Translate this page into:
Comparison of programmed death-ligand 1 (PD-L1) immunostain for nonsmall cell lung carcinoma between paired cytological and surgical specimens
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
programmed death-ligand 1 (PD-L1) is a ligand for the inhibitory programmed cell death protein 1 (PD-L1), which are targeted by several anti-PD-1 and PD-L1 drugs for lung cancer treatment. In clinical practice, many lung cancer cases only have cytology samples available to test PD-L1. Our current study compared the PD-L1 immunohistochemistry (IHC) between paired cytological and surgical samples.
Materials and Methods:
Formalin-fixed lung cancer tissue microarray and paired cell blocks and surgical specimens from the same patients with a confirmed diagnosis of lung squamous cell carcinoma (SCC, n = 29) and adenocarcinoma (AC, n = 23) were sectioned for PD-L1 IHC.
Results:
PD-L1 was expressed on tumor cells in 16 of 29 (55%) SCC surgical specimens and 18 of 29 (62%) paired cytologic specimens with 83% matched immunostains. PD-L1 was expressed on tumor cells in 13 of 23 (57%) AC surgical specimens and in 17 of 23 (74%) paired cytologic specimens with 79% matched immunostains. The PD-L1 was expressed on inflammatory cells in 20 of 23 (87%) AC surgical specimens and in 15 of 23 (65%) paired cytologic specimens with 70% matched immunostains. The PD-L1 was expressed on inflammatory cells in 18 of 29 (62%) SCC surgical specimens and in 12 of 29 (41%) paired cytologic specimens with 79% matched immunostains.
Conclusions:
PD-L1 immunostain in cytology samples matched very well with paired surgical samples in both SCC and AC cases. The cytologic samples present slightly higher sensitivity for PD-L1 immunostain on tumor cells as compared to surgical biopsies.
Keywords
Cytological samples
lung adenocarcinoma
lung squamous cell carcinoma
paired surgical samples
programmed death-ligand 1 expression
PD-L1
PD-1
INTRODUCTION
Lung cancer is the number one leading cause of cancer death in the US.[1] About 80%–85% of the lung cancer cases are non-small cell lung cancer (NSCLC) cases including lung squamous cell carcinoma (SCC) and adenocarcinoma (AC).[12] Although the current treatment options including surgical resection, adjuvant chemotherapy, and targeted therapies can improve the survival for a short period, the overall 5-year survival rate is only about 17%.[13] New strategies for the treatment of NSCLC are constantly pursued.
The immune checkpoint programmed death 1 (PD-1) protein is expressed in tumor-infiltrating T-lymphocytes, B-lymphocytes, natural killer cells, monocytes, and dendritic cells. It is engaged by the tumor expressed ligands programmed death-ligand 1 (PD-L1) and PD-L2, which increase the apoptosis of activated tumor-reactive T-cells and promotes the growth of tumor cells in vivo.[4] Recently, PD-L1 immune checkpoint inhibitor antibodies in multiple clinical trials were used to treat many cancer types, including melanoma,[567] NSCLC,[8910] hepatocellular carcinoma,[11] esophageal cancer,[12] and bladder cancer.[1314] Anti-PD-1 or PD-L1 agents including nivolumab, atezolizumab, and pembrolizumab were approved by the FDA for NSCLC immunotherapy treatment, which improved the overall survival (OS) rate compared to chemotherapy agent docetaxel treatment in advanced NSCLC.[810] There are great variations in the cutoff percentage of the immunoreactive-positive tumor cells for the determination of a positive reaction.[8915161718192021] Another uncertainty is whether PD-L1 evaluation should be performed in tumor cells or tumor-infiltrating immune cells.[22] However, NSCLC patients with relatively higher percentage of PD-L1 expression have demonstrated higher response rates to this immunotherapy.[23]
Three PD-L1 qualitative immunohistochemical (IHC) assays were recently approved by the FDA for diagnostic tests for various anti-PD-1/PD-L1 target immunotherapies. Two assays are from Dako, the PD-L1 IHC 22C3 pharmDx-positive NSCLC patients have good response with KEYTRUDA (pembrolizumab) treatment, and PD-L1 IHC 28–8 pharmDx-positive nonsquamous NSCLC patients showed increased survival to OPDIVO (nivolumab) treatment. The third one is from Roche, Ventana PD-L1 SP263, which the positive expression in urothelial carcinoma cells are likely respond to IMFINZI™ (durvalumab) immunotherapy.[89.102425] In most clinical trials, tumor sample (biopsy) is required for PD-L1 IHC testing. However, in routine clinical practice, almost half of the lung cancer cases do not have surgical biopsy or resection tissue for ancillary testing. Recently, less invasive diagnostic approach such as fine needle aspiration (FNA) of lung tumor is more favored by the patients and doctors, and only cytologic samples are available to test PD-L1 and other targeted therapy biomarkers. If the cytological samples can be incorporated, it may increase the number of potential candidates for clinical assays. At the same time, it is urgent to standardize these cytology tissue specimens for biomarker analysis. So far, there are no standard reporting criteria for the PD-L1 immunostain score in lung NSCLC cytology samples, to the best of our knowledge.[2226]
In our current study, we evaluated PD-L1 IHC scoring on both tumor cells and inflammatory cells in cytologic samples using paired surgical samples as the gold standard. In addition, we used lung NSCLC tissue microarray (TMA) to validate our PD-L1 testing system.
MATERIALS AND METHODS
Construction of tissue microarrays
TMAs were constructed from representative tumor areas with formalin-fixed specimens collected from 2012 in the Department of Pathology and Laboratory Medicine, University of Rochester Medical Center (URMC), Rochester, NY, USA. The TMAs contained 146 lung SCCs and 245 lung ACs.
Paired cytology cell block and surgical samples
Paired formalin-fixed cell blocks from cytological samples and surgical specimens from the same patients with a confirmed diagnosis of lung SCC (n = 29) and AC (n = 23) were found from URMC Soft database. All the paired cytology/biopsy specimens were obtained during the same endobronchial ultrasound-guided or computed tomography-guided procedure or the follow-up surgical resection within 1 month. Both cell blocks from cytological sample and biopsy tissues were fixed in formalin and paraffin embedded as routine standardized protocol in our laboratory. Moreover, all the tissue samples are obtained before chemo/radiation therapies. Slides containing the tissue sections from both cell block and surgical samples from the same patients were selected for immunostain in this study. All patients’ identifiers were removed. This project was approved by Research Subjects Review Board in URMC.
Programmed death-ligand 1 immunohistochemistry
IHC studies were performed on 4-μm thick sections of TMAs, formalin-fixed and paraffin-embedded surgical tissue biopsy, and cell blocks. After deparaffinization and pretreatment the tissue sections using the PD-L1 pretreatment buffer at 99°C for 20 min, ready-to-use mouse monoclonal antibody PD-L1 22C3 PharmDx IHC Kit (Dako, Carpinteria, CA, USA) was applied following the manufacturer's instructions.[9] Appropriate positive and negative controls were evaluated by both Dako and inside control tissue. TMAs were also stained with hematoxylin and eosin and to be used for histologic comparison.
Tumor proportion score and inflammatory cell proportion score
The viable tumor cells showing partial or complete membrane staining at any intensity were defined as positive PD-L1 immunostain [Figure 1]. Tumor proportion score (TPS) was based on evaluating the percentage of PD-L1-positive tumor cells relative to all viable tumor cells present in the specimen. All other cells including infiltrating inflammatory cells, normal lung parenchymal cells, and necrotic cells were excluded for scoring. PD-L1 expression was separated in three categories based on the recommendation from Dako and pembrolizumab clinical trial: (1) if TPS <1%, no expression; (2) if 50%> TPS ≥1%, low PD-L1 expression; (3) IF TPS ≥50%, high PD-L1 expression.[9] In TMA or surgical samples, at least 100 tumor cells were counted; however, in cytologic cell block samples, at least 100 tumor cells were counted by three pathologists. Three reviewers evaluated the PD-L1 staining. For the disagree cases, three of reviewers checked the slides again to get consensus.

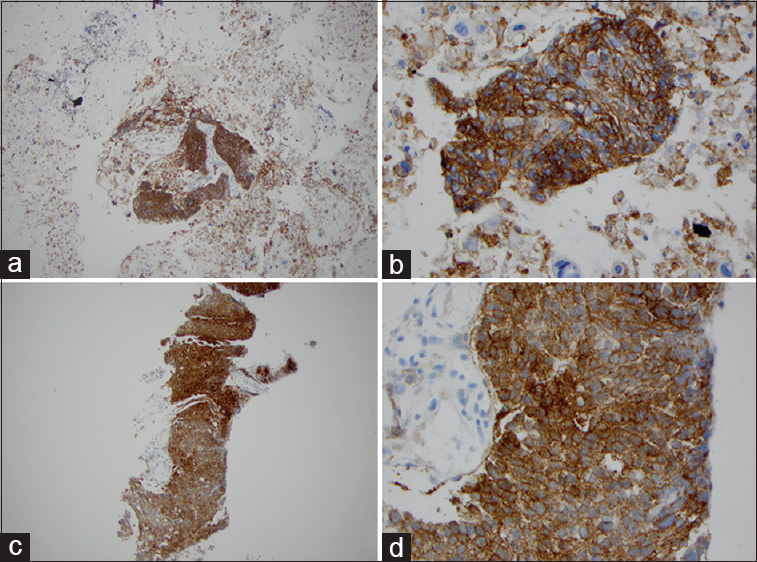
- Programmed death-ligand 1 (PD-L1) immunostaining for lung adenocarcinoma in matched cytologic cell block and surgical samples. Adenocarcinoma cells show membrane staining. High percentage of PD-L1 expression in lung adenocarcinoma in cell block (a: ×100; b: ×400). High percentage of PD-L1 expression in lung adenocarcinoma in matched surgical biopsy specimen (c: ×100; d: ×400)
In surgical samples, we counted the percentage of inflammatory cells surrounding tumor cells, but we did not separate them into high and low percentages since most of inflammatory cells had low percentage of PD-L1 expression. In cell block, it is difficult to evaluate the relationship between tumor cells and adjacent inflammatory cells. Therefore, we did not count the percentage of PD-L1-positive inflammatory cells in cell block, but only diagnosed as positive and negative PD-L1 expression if the inflammatory cells were positive in ≥10 cells. Ten positive inflammatory cells were used for cutoff since most of cases with ≥10 positive inflammatory cells had consensus among three reviewers.
Statistical analysis
The percentages of PD-L1-positive and PD-L1-negative immunostains in both tumor cells and inflammatory cells were analyzed. Fisher's exact test was used as appropriate to compare the percentages of PD-L1 high- and low-expression in both AC and SCC. All statistical tests were two-sided. A P < 0.05 was considered to be statistically significant.
RESULTS
Programmed death-ligand 1 expression in paired lung cytology and surgical samples
The PD-L1 was expressed in 13 out of 23 (57%) AC surgical specimens and in 17 out of 23 (74%) paired cytologic specimens [Figure 1 and Table 1]. Nineteen out of 23 (83%) paired AC cases had matched immunostain results and four cases (17%) unmatched [Table 2]. In four cytologic cases (17%), the tumor cells were positive for PD-L1, but the paired surgical samples were negative. The kappa value between the cytological samples and paired surgical samples was calculated to evaluate the degree of agreement. In AC cases, the kappa value is 0.54; the strength of agreement is considered to be moderate. In SCC cases, the kappa value is 0.35; the strength of agreement is considered to be fair. If we added both SCC and AC cases together, the kappa value is 0.423; the strength of agreement is considered to be moderate.


The PD-L1 was expressed in 16 out of 29 (55%) SCC surgical specimen and in 18 out of 29 (62%) paired cytologic specimens [Figure 2 and Table 1]. Twenty-three out of 29 paired SCC cases (79%) had matched immunostain results and six cases (21%) unmatched [Table 2]. In two cases, the cytologic specimen demonstrated negative expression for PD-L1 immunostain, but the matched surgical samples are positive. In four cases, the surgical specimen demonstrated negative expression for PD-L1 immunostain, but the matched cytologic samples were positive.
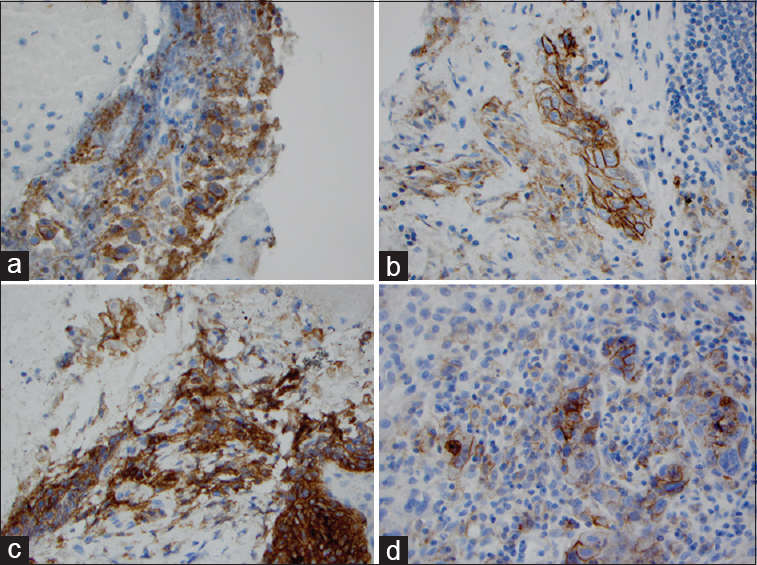
- Programmed death-ligand 1 immunostaining for lung squamous cell carcinoma in matched cytologic cell block and surgical samples. Squamous cell carcinoma cells show membrane staining. High percentage of PD-L1 expression in lung squamous cell carcinoma in cell block (a: ×100; b: ×400). High percentage of PD-L1 expression in lung squamous cell carcinoma in matched surgical biopsy specimen (a: ×100; b: ×400; c: ×100; d: ×400)
Combining SCC and AC cases to analyze PD-L1 expression in NSCLC, the results showed that 29 (56%) of the total 52 NSCLC cases had PD-L1 expression in surgical specimens and 35 (67%) in cytologic specimens. Of 52 paired cases, 42 (81%) had the matched PD-L1 immunostains and 10 (19%) were unmatched. The cytologic samples showed relative higher rate of PD-L1 expression than surgical samples.
Programmed death-ligand 1 expression of inflammatory cells on paired lung cytologic and surgical samples
The PD-L1 was expressed in inflammatory cells in 20 of 23 (87%) AC surgical specimens and in 15 of 23 (65%) paired cytologic specimens [Figure 3a, b and Table 2]. Sixteen out of 23 paired AC cases (70%) had matched immunostain results for the inflammatory cells. In six cytologic cases, the inflammatory cells were negative for PD-L1 immunostain, but they were positive in paired surgical samples. Of six cases, four had only 1%–2% positive inflammatory cells in paired surgical samples. In addition, of the total eight negative cytologic cases, six cases had scant inflammatory cells. In one surgical case, the inflammatory cells were negative for PD-L1 immunostain, but the inflammatory cells were positive in the paired cytologic sample. This surgical case was reviewed and found having relatively scant inflammatory cells around tumor cells.
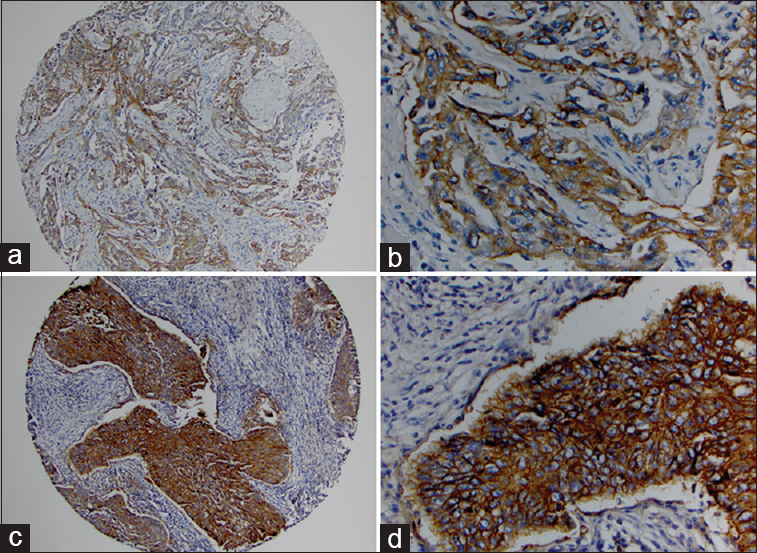
- Programmed death-ligand 1 immunostaining of inflammatory cells surrounding for lung adenocarcinoma and squamous cell carcinoma in paired surgical and cytologic specimen. PD-L1 expression in inflammatory cells of lung adenocarcinoma in both cytologic specimen (a: ×400) and surgical specimen (b: ×400). PD-L1 expression in inflammatory cells of lung squamous cell carcinoma in both cytologic specimen (c: ×400) and surgical specimen (d: ×400)
The PD-L1-expressed inflammatory cells were found in 18 of 29 SCC (62%) surgical specimens and in 12 of 29 (41%) SCC paired cytologic specimens [Figure 3c, d and Table 3]. Twenty-three out of 29 paired AC cases (79%) had matched immunostain results for the inflammatory cells [Table 3]. In six surgical cases, the inflammatory cells were positive for PD-L1 immunostain, but they were negative in paired cytopathologic samples. Of total 17 negative cytologic samples, eight cases had scant inflammatory cells for evaluation. All 12 positive cytologic cases are matched with paired surgical samples.

Programmed death-ligand 1 expression in lung nonsmall cell lung cancer tissue microarray
PD-L1 immunostain with Dako antibody showed membranous and weak cytoplasmic stain on the tumor cells [Figure 4]. Of 245 TMA lung AC cases, tumor cells in 83 (34%) cases were positive for PD-L1 immunostain [Table 4]. Among the PD-L1-expressed lung AC cases, 68 (27.76%) cases had low level of PD-L1 expression (1%–49% tumor cells, positive for PD-L1) and 15 (6.12%) cases had high level of PD-L1 expression (≥50% tumor cells positive for PD-L1) [Table 4]. The average and median percentage of PD-L1 positive tumor cells were 21.61% and 10% in AC cases.
- Programmed death-ligand 1 immunostaining for lung adenocarcinoma and squamous cell carcinoma in tissue microarray. Adenocarcinoma cells show membrane staining. High percentage of PD-L1 expression in lung adenocarcinoma (a: ×100; b: ×400). High percentage of PD-L1 expression in lung squamous cell carcinoma (c: ×100; d: ×400)
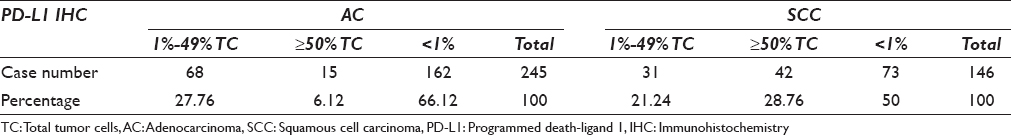
Of 146 TMA lung SCC cases, tumor cells in 73 (50%) cases were positive for PD-L1 immunostain [Figure 4c, d and Table 4]. Among these cases, 31 (21.23%) cases had low expression level of PD-L1 (1%–49% SCC tumor cells were positive) and 42 (28.76%) cases had high expression (≥50% SCC tumor cells were positive) [Table 4]. The average and median percentage of PD-L1-positive tumor cells were 49.18% and 60% in SCC, respectively. The percentage of PD-L1 expression in SCC cases is significantly higher than that in AC cases (P < 0.0001).
In summary, of total 391 NSCLC TMA cases, 156 (39.90%) cases were positive for PD-L1 expression including 99 (25.32%) cases with TPS 1%–49% and 57 (14.58%) cases with TPS ≥50%.
DISCUSSION
In the current study, we used the paired cytological and surgical samples to evaluate the PD-L1 expression in cytological samples. We found that PD-L1 was expressed on tumor cells in 16 of 29 (55%) SCC surgical specimens and 18 of 29 (62%) paired cytologic specimens with 83% matched immunostains. PD-L1 was expressed on tumor cells in 13 of 23 (57%) AC surgical specimens and in 17 of 23 (74%) paired cytologic specimens with 79% matched immunostains.
PD-L1 expression has been reported in multiple clinical trials with various antibodies including Dako 22C3,[915] Dako 28–8,[816171819] Ventana SP142,[1020] and SP263.[27] The various clinical trials also used different definitions of positive PD-L1 test. Using PD-L1 22C3 pharmDx antibody for pembrolizumab tests, 23.3% of patients were in ≥50% group and 37.6% in 1%–49% group.[9] In a large randomized controlled trial also using Dako 22C3 antibody, 1475 of 2222 (66.38%) patients had PD-L1 expression including 623 (28.49%) cases with TPS ≥50% and 842 (37.89%) cases with TPS 1%–49%.[15] Among patients with at least 50% of tumor cells expressed PD-L1, OS and progression-free survival were significantly longer with pembrolizumab than with docetaxel.[15] In clinical trials, eligibility requirements virtually always mandate a tissue biopsy for biomarker testing. However, the biomarker tests proven in trials are rarely validated for cytologic samples. In routine practice, cytologic specimens normally provide the diagnosis in more than half of the patients with lung cancer.[26] Recently, the College of American Pathologists has created guidelines for the validation of IHC on different cytological preparations such as cell blocks, direct smears, and other methods before incorporating the antibodies into clinical practice.[22] In our study, we compared the PD-L1 immunostain in cytologic samples to that in surgical samples from the same patients. In our experiment with paired cytologic and surgical specimens from the same patients, we found that the matched rate in all NSCLC was 81% [Table 2]. Our data showed that the cytologic sample seems well matched to the surgical sample for PD-L1 expression evaluation.
In addition, cytological cell blocks showed relative higher rate of PD-L1 expression (67%) than surgical specimens (56%), which is closer to the percentage of PD-L1 expression in the large clinical trial data (66.38%).[15] Compared with the tissue biopsy, the cytological FNA techniques facilitate to get multiple samples from different areas of the same tumor and/or metastatic sites since multiple passes from different areas within the same tumor usually are performed during FNA procedures.[22] The cytologic sample could represent more tumor heterogeneity.[22] Our study further confirmed that cytologic cell blocks are better samples for PD-L1 expression test since the cytologic cell blocks showed relatively higher rate of PD-L1 expression (67%) than surgical specimens (56%).
Certainly, cytologic samples have some limitations.First, their processing procedures have not been standardized across laboratories.[2829] In addition, the alcohol-fixed specimens lose some degree of immunogenicity. However, the cell block tissue with formalin fixation in our laboratory has a similar immunogenicity to the small biopsy. Another limitation is that cytologic samples do not show the relationship between tumor cells and inflammatory cells. Actually, in some clinical trials,[1025] the PD-L1 expression in tumor adjacent inflammatory cells was required for evaluation. Using the Ventana SP142 PD-L1 antibody for atezolizumab clinical trial with previously treated NSCLC, increasing improvement in OS was associated with increasing PD-L1 expression on both tumor cells and tumor-infiltrating immune cells.[10] In addition, using Dako 22C3 antibody in new clinical trial for gastric and gastroesophageal junctional AC, the combined positive score (CPS = 100 × [tumor positive cells + immune positive cells]/total number of tumor cells) was used to evaluate PD-L1 expression. However, further studies found that atezolizumab treatment resulted in a clinically relevant improvement of OS compared to docetaxel in previously treated NSCLC, regardless of PD-L1 expression or histology.[25] The controversial data about PD-L1 expression evaluation on inflammatory cells or tumor cells may be related to the criteria for the PD-L1 immunostain evaluation on inflammatory cells. It is urgent to further set up the standardized criteria for evaluating the PD-L1 expression in inflammatory cells. In our study, we evaluated the PD-L1 expression on inflammatory cells in both cell block and surgical samples. In cytologic samples, it was difficult to evaluate the relationship between tumor cells and adjacent inflammatory cells due to lack of tissue architecture. In addition, some of our FNA cytological samples came from mediastinal lymph nodes. The tumor cells from the positive lymph node were usually mixed with the background lymphocytes, which was difficult to differentiate them from tumor-associated inflammatory response. Therefore, we did not count the percentage of PD-L1-positive inflammatory cells in our cell block sample cytologic samples instead of we counted any inflammatory cells with positive (≥10 inflammatory cells) or negative PD-L1 immunostain. In surgical samples, we counted the percentage of inflammatory cells surrounding tumor cells. We found that the percentages of PD-L1-positive expression in inflammatory cells were very high in surgical samples (87% in AC and 62% in SCC) compared to those in cytologic samples (65% in AC and 41% in SCC). The percentages of matched samples for inflammatory cells were 70% between paired cytologic and surgical AC cases and 79% between paired cytologic and surgical SCC cases. Most of unmatched cytologic samples were related with scant inflammatory cellularity in both AC and SCC cases. Our finding suggests that the surgical samples are better for evaluation of tumor-associated inflammatory cells as compared to cytologic samples.
Our data from TMA showed relative low PD-L1 expression in both TPS ≥50% group (15%) and TPS 1%–49% group (25%) as compared to the previous clinical trial.[915] The lower PD-L1 expression in our TMA data may be caused by tumor heterogeneity[30] or simply by older tumor tissue.[31] Recently, Gniadek et al. studied the heterogeneous expression of PD-L1 in pulmonary SCC and AC.[30] They found that 28 of the 71 AC cases (39%) stained positive for PD-L1 in at least one TMA core and 43 of the 79 cases (54%) stained positive in at least one SCC TMA. The percentages of positive PD-L1 immunostain in both SCC and AC of our TMA are similar to their results.[30]
CONCLUSIONS
We found that PD-L1 immunostain evaluation in cytologic samples matched very well with paired surgical samples in both SCC and AC cases. The cytological samples are relatively more sensitive to detect PD-L1 expression in tumor cells than surgical biopsy samples, which could be due to the tumor heterogeneity presented in cytological sample. In the future studies, using PD-L1 evaluation in cytological samples and clinical immunotherapy follow-up will help to set up new standard IHC evaluation criteria for PD-L1 testing in cytologic samples. It will help us identify more eligible patients who will be benefit to immune checkpoint inhibitor treatment. We believe that cytology samples are important resources for the ancillary studies including PD-L1 or other molecular biomarker study.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors made no disclosure.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All co-authors have read the manuscript and agree for submission.
ETHICS STATEMENT BY ALL AUTHORS
This project was approved by Research Subjects Review Board in URMC.
LIST OF ABBREVIATIONS (In alphabetic order)
AC – Lung adenocarcinoma
IHC – Immunohistochemistry
NSCLC – Non-small cell lung cancer
PD-1 – Programmed cell death protein-1
PD-L1 – Programmed death-ligand 1
SCC – Lung squamous cell carcinoma; TMA: tissue microarray.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENTS
We want to thank all support from cytology technologists and IHC (Qi Yang and Loralee McMahon) at URMC.
REFERENCES
- Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584-94.
- [Google Scholar]
- Intercalated chemotherapy and epidermal growth factor receptor inhibitors for patients with advanced non-small-cell lung cancer: A systematic review and meta-analysis. Clin Lung Cancer. 2017;18:23-330.
- [Google Scholar]
- Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793-800.
- [Google Scholar]
- Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- [Google Scholar]
- Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-9.
- [Google Scholar]
- Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-30.
- [Google Scholar]
- Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627-39.
- [Google Scholar]
- Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-28.
- [Google Scholar]
- Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837-46.
- [Google Scholar]
- Immune checkpoint inhibition in hepatocellular carcinoma: Basics and ongoing clinical trials. Oncology. 2017;92(Suppl 1):50-62.
- [Google Scholar]
- Nivolumab treatment for oesophageal squamous-cell carcinoma: An open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631-9.
- [Google Scholar]
- Atezolizumab (Tecentriq) for bladder cancer and NSCLC. Med Lett Drugs Ther. 2017;59:e40-1.
- [Google Scholar]
- Bladder cancer: Atezolizumab effective against advanced-stage disease. Nat Rev Urol. 2016;13:238.
- [Google Scholar]
- Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540-50.
- [Google Scholar]
- Overall survival and long-term safety of nivolumab (Anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004-12.
- [Google Scholar]
- Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2969-79.
- [Google Scholar]
- Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-35.
- [Google Scholar]
- Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31-41.
- [Google Scholar]
- PS01.56: IMpower110: Phase III trial comparing 1L atezolizumab with chemotherapy in PD-L1-selected chemotherapy-naive NSCLC patients: Topic: Medical oncology. J Thorac Oncol. 2016;11:S304-05.
- [Google Scholar]
- Programmed death ligand-1 immunohistochemistry: Friend or foe? Arch Pathol Lab Med. 2016;140:326-31.
- [Google Scholar]
- Lung carcinoma predictive biomarker testing by immunoperoxidase stains in cytology and small biopsy specimens: Advantages and limitations. Arch Pathol Lab Med. 2016;140:1331-7.
- [Google Scholar]
- Assessment of the PD-L1 status by immunohistochemistry: Challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016;468:511-25.
- [Google Scholar]
- Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67-76.
- [Google Scholar]
- Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-65.
- [Google Scholar]
- Non-small cell lung cancer, PD-L1, and the pathologist. Arch Pathol Lab Med. 2016;140:249-54.
- [Google Scholar]
- Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol. 2016;17:299-308.
- [Google Scholar]
- Respiratory cytology – Current trends including endobronchial ultrasound-guided biopsy and electromagnetic navigational bronchoscopy: Analysis of data from a 2013 supplemental survey of participants in the College of American Pathologists interlaboratory comparison program in nongynecologic cytology. Arch Pathol Lab Med. 2016;140:22-8.
- [Google Scholar]
- Immunohistochemistry practices of cytopathology laboratories: A survey of participants in the college of American pathologists nongynecologic cytopathology education program. Arch Pathol Lab Med. 2014;138:1167-72.
- [Google Scholar]
- Heterogeneous expression of PD-L1 in pulmonary squamous cell carcinoma and adenocarcinoma: Implications for assessment by small biopsy. Mod Pathol. 2017;30:530-8.
- [Google Scholar]
- PD-L1 biomarker testing for non-small cell lung cancer: Truth or fiction? J Immunother Cancer. 2016;4:48.
- [Google Scholar]








