Translate this page into:
Constructing a modern cytology laboratory: A toolkit for planning and design
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Constructing or renovating a laboratory can be both challenging and rewarding. UAB Cytology (UAB CY) recently undertook a project to relocate from a building constructed in 1928 to new space. UAB CY is part of an academic center that provides service to a large set of patients, support training of one cytotechnology program and one cytopathology fellowship training program and involve actively in research and scholarly activity. Our objectives were to provide a safe, aesthetically pleasing space and gain efficiencies through lean processes.
Methods:
The phases of any laboratory design project are Planning, Schematic Design (SD), Design Development (DD), Construction Documents (CD) and Construction. Lab personnel are most critical in the Planning phase. During this time stakeholders, relationships, budget, square footage and equipment were identified. Equipment lists, including what would be relocated, purchased new and projected for future growth ensure that utilities were matched to expected need. A chemical inventory was prepared and adequate storage space was planned. Regulatory and safety requirements were discussed. Tours and high level process flow diagrams helped architects and engineers understand the laboratory daily work. Future needs were addressed through a questionnaire which identified potential areas of growth and technological change. Throughout the project, decisions were driven by data from the planning phase. During the SD phase, objective information from the first phase was used by architects and planners to create a general floor plan. This was the basis of a series of meetings to brainstorm and suggest modifications. DD brings more detail to the plans with engineering, casework, equipment specifics, finishes. Design changes should be completed at this phase. The next phase, CD took the project from the lab purview into purely technical mode. Construction documents were used by the contractor for the bidding process and ultimately the Construction phase.
Results:
The project fitted out a total of 9,000 square feet; 4,000 laboratory and 5,000 office/support. Lab space includes areas for Prep, CT screening, sign out and Imaging. Adjacent space houses faculty offices and conferencing facilities. Transportation time was reduced (waste removal) by a Pneumatic Tube System, specimen drop window to Prep Lab and a pass thru window to the screening area. Open screening and prep areas allow visual management control. Efficiencies were gained by ergonomically placing CT Manual and Imaging microscopes and computers in close proximity, also facilitating a paperless workflow for additional savings. Logistically, closer proximity to Surgical Pathology maximized the natural synergies between the areas.
Conclusions:
Lab construction should be a systematic process based on sound principles for safety, high quality testing, and finance. Our detailed planning and design process can be a model for others undertaking similar projects
Keywords
Cytolaboratory design
laboratory design
lean methods
quality control
laboratory construction
cytopathology equipments
cytopathology procedures
INTRODUCTION
Constructing or renovating a laboratory can be both challenging and rewarding. The UAB Cytology Section recently had the opportunity to relocate services from a building constructed in 1928 to a new space. The laboratory provides diagnostic and screening services for UAB Medicine patients, which include a 1000-bed hospital and a large Outpatient Clinic. Over 20,000 tests, including both conventional and liquid-based gynecological cases, 1000 fluid specimens, 2000 Fine Needle Aspirations (FNA), and 1000 pulmonary cases are processed annually. Internally, the section supports an onsite rapid FNA service for Endoscopic Procedures (EUS) as well as for Mammography and Bronchoscopy (EBUS). The technical and professional staff attend and perform FNAs throughout the inpatient and outpatient areas. The laboratory serves as a clinical affiliate site for Cytotechnology Programs, supports two Cytopathology Fellowships, Residents, and a Professional staff, active in research and publication. As an academic institution, our mission includes patient care, research, and education; and our facilities must be able to accommodate these functions seamlessly.
Our challenge in the laboratory design and building project has been to develop a plan that demonstrated flexibility, safety, quality of environment, and cost efficiency. Flexibility is important, as laboratory testing is constantly changing and to remain competitive, we must be able to accommodate future growth in both test volume and diversity. Safety of the employees is a major concern in areas with potential chemical exposure and biohazards. Additionally, there are numerous agencies, with applicable safety mandates, which require compliance. A well-designed, high-quality work environment is evidence of our commitment to patients and employees and fosters satisfaction and high performance among the staff. As a teaching facility, recruiting the best staff available is a priority and top-level facilities attract top-level staff. Cost efficiency is always a concern and can be a limiting factor, if not carefully monitored throughout the planning and design.[1]
METHODOLOGY
Once a project has been approved in concept, the core stakeholders must be identified and assembled as a project team. The composition of this group will vary depending on the facility type. Even as academic institutions, corporate laboratories, and hospitals have variable business models, there are common roles and areas of expertise that must be represented in the composition of the project team. Each member will have expectations to be met during specific phases of the project[2] [Figure 1].
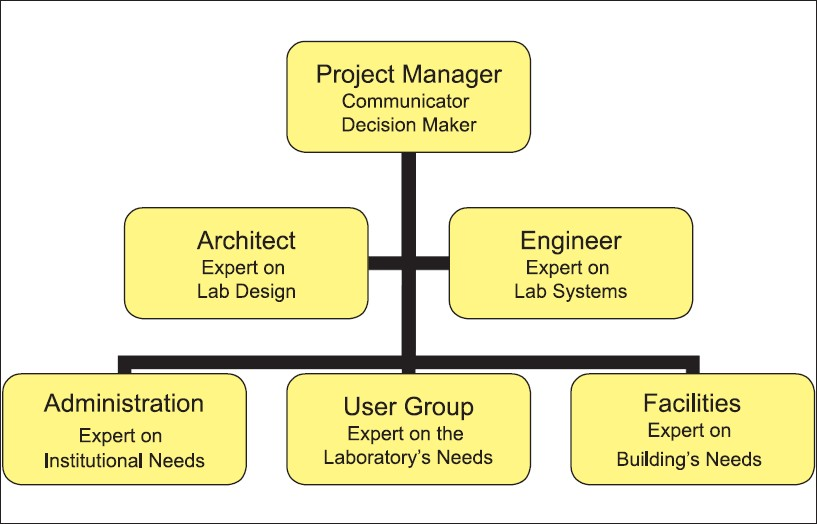
- Project team composition
The phases of project design are Planning, Schematic Design (SD), Design Development (DD), Construction Documents (CD), and Construction.[3]
PLANNING
Laboratory participation (User Group) is the most critical in the planning phase. Detailed information regarding the methodologies and scope of testing performed by the laboratory must be compiled and conveyed to the others on the project team. Administrative and facility members also participate, to ensure that the plans remain within the financial and physical constraints and are aligned with long-term institutional strategies.
Tours and high-level process flow diagrams can bring an understanding of the daily work in the laboratory to architects and engineers. Awareness of the appropriate relationships and adjacencies are essential to the design for a smooth flow of personnel, supplies, and equipment. The strength and complexity of these relationships can be illustrated through a bubble diagram [Figure 2].
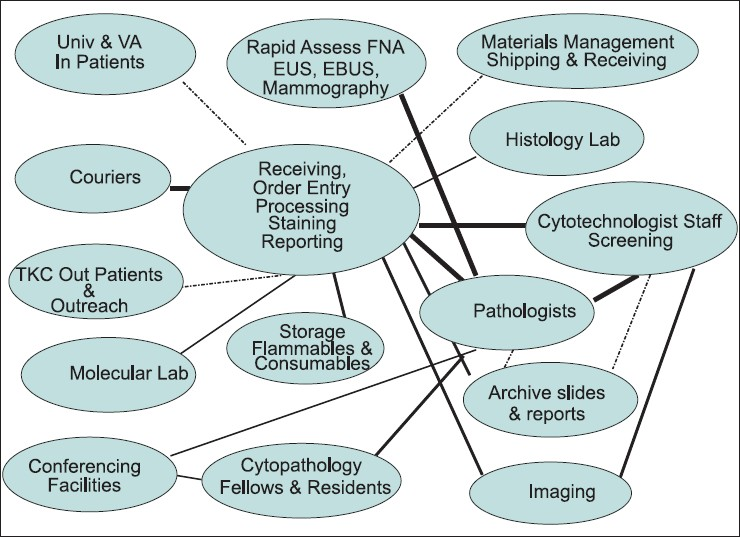
- Bubble diagram illustrating strength and complexity of relationships, both internal and external, to the laboratory
Any discussion of an efficient facility design must include planning for sustainable design and Lean operations. For example, materials handling is estimated to consume 20-50% of the operating expense in a variety of production and manufacturing settings, not atypical of laboratories. Efficient facility design can reduce these costs by 10-30%; an excellent return on investment over the life of the facility.[4] Objectives of Lean facility planning are, obtaining a smooth work flow, reducing the walking distances and work in progress, improving visibility for effective management of operations, and improving the work environment and inventory management.[5]
Along with Lean design concepts, this detailed planning information covers personnel, equipment, and future growth. The more information compiled at this stage, the more completely the outcome will meet the user group needs.
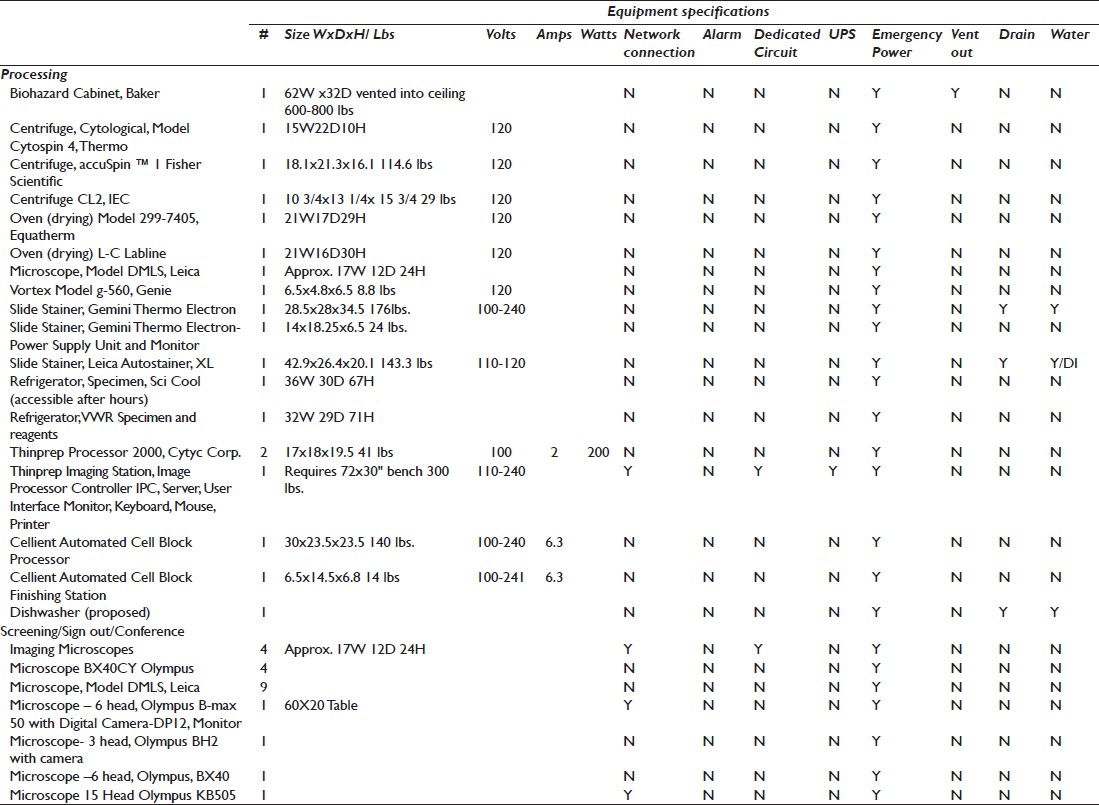
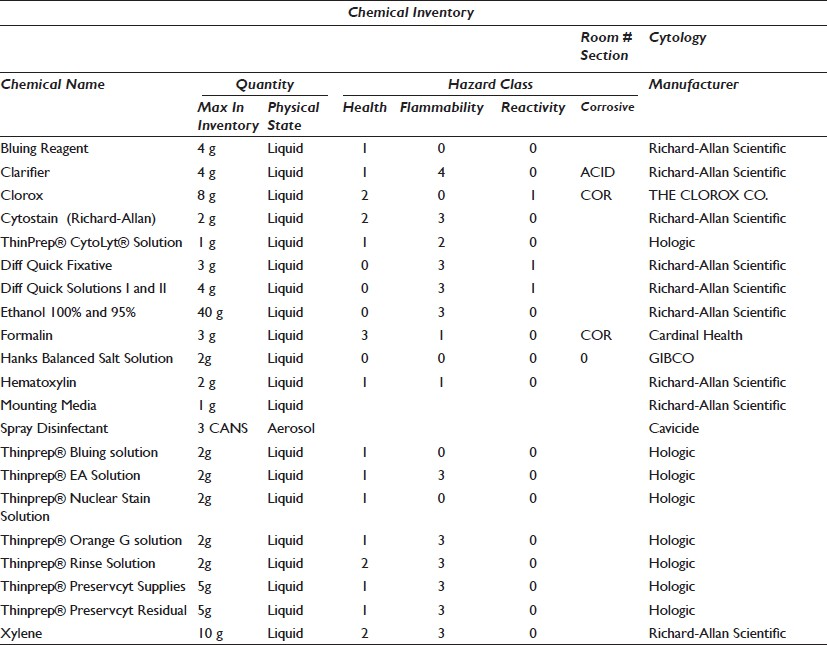
An equipment list with specifications, including what will be relocated, newly purchased, and projected for future growth, ensures that utilities are matched to need. The list must include: Footprint (dimensions and weight), electrical requirements, computer or interface requirements, uninterruptible power supply (UPS), alarm, dedicated circuit, emergency power, ventilation or exhaust, and a plumbing drain or deionized (DI) water [Table 1]. Cut sheets must also be available for each piece of equipment, conveying any information needed to install or use the product. A quantitative chemical inventory is essential to provide the appropriate storage space. This inventory must include the chemical name, storage requirements, and volume stored [Table 2]. Storage of used reagents and residual specimen containers/fixatives must also be included in this volume. The National Fire Protection Association (NFPA) provides ratings for areas based on volume and potential hazards. Anatomic pathology laboratories, because of the high volumes of xylene and formalin, are typically rated as Class A.
Regulatory and safety requirements must be discussed as they relate to the specimen sources and testing to be performed. A BioSafety level is the level of bio-containment precautions required to isolate dangerous biological agents in an enclosed facility. The levels of containment range from the lowest, BioSafety level 1, to the highest, level 4. In the United States, the Centers for Disease Control and Prevention (CDC) have specified these levels.[6] Once determined, this level will dictate many of the safety requirements for the laboratory. Our project and the typical cytology wet laboratory space is designated as BioSafety Level 2 (BSL2).
Future utilization must be addressed through a questionnaire and/or discussions among stakeholders. Even as future needs are difficult to predict, this exercise can identify the potential areas of growth and direction of technological change. A universally accepted growth area in laboratories is automation in processing and specimen tracking, which will necessitate more electrical and data capacity than in the previous workplace.
DESIGN
Throughout the Schematic Design, data and information from the planning phase is used by architects and planners to create a general floor plan. This design provides the groundwork for a series of meetings, to brainstorm and modify the plan. To ensure maximum efficiency, the workflow (movement of specimens) and traffic flow (movement of personnel) must be carefully evaluated during this phase [Figure 3].
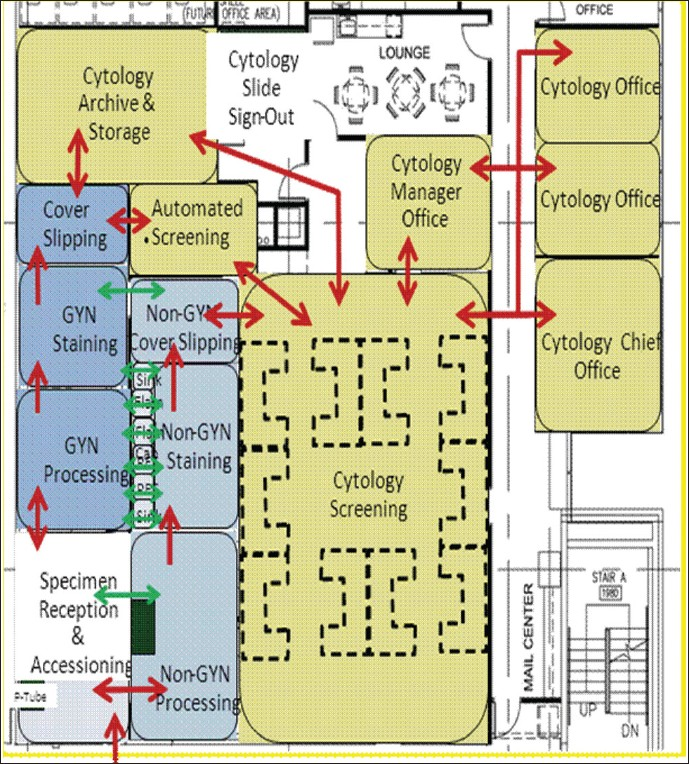
- Schematic design illustrating movement of people and materials
Specimen preparation and result reporting are bookends to the cytological testing process, providing both pre-analytic and post-analytic support. One of the objectives in the design of the preparation area is to allow two to three operators to effectively and simultaneously cover several instruments and functions. To achieve this, handling and walking distances are minimized and open areas are provided to facilitate visual management. Open work areas also allow managers to quickly assess the state of operations, inventory levels, and communication among workers, a key feature of Lean design. Another key requirement of this area is broad accessibility to other functional areas, specimen couriers at all hours, pathology sign out area, cytotechnologist screening, and imaging and archive.
Many of the laboratory safety mandates directly impact the preparation area and must be built into the design for appropriate plumbing and electrical support and ease of access for staff.[78] A safety checklist is provided in Table 3.
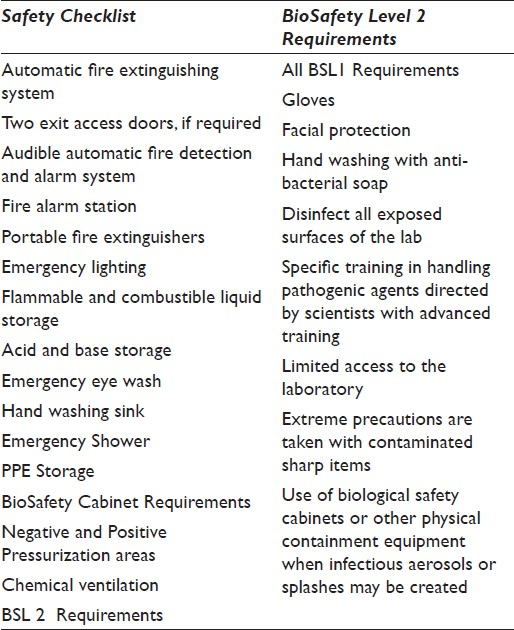
The Occupational Safety and Health Administration (OSHA) regulates and the American National Standards Institute (ANSI) provides standards (ANSI/ISEA Z358.1-2009) for emergency shower and eye wash station equipment. OSHA's general regulation ([29 CFR 1910.151 (c)]) states that: “Where the eyes or body of any person may be exposed to injurious corrosive materials, suitable facilities for quick drenching or flushing of the eyes and body shall be provided within the work area for immediate emergency use.”[9] Table 4 lists these standards.

In contrast to the preparation area, cytotechnologists performing the analytic phase of testing, screening, and diagnostics, require a workspace with more restricted access, to minimize interruptions. Multifunction workstations, with carefully considered ergonomics, must be designed, due to the repetitive nature of screening.[10] To reduce the movement of personnel and specimens; the workspace is designed to accommodate both manual and automated microscopes as well as computers. An open area, with individual work cubicles and a common resource area for educational materials, meets the needs of rotating trainees. Slides ready to be screened are transferred from the preparation to the screening area using a ‘pass thru’ cabinet. This minimizes interruptions and also helps to maintain the positive/negative airflow between the two areas.
Conferencing facilities are a high priority for academic institutions. A multipurpose space is designed, allowing a multihead microscope review as well as overhead projection and presentation resources. A moveable wall provides the capability of separating the two functions as needed. Locating this adjacent to the lounge facilitates food service, often a component of functions and conferences.
The Americans with Disabilities Act (July 26 1990) requires that public entities ensure that newly constructed buildings and facilities are free of architectural and communication barriers that restrict access or use by individuals with disabilities. The accessibility guidelines (ADAAG) are numerous and detailed, however, compliance is relatively simple if addressed early in the project [Table 5].[11]

Design development, the third phase, brings more detail to the schematic design, with addition of engineering, casework, equipment specifics, and finishes. For long-term savings, requirements for sustainable design and energy efficiency must be specified.[12] Examples of this are motion sensor, timed lighting, and planned air conditioner zones, to accommodate the heat generating equipment. Moveable casework will allow for adaptation to changing instrumentation. Use of open casework promotes the Lean concept of visual assessment [Figures 4–7].
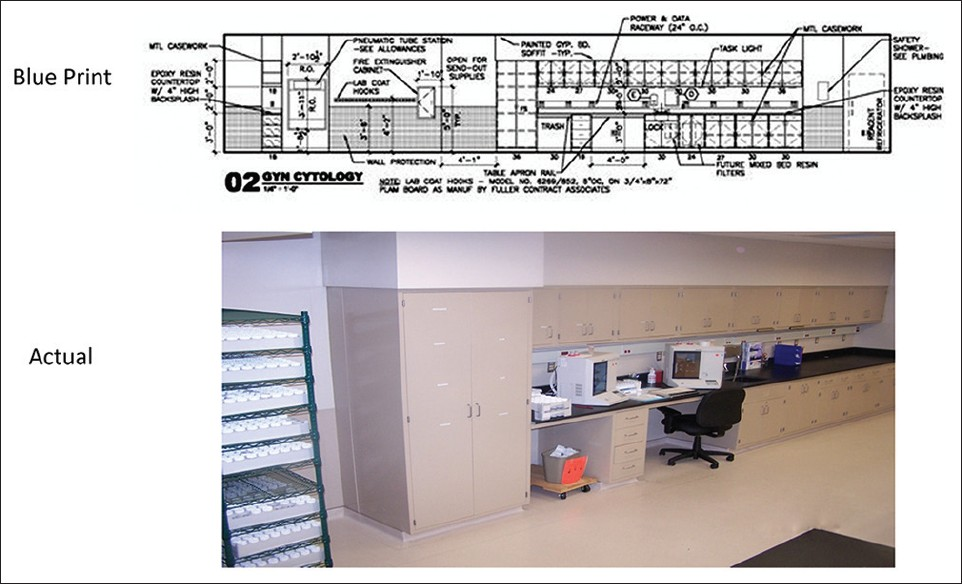
- Gyn-cytology processing elevation

- Accessioning and staining coverslipping in an open work area
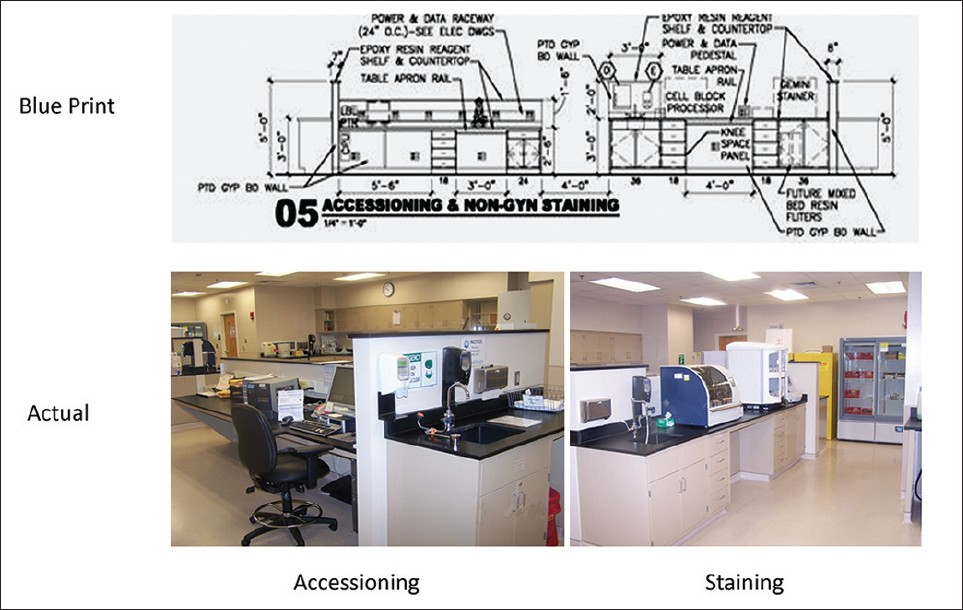
- Accessioning, staining, flammable cabinet, and refrigerated storage
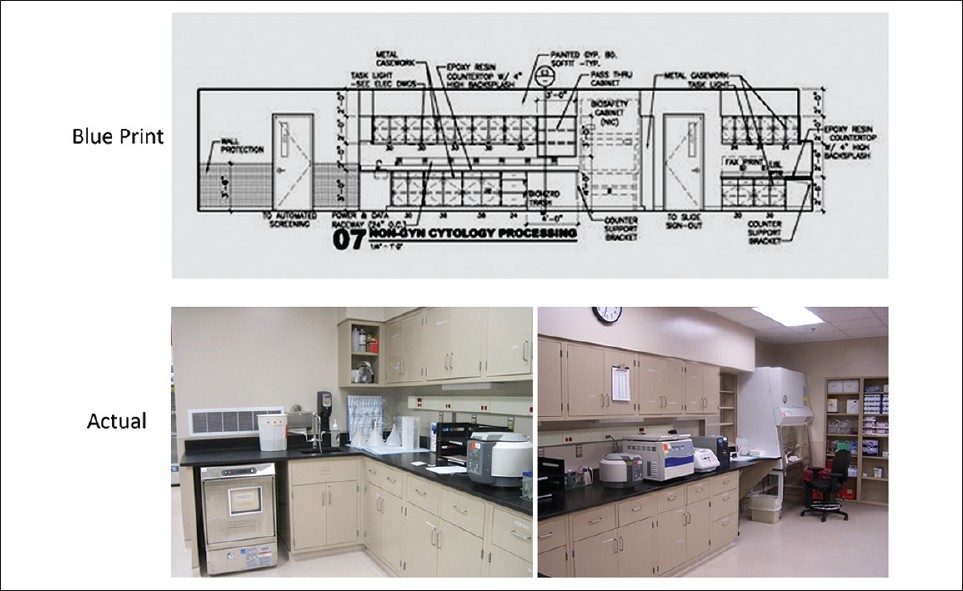
- Elevation 07: Non-Gyn processing
As the project moves to Construction Documents, the work transfers from the laboratory purview to a purely technical mode. At this point, due to costs, design changes must not be made. Architects and engineers provide more detail and specifications to allow qualified contractors to participate in the bid process.
During the construction phase, architects and engineers serve as liaisons between the construction team and owners, to ensure compliance with the construction documents. The user group tours the space periodically to verify that the construction meets the expectations of the planning and design phases. Change orders may be generated during this phase, however, they add to the project cost and must be minimized.
Although not a phase of construction and beyond the scope of this article, it must be noted that detailed plans for the actual move-in must begin early, as they can significantly impact the workflow and the project budget. Vendors must be notified well in advance for quotes and schedules for equipment transport and set up. Carefully developed detailed crosswalks from the old to the new space for phones and computers, ensure a seamless transfer of this equipment. Customers and supply chains must be informed of the potential interruption of service. The vacated space must be cleared of all chemical and biological hazards as well as any Protected Health Information (PHI).
RESULTS
At completion, the project fitted out a total of 9000 square feet; 4000 square feet of laboratory space and 5000 square feet of office/support space. The laboratory space included areas for preparation, CT screening, sign out, and imaging. The adjacent space housed faculty offices, support staff, and conferencing facilities.
Our objectives for building a Lean facility were essentially met. The workflow was improved by moving specimens and their derivatives in a linear motion, from receipt to final verification. Walking distances (transportation) were greatly reduced by the installation of a Pneumatic Tube System (PTS), specimen drop window directly into the Prep area, and a pass thru cabinet to the screening area. Logistically, closer proximity to Surgical Pathology maximized the natural synergies between these areas. Open floor plans for the main functional areas and screening and preparation, improved visibility for a more effective management of operations. The overall work environment was improved with better lighting, aesthetics, conferencing facilities, information technology, and employee comforts. Inventory management was more efficient, with accessible areas appropriate for short-term and long-term storage. Security was managed by key-code and card access entry and the slide archive area could only be accessed by authorized individuals. Surveillance cameras were also strategically located for added security. Efficiencies were gained by ergonomically placing manual and imaging microscopes in customized work cells for cytotechnologists. Location of computers in close proximity and the addition of bar code scanners facilitated a new paperless workflow for additional savings.
SUMMARY
For Cytology, the past decade has brought tremendous technological change to what for half a century, has largely experienced predictable and constant practice. Automated stainers and cover slippers, liquid-based processors, and imaging systems are now a standard of practice in most laboratories. These advances in instrumentation require bench space and facility modifications not seen in traditional cytology laboratories. With the expected decrease in volume of routine Pap tests and growth and integration of molecular testing, future cytology laboratories will demand an increasingly more technologically sophisticated workplace. Planning, design, and construction must incorporate the flexibility to allow this change at a minimal time and cost. Infrastructure and information technology must be able to support networked instruments, mobile devices, wireless connectivity, digital projection, and high quality audio-visual technology.
Successful laboratory construction must be a systematic process, based on sound principles, to provide flexibility, safety, quality of environment, and cost efficiency. Throughout the project, decisions must be driven by data gathered in the planning phase. This careful planning and design can ensure that the laboratory we build today will adapt to the laboratory we need tomorrow.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author aknowledges that this final version was read and approval.
ETHICS STATEMENT BY ALL AUTHORS
This article content does not require approval from Institutional Review Board (IRB) at out institution. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/3/107983
REFERENCES
- Research Laboratory Design Guide, Department of Veterans Affairs. 1995. Office of Research and. Development, Office of Facilities Management. Available from: http://www.wbdg.org/ccb/VA/VADEGUID/lab.pdf
- [Google Scholar]
- Laboratory Design for Today's Technologies, MedTechNet. 1997. p. :1-14.
- Clinical and Laboratory Standards Institute. In: Laboratory Design; Approved Guideline -Second Edition. 940 West Valley Road, Suite 1400. Wayne, Pennsylvania 19087-1898 USA: CLSI document GP18-A2 [ISBN 1-56238-631-X]. Clinical and Laboratory Standards Institute; 2007.
- [Google Scholar]
- Design Your Laboratory of the Future. Available from: http://www.sprickstegall.com
- Lean Boot Camp: CLMA Immersion Day, presented by Susan Stegal www.sprickstegall.com
- Centers for Disease Control and Prevention, Summary of BioSafety Levels-1 and 2. Available from: http://emergency.cdc.gov/documents/PPTResponse/table3abiosafety.pdf
- [Google Scholar]
- College of American Pathologist. http://www.cap.org Lab General Checklist 7/11/2011
- [Google Scholar]
- NCCLS. In: Clinical Laboratory Safety: Approved Guideline-Second Edition. 940 West Valley Road, Suite 1400. Wayne, Pennsylvania 19087-1898 USA: NCCLS; 2004. NCCLS document GP17-A2 [ISBN 1-56238-530-5]
- [Google Scholar]
- Eyewash and Safety Shower Requirements. Available from: http://gesafety.com/ansi/index.shtml
- [Google Scholar]
- Ergonomics Checklist. Available from: http://www.ehs.ucr.edu/ehsacademy/presentations/ergonomicslaboratorychecklist.pdf
- [Google Scholar]
- Available from: http://www.ada.gov/2010ADAstandards
- Supporting Integrated Design through Interlinked Tools: The Labs21 Toolkit. Available from: http://www.labs21century.gov/
- [Google Scholar]








