Translate this page into:
Cervical cytology: Radiation and other therapy effects

*Corresponding author: Jasvinder Kaur Bhatia, Professor and Head, Department of Pathology, Command Hospital, Kolkata, West Bengal, India. drjkbhatia@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Poflee SV, Bhatia JK. Cervical cytology: Radiation and other therapy effects. CytoJournal 2022;19:32.
Abstract
The different treatment options for carcinoma cervix include radiation, chemotherapy, and surgical treatments. Cytological analysis of smears is crucial for patient follow-up to determine response to therapy and to diagnose the persistence or recurrence of malignancy. Anatomical alterations and changes in cell morphology following radiation or chemotherapy make collecting and interpreting cervical cytology samples difficult. These issues can be mitigated by liquid-based cytology. Ionizing radiation is used in radiotherapy (RT) to kill cells. It is important that cytologists are aware of alterations in morphology of the cells. Radiation can cause cytoplasmic and nuclear changes. Cellular enlargement, vacuolation, granularity loss, and other changes linked with cell death are examples of cytoplasmic alterations. Nuclear enlargement and multinucleation are the most frequent nuclear alterations. These changes are determined by the amount of time that has passed since radiation. It should be emphasized that no one characteristic is pathognomonic. Post-irradiation dysplasia is a condition described as abnormal cellular changes in non-neoplastic epithelial cells after RT. Chemotherapy causes comparable alterations as radiation but impacts fewer cells. Busulfan and other chemotherapeutic treatments may produce morphological alterations, which cytologists must be aware of and able to identify. Immunosuppressive treatments, hormonal therapy, and tamoxifen are some of the other drugs that might cause changes in cervical morphology. Surgical methods used in the detection and treatment of cervical cancer may potentially cause alterations as a result of thermal damage and healing. For the treatment of cervical lesions, electrocautery and the loop electrosurgical excisional procedure are available. These procedures employ electric current ablation leading to ischemic changes in the cervical smear. Cytological analysis of smears following treatment with these modalities necessitates a comprehensive history, kind of therapy, and duration of treatment.
Keywords
Radiation
Cervical Smear
Uterine Cervical Neoplasms
Cytology
Pap smear

INTRODUCTION
Cervical cancer is the most common cause of cancer deaths among women in developing countries, with India having the highest age standardized incidence of cervical cancer in South Asia.[1] In addition, there is lack of effective screening programs in low and middle income countries.[1,2] Majority of the cases of carcinoma cervix present in late stages (80%) with the most common stage being Stage III in moderately differentiated (41.6%) and in poorly differentiated (70%) types.[3]
Various treatment modalities are available for the treatment of patients with carcinoma cervix. These include radiotherapy (RT), chemotherapy, and surgical procedures. These techniques and procedures lead to changes in the morphology of cells and cytologists need to be aware of these as they can be mistaken for malignancy. In addition, treatment of other malignancies with chemotherapeutic agents may also induce morphological changes in the cervix which need to be kept in mind while examining a Pap smear.
Recent times have seen important advances in management of carcinoma cervix. About 80–95% of the patients of early stage disease and 60% of Stage III disease can be cured by surgery or chemo-RT. Chemo-RT is the standard of care for locally advanced and early stage cancers with poor prognostic factors.[2]
RADIATION THERAPY
The aim of radiation therapy is to destroy susceptible malignant lesions without any significant damage to the surrounding normal tissue.
HOW DOES RT WORK?
Ionizing radiation basically comprises of electromagnetic waves which have energy. These waves interact with atoms and molecules and lead to detachment of electrons from them leading to ionization. DNA of the cell gets damaged by lysis and gene mutation due to both direct effect (ionizing) and indirect (free radical formation) of radiation.[4]
RT uses this property of radiation to destroy tumor cells by the use of ionizing radiation which die due to inactivation of their vital systems. The dose is calculated depending on the volume of tissue to be destroyed and the strength of the radiation. Response of tissue depends on the sensitivity of the tissue, the tumor volume or burden, tumor location, oxygenation, the type and amount of radiation, and the total time for which it is administered.[4,5]
Although RT is a necessary and frequently used procedure for the treatment of gynecological cancer, it has adverse effects on the lower genital tract. This leads to a change in morphology of squamous cells and causes difficulty in proper diagnosis.[6]
RT is used either alone or along with chemoradiation or surgery depending on the stage of the disease and other clinical factors. Staging of carcinoma cervix is given in Table 1. In addition to the stage of the tumor, the following are points to be remembered: (a) Imaging and pathology can be used, where available, to supplement clinical findings with respect to tumor size and extent, in all stages. (b) The involvement of vascular/lymphatic spaces, seen in histopathology, does not change the staging. (c) Adding notation of r (imaging) and p (pathology) to indicate the findings that are used to allocate the case to Stage IIIC.
| Stage | Description |
|---|---|
| I | The carcinoma isconfinedto the cervix (extension to the uterine corpus shouldbe disregarded) |
| IA | Invasive carcinoma that canbe diagnosedonly by microscopy, with maximum depthof invasion <5 mm |
| IA1 | Measured stromal invasion <3 mm in depth |
| IA2 | Measured stromal invasion ≥3 mm and <5 mm in depth |
| IB | Invasive carcinoma with measured deepest invasion ≥5 mm (greater than Stage IA),lesionlimited to the cervix uteri |
| IB1 | Invasive carcinoma ≥5 mm depth of stromal invasion, and <2 cm in greatest dimension |
| IB2 | Invasive carcinoma ≥2 cm and <4 cm in greatest dimension |
| IB3 | Invasive carcinoma ≥4 cm in greatest dimension |
| II | The carcinoma invades beyond the uterus, but has not extended onto the lower third of the vagina or to the pelvic wall |
| IIA | Involvement limited to the upper two-thirds of the vagina without parametrial involvement |
| IIA1 | Invasive carcinoma <4 cm in greatest dimension |
| IIA2 | Invasive carcinoma ≥4 cm in greatest dimension |
| IIB | With parametrial involvement but not up to the pelvic wall |
| III | The carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/or causes hydronephrosis or non-functioning kidney and/or involves pelvic and/or para-aortic lymphnodes |
| IIIA | The carcinoma involves the lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or non-functioning kidney (unless known to be due to another cause) |
| IIIC | Involvement of pelvic and/or para-aortic lymph nodes, irrespective of tumor size and extent (with r and p notations) |
| IIIC1 | Pelvic lymph node metastasis only |
| IIIC2 | Para-aortic lymph node metastasis |
| IV | The carcinoma has extended beyond the true pelvis or has involved (biopsyproven) the mucosa of the bladder or rectum. (A bullous edema, as such, does notpermita case tobe allottedto Stage IV) |
| IVA | Spread to adjacent pelvic organs |
| IVB | Spread to distant organs |
The stage-wise treatment recommended is as follows:
International Federation of Gynecology and Obstetrics (FIGO) Stage IA, IB1, IB2, and IIA1: Both surgery and RT are viable options for early stage disease. RT gives good control where surgery or anesthesia is contraindicated.
Adjuvant RT: This is given for patients who have adverse pathologic factors such as positive nodes and positive margins after radical hysterectomy.
FIGO Stage IB3 and IIA2: Concurrent chemo-RT (CCRT) is the treatment of choice. CCRT includes external radiation and intracavitary brachytherapy.
FIGO Stage IIB–IVA: Concurrent chemoradiation is the standard treatment for patients with locally advanced cervical cancer. Cisplatin is given weekly for 5–6 cycles during external beam therapy.[7]
CYTOLOGICAL EXAMINATION OF POST-RADIATION SMEARS
On the part of cytologists, awareness and knowledge is essential about the effects of radiation on non-neoplastic and malignant cells and of the changes produced by radiation in the overall pattern of smears.
The earliest correlation between clinico-radiological and cytological findings was given by Graham and Graham in 1955 by study of vaginal smears.[8] Radiation response in nonneoplastic cells was an indicator of response to therapy and was characterized by morphological changes in these cells.
McLennan and McLennanstressed that finding of malignant cells in smears after completion of RT was of grave significance and should prompt the clinicians to plan different treatment strategies.[9]
Changes seen in cells in post radiation therapy smears can be seen in the cytoplasm or nuclei or both.
Cytoplasmic changes
Increase in cell size about 3-fold to 4-fold than its normal counterpart with nucleus-cytoplasm ratio remaining unchanged.
Loss of granularity of cytoplasm with cytoplasm becoming amorphous, dense, and eosinophilic or polychromatic.
Indistinct cell outline.
Cytoplasmic vacuolization: Majority of radiation-induced vacuoles are multiple but single large vacuoles are also seen.
Multiple vacuoles with thick border surrounding the nucleus without distorting it and containing no inclusions.
Rupture and fragmentation of cytoplasmic membrane.
Changes in the nuclei
Nuclear enlargement – increase in nuclear size by 2–10 times.
Well-defined perinuclear halo.
Nuclear vacuolization.
Multinucleation with nuclei varying in size and shape.
Appearance of multilobulated nucleus.
Nucleoli – increase in number and size.
Condensation of chromatin at periphery, nuclear pyknosis, and fragmentation or karyorrhexis.
Other changes
A large no of inflammatory cells – leukocytes and an increased number of epithelial cells showing features of repair.
An increase in the number of histiocytes.
An increase in the number of foreign body giant cells.
An increase in the amount of endocervical mucous secretion and cellular debris deposited in the background of the smear.
Additional changes which may be seen are polychromasia, eosinophilia, hyperchromasia, nuclear membrane blebbing and nuclear vacuolization, as well as repair cells, atypical stromal cells, endothelial cells, and macrophages.[4]
It should be remembered that no single feature is pathognomonic.
Vacuolation, nuclear pyknosis, and karyolysis are features of cellular degeneration.
In most cases, these effects subside 3–6 months following treatment.
LIMITATIONS OF CYTOLOGY
In addition to destruction of tumor cells, radiation also induces anatomical changes in the vagina, including atrophy, adhesion formation, stenosis, and ulceration that often hamper sample collection. Therefore, radiation compromises the accuracy of cytology by both making the collection of representative material difficult leading to paucicellular smears and further difficulty in interpretation.
Another limitation is radiation-induced atypia which simulates malignancy. Irradiation-induced atypia can interfere with cytological analysis and thus detection of a local recurrence. As it simulates malignant atypia, it may lead to misinterpretation as recurrence of malignancy.[10]
SMEAR FREQUENCY
After the primary treatment, recurrence occurs after a median period of 7–36 months. Therefore, it is pertinent to follow up these patients clinically at closer intervals for the first 3 years. Routine follow-up visits are recommended every 3–4 months for the first 2–3 years, then 6 monthly until 5 years, and then annually for life.[4,7]
CYTOLOGICAL EXAMINATION IN A SATISFACTORY SMEAR[11]
From the beginning of RT to between 4 and 8 weeks, examination of cervical smears reveals malignant cells along with inflammatory cells and necrotic debris.[4]
Majority of malignant cells disappear by the end of one month of radiation therapy. Therefore, the cervical smear taken at 6–8 weeks is preferred for the assessment of treatment response.[12]
Interpretation of these radiation cell changes in relation to duration of RT is described as acute or early and chronic or late changes by most workers on radiation cytology.[11,13,14] Cytopathic effects because of RT can be categorized as follows:
Acute radiation changes
Acute changes are seen in the first 6 months after RT. These are cellular and nuclear enlargement with preserved N: C ratio, cytoplasmic vacuolation, chromatin condensation, and presence of multinucleated and bizarre-shaped cells. These cellular changes diminish with time and are rarely observed after 6 months.[9]
On commencement of RT, the smears are crowded with viable and non-viable epithelial cells with necrosis, debris, inflammatory, and repair cells in the smear background. Cytoplasmic vacuolation is first seen in parabasal cells and then in intermediate and superficial cells. Size of vacuoles varies from small multiple vacuoles to single large vacuole. These vacuoles can also be seen in the nucleus. Additional changes are altered staining and amphophilic change. Nucleus shows multinucleation, pyknosis, and karyorrhexis. Background shows neutrophils, necrotic debris, and other inflammatory cells. Inflammatory cells are predominantly polymorphs present in the background in large numbers. Another finding often seen in the smears is invasion of epithelial cells by the polymorphs.
Variable number of giant cells is seen. These giant cells have a large number of nuclei and show features of radiation like cytoplasmic vacuolation. The nuclei of these cells are eccentrically located. Figures 1-5 show changes due to radiation in conventional Pap Smear and LBC.
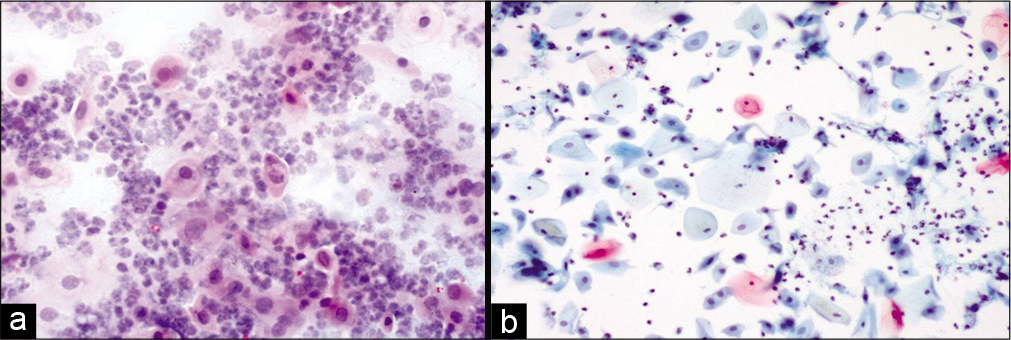
- (a) Conventional Pap smear and (b) liquid-based cytology (LBC) preparation showing acute radiation changes: Superficial and intermediate cells along with inflammation, necrotic debris in the background (×10). LBC smear shows a cleaner background.
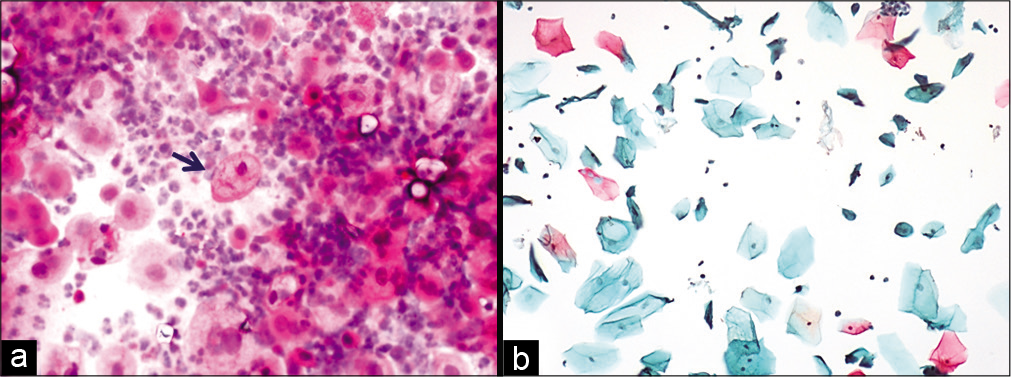
- (a and b) Conventional smear showing multiple cytoplasmic vacuolation and LBC preparation showing a single large vacuole indenting the nucleus (×10).
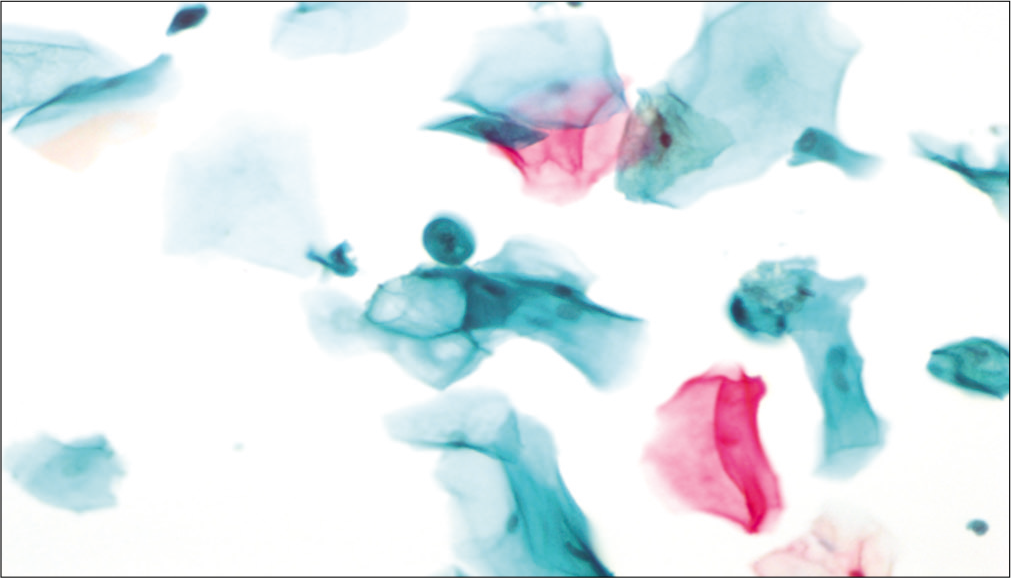
- LBC preparation: Large cell size with maintained N: C ratio. Multiple cytoplasmic vacuoles (×40).
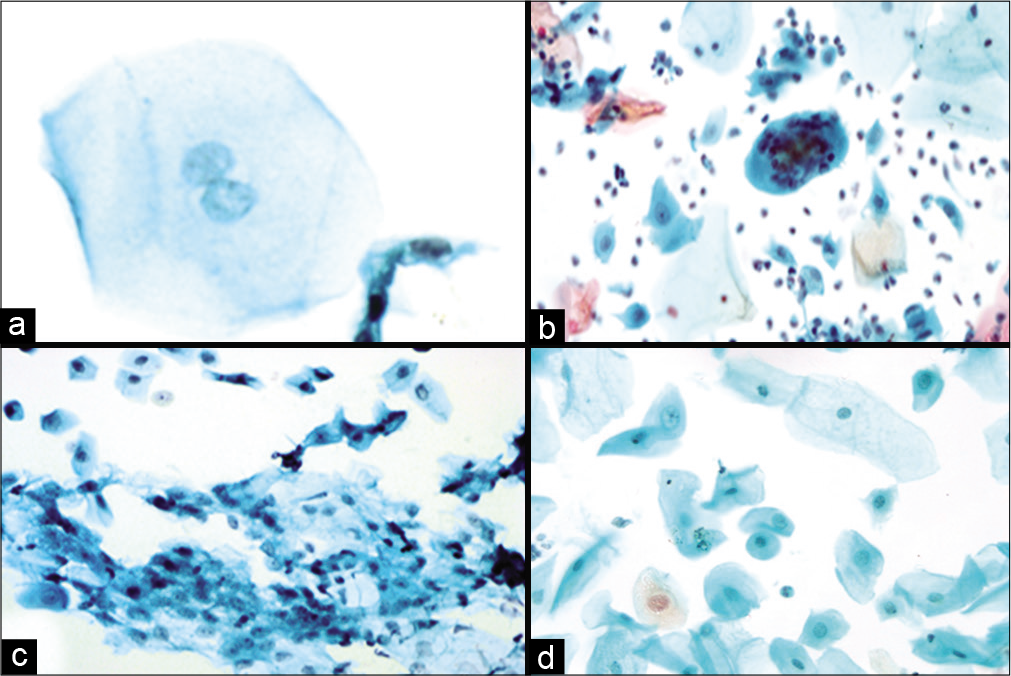
- (a-d) LBC preparation showing binucleation (a, ×40), multinucleation (b, ×10), nuclear smudging (c, ×10), cellular gigantism with bland nucleus (d, ×10).
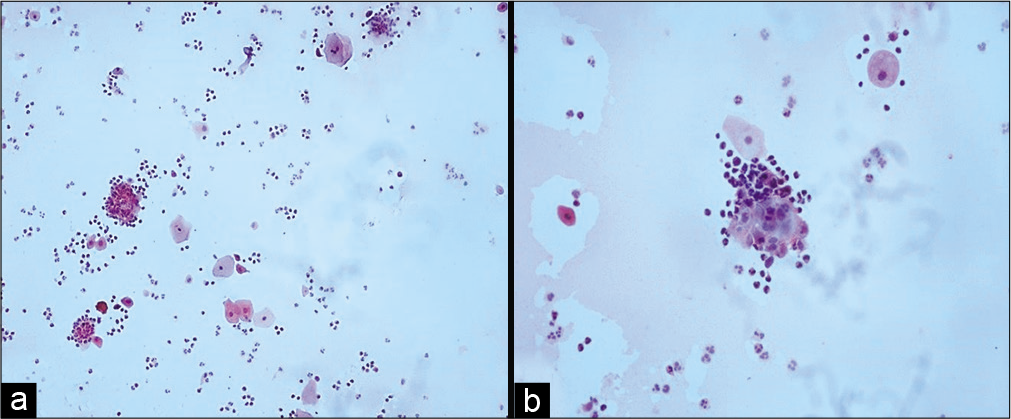
- (a and b) Conventional smear showing pus balls, that is, invasion of epithelial cells by polymorphs (a-×10, b-×40).
Changes in malignant cells
Malignant cells show the same changes as non-neoplastic cells including vacuolization of both the cytoplasm and the nucleus. The nuclear enlargement is more than the cytoplasmic swelling leading to a higher N:C ratio [Figure 6a and b].
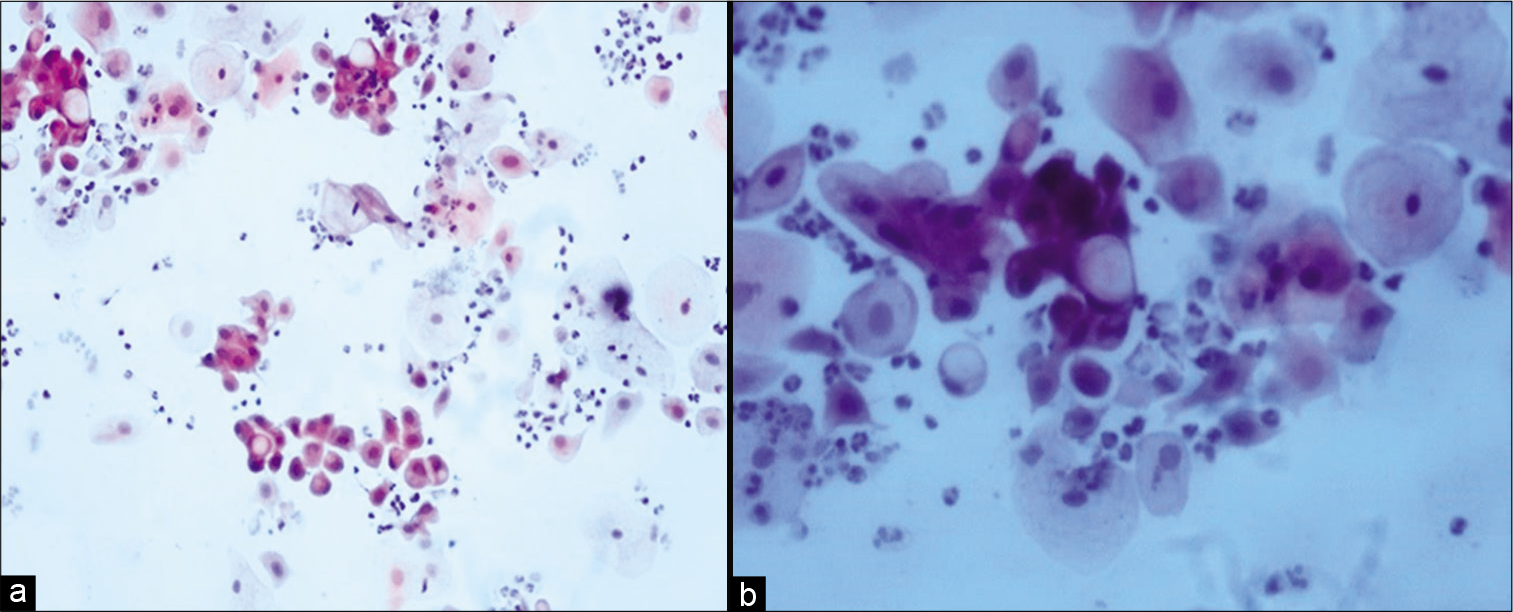
- (a and b) Conventional Pap smear showing cytoplasmic vacuolation in malignant cells (a-×20, b-×40).
Nuclei show fragmentation.[12,13] Background reflects the tissue response which is of inflammation and necrosis.[12] As these malignant cells are more sensitive to radiation, they disappear within 4 weeks after completion of therapy. After 6–8 weeks following RT, smears can be evaluated for tumor cells, radiation reaction and presence of residual carcinoma can be diagnosed.
Till about 8 weeks, the smears show necrosis, many inflammatory cells, and occasional malignant cell. Following this atrophic pattern is seen.[4]
Chronic radiation changes
Cellular changes due to radiation seen after 6 months to 1 year are defined as chronic radiation changes. Smears obtained after 6 months of radiation therapy show an atrophic smear pattern [Figures 7 a-c]. Pleomorphic or bizarre cell shapes and polychromasia are considered characteristic of chronic radiation changes. Cytoplasmic and nuclear vacuolation and necrosis disappear. There is slight enlargement of the cell and nuclei.[13]
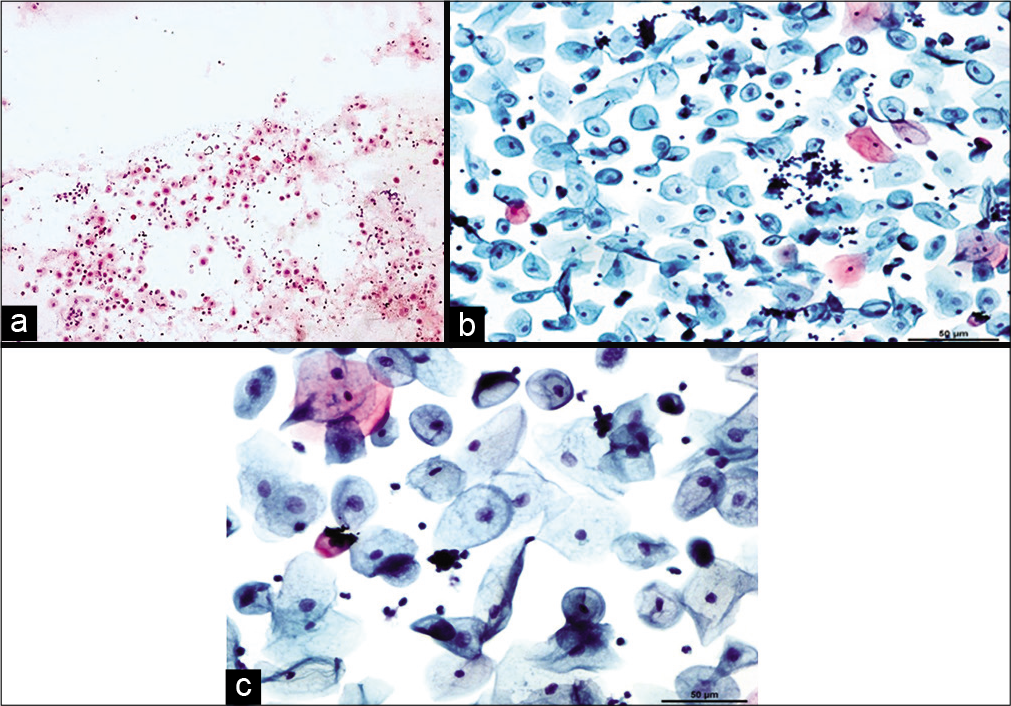
- Conventional smear (a and b) showing chronic radiation changes in the form of atrophy in LBC preparation (c): Atrophic smear pattern in 45-year-old women 18 months after radiation (×10).
Variable number of squamous cells, parabasal and basal cells are seen scattered in an inflammatory background.
WHAT IS PERSISTENT CARCINOMA?
Some patients continue to have malignant cells in the smears without a tumor-free interval. Persistence of unaffected cancer cells in smears during and after treatment suggests that a tumor is not responding to radiation.[13]
If the malignant cells with minimum irradiation changes are identified in the smear obtained after 4 weeks, the disease should be called as persistent.[15] Single, small undifferentiated cells are present with superficial and intermediate cells without radiation changes.[16,17] There is little or no radiation effect in the malignant cells. When unaffected malignant cells are identified in a post-irradiation smear, a thorough colposcopy and multiple biopsies should be performed to rule out any persisting or recurrent lesions [Figure 8 a and b].[18]
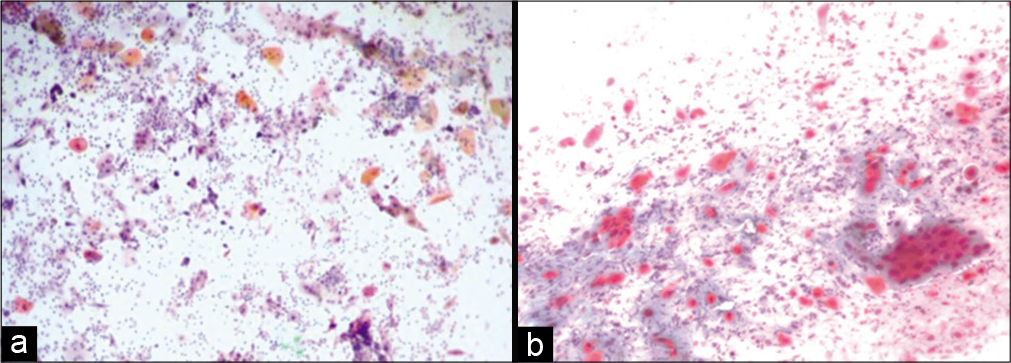
- (a and b) Conventional smear from a patient with persistent carcinoma, malignant cells without radiotherapy effect (a-×10, b-×20).
WHAT IS RECURRENT CARCINOMA?
The presence of intact tumor cells immediately after treatment is of no prognostic significance, as malignant cells can be seen in smears after treatment.[19]
A period of disappearance of malignant cells, of 2–3 months, and reappearance of malignant cells in the smears should make one suspect recurrent carcinoma [Figure 9 a and b].[12] The criteria of malignancy are similar to a de novo malignancy with increased nuclear cytoplasmic ratio, thickened nuclear margin, coarsely distributed chromatin, and presence of mitosis. The cells do not show any radiation changes. Finding of mitosis is the most dependable finding for viable cells and malignancy.[4]
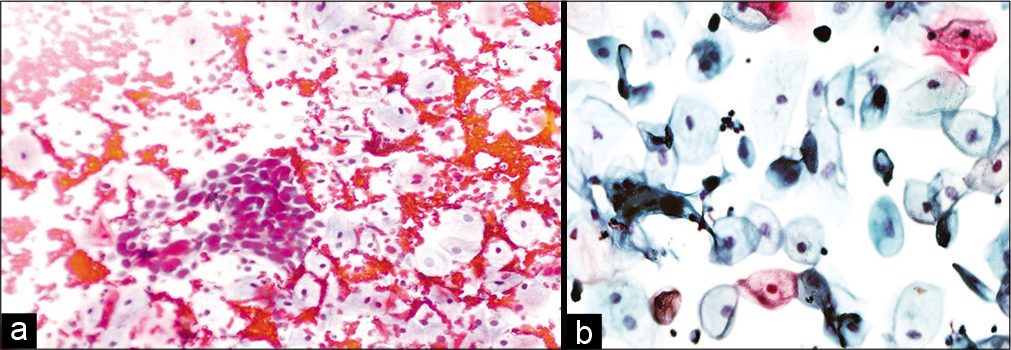
- (a and b) Conventional smear: Recurrent squamous cell carcinoma 10 months after radiation therapy in a 45-year-old woman (×10).
POST-IRRADIATION DYSPLASIA (PRD)
PRD cytology was initially described by Kaufman et al. as “late irradiation changes”[20] The term “post-radiation dysplasia” was used by Patten.[21]
PRD is the presence of abnormal cellular changes in nonneoplastic epithelial cells after RT.[17] These changes comprise cytoplasmic and nuclear enlargement with an altered N: C ratio, hyperchromatic nuclei with fine to coarsely granular cytoplasm, and eosinophilic or amphophilic staining of the cytoplasm.
This occurs after successful completion of RT for invasive carcinoma cervix. A few studies have reported that the probability of developing recurrent cancer is much higher for patients who developed the post-irradiation changes within 3 years or less, than for the patients with a delayed onset.[13] The cells are often arranged in groups having a hyperchromatic, finely granular chromatin pattern. Thorough examination of the peripheral cells of the group and identification of radiation changes in the cells helps to avoid misdiagnosing these as recurrent carcinoma.[22] Figure 10 shows features of post-radiation dysplasia on LBC preparation.
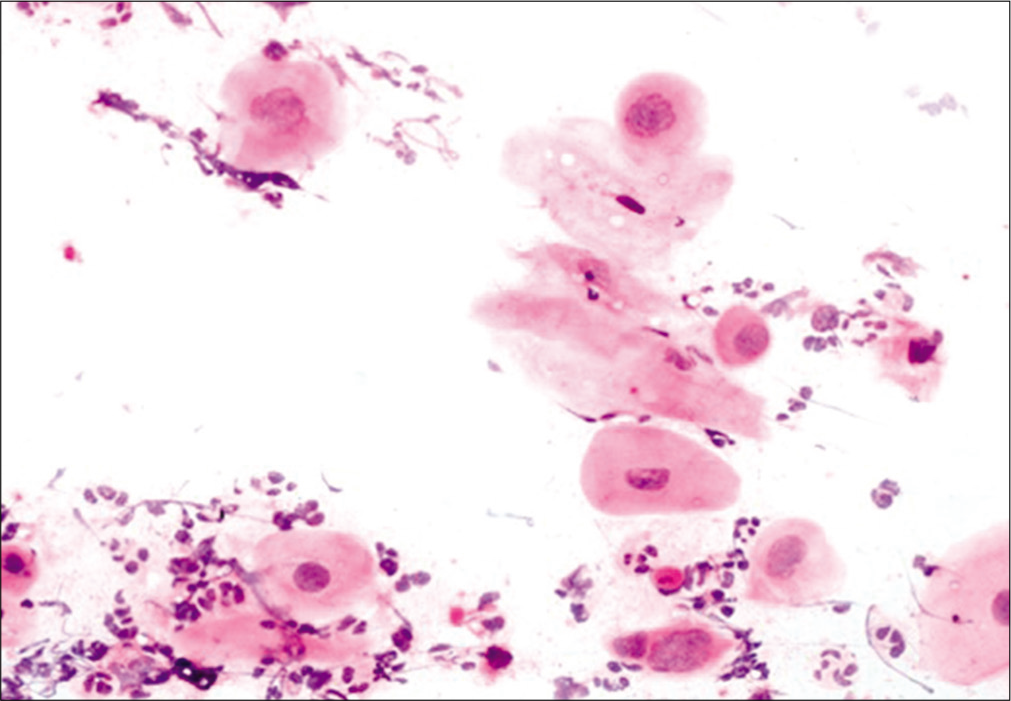
- LBC preparation: Cells showing post-radiation dysplasia in the lower half of the field.
It has been observed that LBC decreases the incidence of false-negative and equivocal cytologic interpretation. LBC improves sample quality by decreasing the number of obscuring cells and the amount of cellular debris present.[23] Patients treated for cancer cervix with RT can be better followed up with LBC.[24]
CHEMOTHERAPY
Chemotherapeutic changes are similar to changes due to radiation but affect a smaller number of cells than RT.[18]
BUSULFAN
Just like RT, certain drugs such as busulfan and other systemic chemotherapeutic agents may also induce morphologic changes in smears that are difficult to distinguish from neoplasia and it is important for cytologist to be aware of these. Busulfan is an alkylating agent that binds to nucleophilic sites of DNA bases. Morphological changes due to treatment with busulfan include, increase in nuclear size with irregular nuclear margin and presence of smudged chromatin or coarsely granular cytoplasm. Mitotic figures are not seen.
IMMUNOSUPPRESSIVE THERAPY
Cervical neoplasia shows a 14-fold increase in the immunosuppressed population compared with the general population, therefore, it is recommended for women to have a baseline cervicovaginal smear before immunosuppressive therapy and at regular intervals thereafter to monitor for early dysplastic changes. Women who are immunosuppressed because of AIDS or organ transplant/bone marrow transplant, are particularly susceptible to human papillomavirus infection. Due to immunosuppression, the virus is not cleared adequately. This leads to higher incidence of squamous abnormalities in these patients.[25]
HORMONAL THERAPIES
Oral contraceptives (OC) and hormone replacement therapy, depending on the proportion of estrogens and progesterone, result in maturation of the epithelium that may not correlate with the menstrual phase. They may be a cause of microglandular endocervical hyperplasia, reactive or degenerative atypia in glandular cells, or Arias–Stella like changes (nuclear hyperchromasia and cytoplasmic vacuolation). The latter is particularly associated with prolonged progesterone therapy. Exfoliation of “decidual cells”: cells with abundant hyaline cytoplasm and a central nucleus, may also be long-term effect of OC pills.[26] Diethylstilbestrol, a synthetic estrogen can lead to vaginal adenosis and ectropion.[27] In addition, oral contraceptive pills can disrupt the vaginal flora and allow the proliferation of Candida group of organisms. Recurrent appearance of these organisms in the Pap smear should therefore be a reason to discontinue the OC pills.
DRUG THERAPY
Tamoxifen-associated changes
Tamoxifen is the endocrine drug of choice for breast cancer. Tamoxifen has a partial estrogenic effect on the cervicovaginal lining epithelium. Changes due to tamoxifen may be a diagnostic pitfall and lead to an erroneous diagnosis of carcinoma. It must be noted that these cells do not show nuclear membrane irregularity and there are no dissociated cells. Tamoxifen use may be associated with benign squamous atypia in cervical smears which is not associated with intraepithelial lesions. Cytologist should be aware of these and exercise caution while interpreting these findings to avoid pitfalls.[28]
A study from a tertiary care referral center noted marked crowded sheets of glandular cells with nuclear overlapping with enlarged nuclei, anisonucleosis, and a finely granular chromatin pattern. Numerous large superficial squamous cells with absence of intermediate and parabasal cells were also seen. To distinguish changes due to tamoxifen from adenocarcinoma, it has been suggested to discontinue tamoxifen for a period of 6–11 months as endometrial thickness diminishes significantly in the majority of women taking tamoxifen.[29]
SURGICAL PROCEDURES
Biopsies/loop electrosurgical excision procedure (LEEP)/ cryotherapy/electrodiathermy
A Pap smear taken within a week of any of these procedures may show the following cytologic features:
A necrotic granular background because of coagulative necrosis along with acute inflammatory cells.
Ischemic cells appear spindly or elongated with cytoplasmic vacuolation or dense cytoplasm at times. Cytoplasmic margins may appear frayed or may show cytoplasmic tails (due to thermal injury) (Figure 11).
Nuclear pyknosis appears as dense dark nuclei and karyolysis may also be appreciated.
Regenerative/reparative features set in within a week and continue thereafter till around 6 weeks. Smears show plenty of cells with features of immature metaplasia having enlarged hyperchromatic nuclei mimicking dysplastic cells. Important is that when one is in doubt, the history of any surgical procedure being done in the past 15 days needs to be extracted carefully.
“Pencil thin” endocervical cells can also be seen after thermal injury or when a smear is collected after painting the cervix with dilute acetic acid solutions or Lugol’s iodine (used for visual inspection with acetic acid/visual inspection with Lugol’s iodine during colposcopy).[30]
Long-term consequences of cone biopsy include “tubal metaplasia.” These cells are columnar with ciliated cytoplasm.
Massive exfoliation of ischemic endometrial cells (post-uterine artery embolization) also show similar features as above.[31]
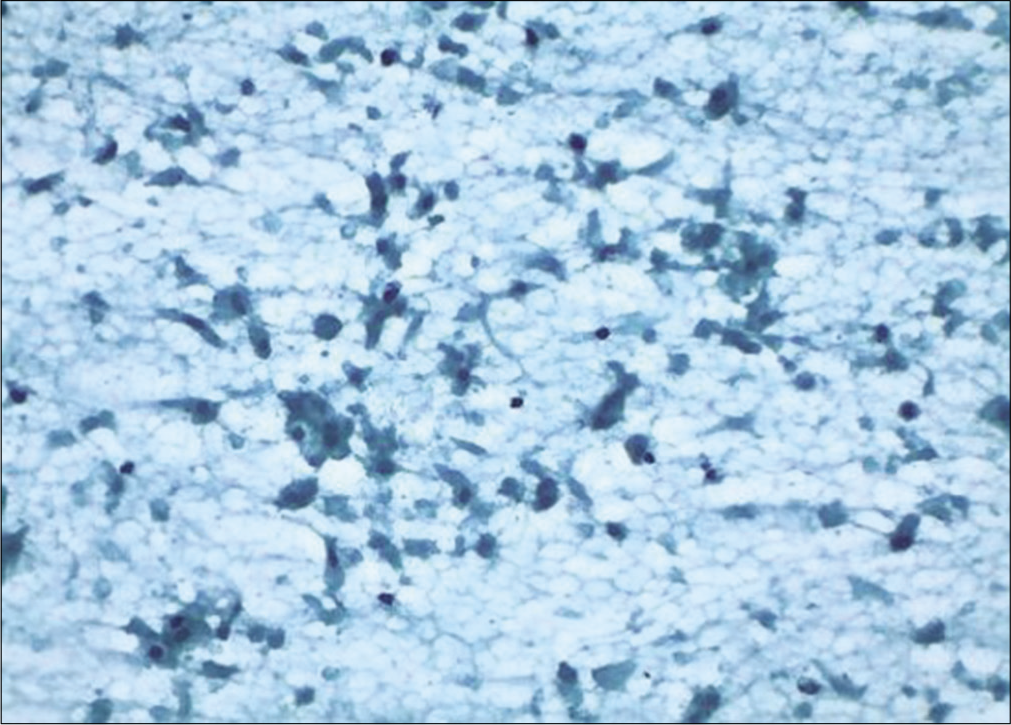
- Conventional smear: Granular background and disintegrating squamous and glandular cells showing features of ischemia after cautery (×10).
ELECTROCAUTERY
Electrocautery achieves tissue ablation using electric current. There is coagulative thermal necrosis of tissues. It is used to treat varied conditions such as cervicitis, chronic or large erosions, pre-neoplastic, and neoplastic conditions. It causes a host immune reaction where lymphoid cells act against the host epithelial cells. Acute phase shows marked inflammatory response predominantly composed of neutrophils with necrosis and diathesis in the background. There is presence of abnormal cells which are atypical parabasal cells and intermediate cells. N: C ratio remains normal.[18] Chronic phase shows features of regeneration and repair as described above.
In neoplastic lesions, a repeat cervicovaginal smear is recommended 4–6 weeks after therapy. This interval allows the necrotic inflammatory background to clear and not obscure persistent neoplastic cells.
LOOP ELECTROSURGICAL EXCISIONAL PROCEDURE
Loop electrosurgical excisional procedure uses low-voltage, high-frequency wire loop electrodes to remove cervical lesions. An electric cutting arc is created between the loop and the tissue, and it rapidly heats the cells to temperatures exceeding 100°C, causing them to vaporize. Therefore, the method of tissue ablation is the same as with electrocautery and laser therapy (i.e., thermal injury). The changes due to LEEP are similar to that observed after cautery and cryotherapy.
SUMMARY
Cytological examination of smears after treatment with the above modalities requires a detail history, type of therapy, and its duration for interpretation of cervico-vaginal cells.
Awareness of these cellular changes and varied composition of post-irradiation smears would improve the interpretation of the cytological findings in cases under follow up.
Anatomical changes and changes in morphology of cells after treatment with radiotherapy or chemotherapy makes collection and interpretation of cervical cytology smears challenging. LBC can minimize these difficulties.
LIST OF ABBREVIATIONS (In alphabetic order)
CCRT – Concurrent Chemo radiotherapy
FIGO – International Federation of Gynecology and Obstetrics
LBC – Liquid Based Cytology
LEEP – Loop Electrosurgical Excisional procedure
OC – Oral contraceptive pills
PRD – Post Irradiation Dysplasia
RT – Radiotherapy.
References
- Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015;7:405-14.
- [Google Scholar]
- Clinicopathological profile of cervical carcinoma: An experience of tertiary care cancer centre. Asian Pac J Cancer Care. 2019;4:83-6.
- [Google Scholar]
- Cytopathologic evaluation of patients submitted to radiotherapy for uterine cervix cancer. Rev Assoc Medica Bras 1992. 2017;63:379-85.
- [Google Scholar]
- Biological response of cancer cells to radiation treatment. Front Mol Biosci. 2014;1:24.
- [Google Scholar]
- Pelvic radiotherapy for cervical cancer affects importantly the reproducibility of cytological alterations evaluation. BMC Clin Pathol. 2018;18:11.
- [Google Scholar]
- Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet. 2019;145:129-35.
- [Google Scholar]
- Cytological prognosis in cancer of the uterine cervix treated radiologically. Cancer. 1955;8:59-70.
- [Google Scholar]
- Significance of cervicovaginal cytology after radiation therapy for cervical carcinoma. Am J Obstet Gynecol. 1975;121:96-100.
- [Google Scholar]
- PAP smear after radiation therapy for cervical carcinoma. Anticancer Res. 1997;17:3747-50.
- [Google Scholar]
- Diagnostic cytopathology of the uterine cervix In: Monographs in Clinical Cytology Vol 3. (2nd ed). Basel, Switzerland: Karger Publishers; 1978.
- [Google Scholar]
- Koss’ Diagnostic Cytology and Its Histopathologic Bases. Available from: https://www.ovid.com/product-details.5210.html [Last accessed on 2020 Oct 22]
- [Google Scholar]
- The Art and Science of Cytopathology (2nd ed). Chicago: American Society of Clinical Pathologists Press; 2012.
- [Google Scholar]
- Cytologic Diagnosis of Cancer (3rd ed). Philadelphia, PA: WB Saunders; 1972. p. :177-83.
- [Google Scholar]
- Benign proliferative reactions, intraepithelial neoplasia, and invasive cancer of the uterine cervix In: Bibbo M, Wilbur DC, eds. Comprehensive Cytopathology (3rd ed). Philadelphia, PA, USA: Saunders/Elsevier; 2008. p. :131-212.
- [Google Scholar]
- Post-irradiation cytology of cervical cancer patients. Cytopathology. 1992;3:167-82.
- [Google Scholar]
- Postirradiation dysplasia of uterine cervix and vagina: An analytical study of the cells. Cancer. 1963;16:173-82.
- [Google Scholar]
- Increased detection of epithelial cell abnormalities by liquid-based gynecologic cytology preparations. A review of accumulated data. Acta Cytol. 1998;42:178-84.
- [Google Scholar]
- Comparative study between liquid-based cytology and conventional Pap smear for cytological follow up of treated patients of cancer cervix. Indian J Med Res. 2018;147:263-7.
- [Google Scholar]
- Abnormal cervical cytology in bone marrow transplant recipients. Bone Marrow Transpl. 2001;28:393-7.
- [Google Scholar]
- Ectopic decidual reaction of the uterine cervix: Frequency and cytologic presentation. Acta Cytol. 1981b;25:616-22.
- [Google Scholar]
- Effects of tamoxifen on the cytology of the uterine cervix in breast cancer patients. Diagn Cytopathol. 1998;19:417-22.
- [Google Scholar]
- Cytodiagnostic problems in cervicovaginal smears from symptomatic breast cancer patients on tamoxifen therapy. Acta Cytol. 2009;53:383-8.
- [Google Scholar]
- Vaginal and cervical cytologic changes following laser treatment. Acta Cytol. 1976;20:290-6.
- [Google Scholar]
- Cellular changes following uterine artery embolization for the treatment of adenomyosis (letter) Cytopathology. 2001;12:270-2.
- [Google Scholar]








