acta Cytol. 2007;51:711-20.
- [CrossRef] [PubMed] [Google Scholar]








