Translate this page into:
Fine needle aspiration cytology in diagnosis of salivary gland lesions: A study with histologic comparison
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Fine needle aspiration cytology (FNAC) has been employed in pre-operative diagnosis of salivary gland lesions for many years. Various studies in the existing literature have shown a wide range of sensitivity and diagnostic accuracy of cytologic diagnosis. This study was aimed at evaluating salivary gland FNAC for sensitivity, specificity and diagnostic accuracy at a tertiary care center.
Materials and Methods:
This study included 80 patients who underwent pre-operative FNAC followed by surgical procedure and histologic examination. The histologic diagnosis was considered as the gold standard. FNAC diagnosis was compared with the final histologic impression and concordance assessed. Sensitivity, specificity and diagnostic accuracy of FNAC for malignant lesions were calculated.
Results:
Of the 80 cases, majority (67.5%) involved the parotid gland. Eight cases (10%) were non-neoplastic lesions, comprised of sialadenitis, retention cyst and sialadenosis. Of a total of 72 neoplasms, 58 were benign and 14 were malignant salivary gland tumors. A cyto-histologic concordance of benign diagnosis was achieved in 85.7% of cases and for malignant lesions in 92.8% of the malignant tumors. FNAC showed a sensitivity of 92.8%, specificity of 93.9%, a positive predictive value of 81.2% and negative predictive value of 98.4% for malignant salivary gland tumors. There was one false-negative diagnosis and four false-positive cases diagnosed on FNAC.
Conclusion:
FNAC continues to be a reliable diagnostic technique in hands of an experienced cytopathologist. The sensitivity of diagnosis of malignant lesions is high, though the rate of tumor type-specific characterization is lower, due to variable cytomorphology. In difficult cases, histologic examination may be employed for accurate diagnosis.
Keywords
Fine needle aspiration
histologic diagnosis
salivary gland
sensitivity
specificity
INTRODUCTION
Fine needle aspiration cytology (FNAC) is a popular method for diagnostic evaluation of salivary gland masses due to their superficial nature and easy accessibility for the procedure. This technique assumes greater importance considering the lack of characteristic clinical or radiologic features that may suggest a particular diagnosis. Though, few symptoms and signs may suggest malignancy, most malignant salivary gland lesions cannot be differentiated from their benign counterparts on clinical criteria alone.[1]
The characteristic cytologic features of the common salivary gland lesions are well-delineated in literature. However, there also exist cytologic pitfalls and overlapping features that make an accurate diagnosis difficult in few cases. This has led to a wide-range of sensitivity (62-97.6%) and specificity (94.3-100%) of cytologic diagnosis.[2–7] The reported diagnostic accuracy is high for benign neoplasms, but lower for malignant tumors. The accuracy of type-specific diagnosis of malignant salivary gland tumors is quite poor, as reported in the literature.[2–7]
The present study was designed to compare the cytologic findings of salivary gland lesions with the histologic diagnosis, in order to assess the sensitivity, specificity and diagnostic accuracy of FNAC, with emphasis on discordant cases.
MATERIALS AND METHODS
This prospective study included 80 cases of various salivary gland lesions that underwent both FNAC and surgical excision over a period of 3 years (2006-2008). Patients with recurrent lesions and patients who did not undergo surgical excision (34 patients) during the period of study were excluded. Relevant clinical details were elicited in all the cases and findings of local examination noted. All the patients underwent FNAC, which was performed using 23 G needle with suction provided by 20 ml syringe. The character of aspirate was noted, routine smears prepared, air-dried and stained with May-Grünwald-Giemsa stain.
Following the cytological diagnosis, patients underwent appropriate surgical procedure and specimen submitted for histopathologic diagnosis. Special stains and immunohistochemistry were performed, wherever required.
The cytologic and histologic slides were reviewed by two pathologists (Sompal Singh and Madhur Kudesia) in a blinded fashion. The histologic diagnosis was considered as the gold standard for assessment of sensitivity and specificity of FNAC. Cyto-histologic correlation was done and appropriate statistical tests applied.
RESULTS
The study included 80 patients with no gender predilection (42 males, 38 females, M: F 1.1:1). The male to female ratio was 1.2:1 for benign neoplasms and 1.3:1 for malignant neoplasms. The mean age was 35.3 years (±13.6 years) for all lesions considered together. Non-neoplastic lesions were seen in younger patients (mean age 20.5 ± 8.7 years). The mean age for benign neoplasms was 37.4 years (±14.1 years) while that for malignant neoplasm was 33.60 years (±11.6 years).
Of the 80 cases, 54 (67.5%) occurred in parotid gland, 24 (30%) in submandibular gland and 2 (2.5%) in minor salivary glands (palate). The non-neoplastic lesions involved only submandibular gland. Based on the final histologic diagnosis, eight cases (10%) were non-neoplastic and the rest were neoplasms. Among neoplastic lesions, 58 (80.5% of 72 cases) were benign and 14 (19.5%) were malignant.
Non-neoplastic lesions
There were eight cases of non-neoplastic salivary gland masses. These included four cases of chronic sialadenitis, two retention cyst, and two cases of sialadenosis. Cyto-histologic correlation was established in 50% (two cases each of chronic sialadenitis and retention cyst). In the other four cases, FNAC was evaluated as inadequate for opinion [Table 1].
Benign neoplasms
Of the 58 cases of benign neoplastic lesion, cytologic material was inadequate in two cases. Among the rest 56 cases with evaluable cytologic features, 38 (67.8%) had concordant cytologic diagnosis and tumor characterization. In additional 10 cases, the cytology smears were suggestive of a benign tumor; though further characterization could not be done. Thus, a cyto-histologic correlation of benign neoplasm was established in 85.7% of the evaluable cases [Table 1].
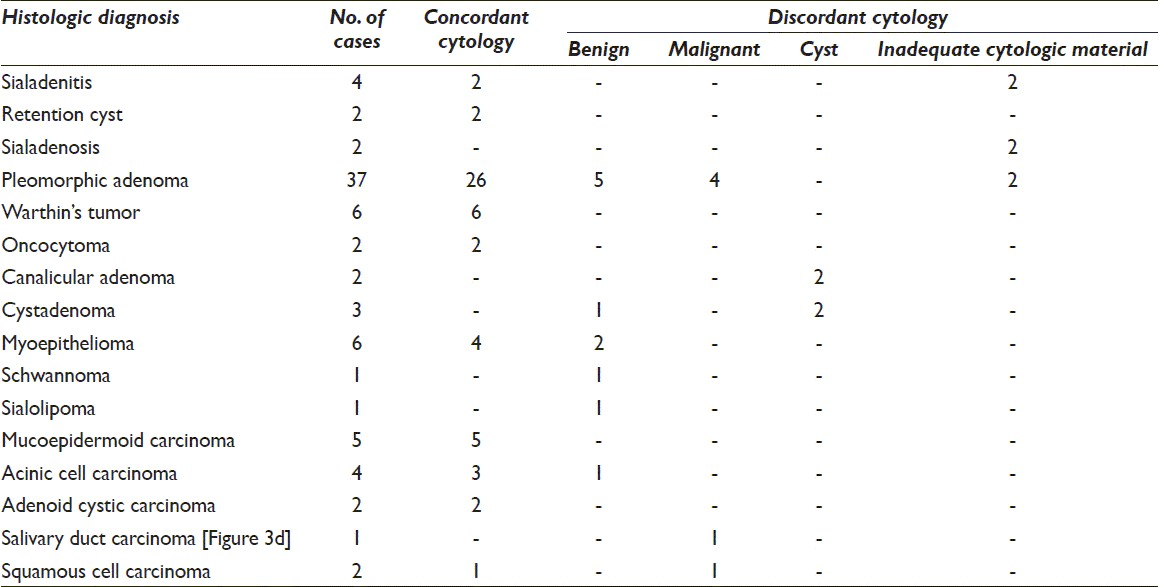
The remaining cases had discordance between the cytologic and histologic diagnoses. In these cases, the final diagnosis could be made only on histology. In four cases (two each of canalicular adenoma and cystadenoma), a cytologic diagnosis of cystic lesion was rendered and the final diagnosis was made on histologic material. Four cases of pleomorphic adenoma (PA) were misdiagnosed as malignant (adenoid cystic carcinoma [ACC]) on cytologic smears due to presence of hyaline globules and lack of characteristic chondromyxoid stroma [Figure 1].
- Photomicrograph from a case of pleomorphic adenoma (PA) diagnosed as adenoid cystic carcinoma on cytology due to hyaline globules seen in inset (a: Giemsa, ×200) while histology showed features of PA (b: H and E, ×100)
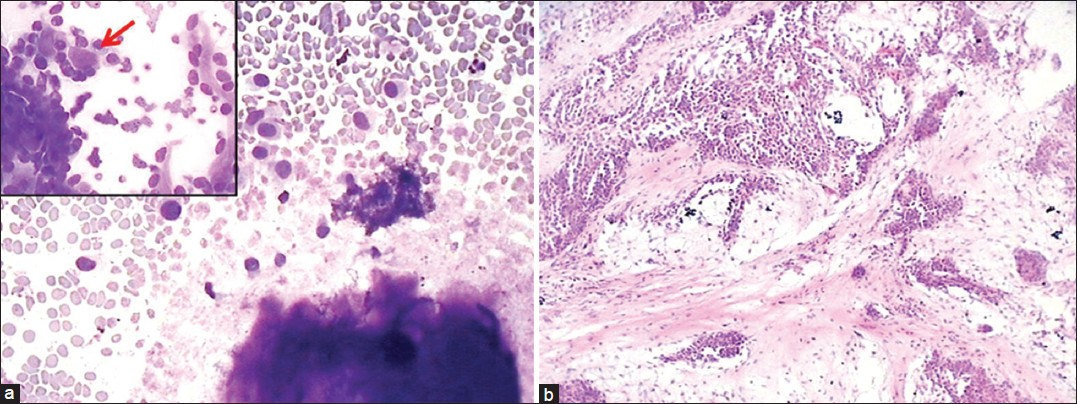
Of the 10 cases with cytologic impression of benign neoplasm, five cases was confirmed as PA on histology (cytology showed only stromal material without epithelial cells); two tumors were diagnosed as myoepithelioma (cytology reported as PA due to presence of chondromyxoid material [Figure 2]); one case of cystadenoma (cytology interpreted as Warthin's tumor due to presence of few epithelial cells, lymphocytes and fibrillary stroma) and one case each of schwannoma and sialolipoma.
- A case of myoepithelioma with abundant pink stromal material (a: Giemsa, ×100) and enmeshed myoepithelial cells (b: Giemsa, ×400) leading to misdiagnosis as pleomorphic adenoma. Histological section showing sheets of myoepithelial cells (c: H and E, ×400), and diffuse positivity for S-100 antigen (d: ×400) confirming the diagnosis of myoepithelioma
Malignant neoplasms
There were 14 cases of malignant salivary gland neoplasm in our study. A concordant cytologic diagnosis with accurate characterization was made in 10 (71.4%) cases. In three cases, a cytologic diagnosis of malignancy was made without further tumor typing. Thus, cyto-histologic concordance of malignant diagnosis was achieved in 92.8% of the cases [Table 1].
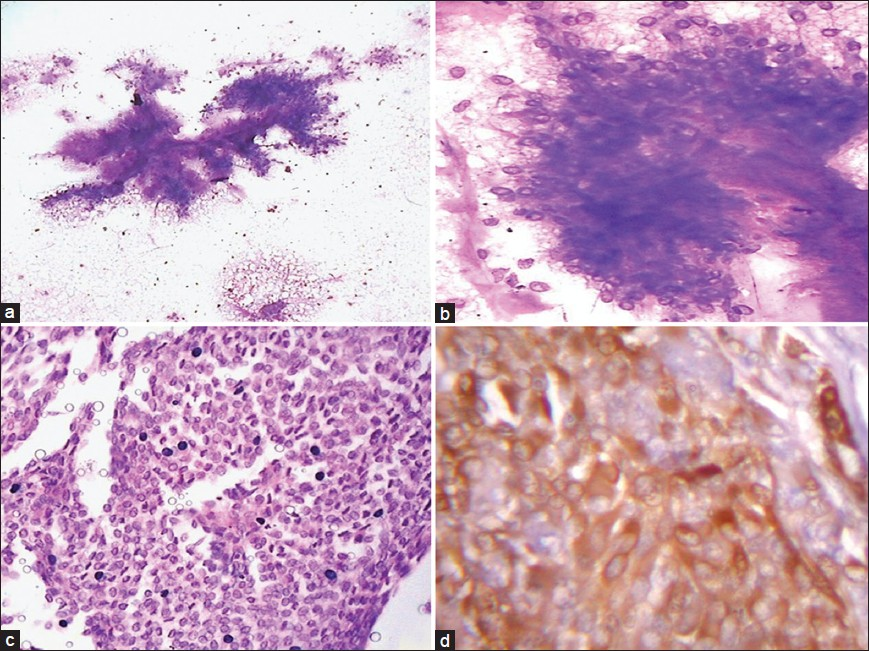
The discordant cases included one case of acinic cell carcinoma misdiagnosed as oncocytoma on cytology due to presence of oncocytes [Figure 3a and b] with few lymphoid cells and absence of characteristics acinic cells with finely vacuolated cytoplasm. One case of squamous cell carcinoma was interpreted as adenocarcinoma on aspiration cytology due to presence of sheets of cells with vacuolated cytoplasm and lack of evidence of keratinization [Figure 3c]. Histology provided the correct tumor characterization.
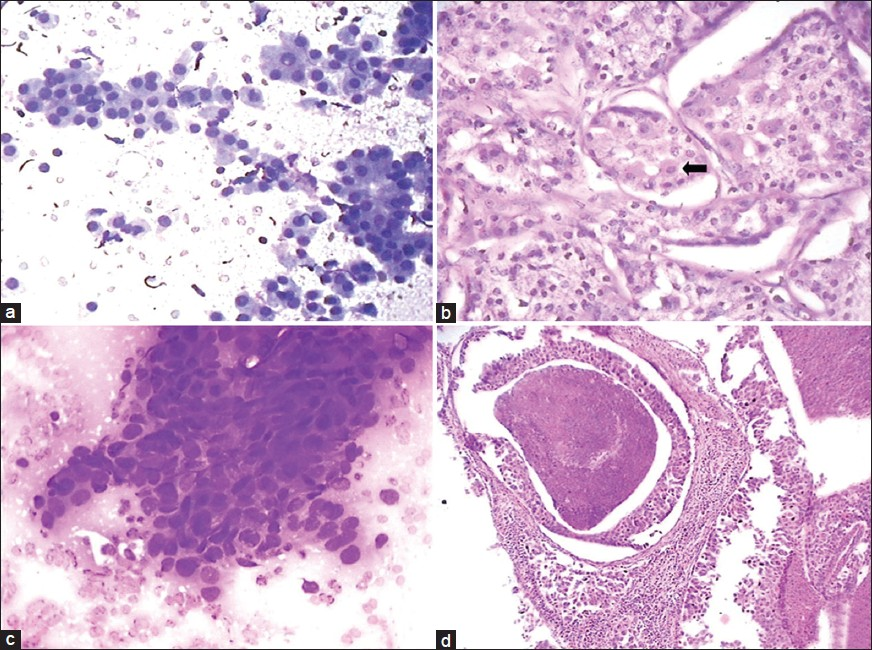
- Aspiration smear composed of oncocytic cells with dense granular cytoplasm (a: Giemsa, ×400) diagnosed as oncocytoma. Histology of the same case as (a) showing vacuolated acinar cells along with few oncocytic cells (b: Arrow, H and E, ×400) and final diagnosis of acinic cell carcinoma. A case of squamous cell carcinoma diagnosed as adenocarcinoma due to vacuolated cells and lack of keratinisation (c: Giemsa, ×400). Histology of a case of salivary duct carcinoma showing comedonecrosis (d: H and E, ×100)
DISCUSSION
FNAC is a widely used, safe and less traumatic diagnostic procedure capable of providing important information to the treating physician. In salivary gland masses, FNAC serves to determine the nature of the lesion (inflammatory/neoplastic – benign or malignant) and in some cases, the specific diagnosis. Though the management of almost all neoplastic salivary gland lesions is surgical excision, a pre-operative diagnosis of benign or malignant assists the clinician in planning the extent of surgery.[8] However, FNAC of salivary gland lesions often poses a diagnostic problem since various pathologic processes exhibit diverse and somewhat overlapping cytologic features. In doubtful cases, histologic examination of the resected specimen provides the accurate diagnosis.
Various studies in literature have reported a diagnostic accuracy of 86-98% for cytologic diagnosis of salivary gland neoplasms. The sensitivity has ranged from 62% to 97.6% and specificity from 94.3% to 100%.[2–7] In a recent study, the sensitivity of FNAC for diagnosis of malignant salivary gland lesions was 88.2% with extremely low cyto-histological type agreement rate of 30%.[5] Another study reported sensitivity for diagnosis of malignant lesions as 77.77%, specificity of 98.78% and positive predictive value of 93.33%.[9] In the present study, a high sensitivity of 92.8% was achieved for diagnosis of malignant lesions of salivary gland with a specificity of 93.9%. A cyto-histologic type correlation was seen in 64.2% of the malignant tumors, with a kappa value of 0.6 for cyto-histologic agreement.
The inadequate sampling rate in the present study was 7.5%, which is in concordance with the 5-10% inadequacy rate reported in literature.[5610] A recent study evaluated the utility of repeat FNAC in cytologic diagnosis of salivary gland tumors and found the sensitivity and specificity of repeat FNAC similar to the initial procedure.[11]
Non-neoplastic lesions constituted 10% of all salivary gland aspirates in our study and included sialadenitis, retention cyst, and sialadenosis. This proportion is in accordance with the existing literature (11-66%).[89] Two cases of sialadenitis revealed only normal salivary gland parenchyma. Such an aspirate can be judged as false-negative, since these features are also seen in pathologic condition like sialadenosis. However, the diagnosis of sialadenosis should be based on clinical and cytologic correlation with reasonable follow-up of the patient.[12]
In the present study, 80.5% of salivary gland tumors were benign and 19.5% were malignant. This is similar to previous reports.[589] Among benign neoplasms, PA was the most common (63.8%) followed by Warthin's tumor and myoepithelioma. A cyto-histologic agreement for diagnosis of PA was achieved in 70.2% of cases while the accuracy of benign neoplastic diagnosis was 83.8%. This is in agreement with the existing literature.[5813] A cytologic smear representative of PA includes three components: extracellular matrix, myoepithelial, and ductal cells in varying proportions and metachromatic chondromyxoid stroma.[14] However, considerable variation of the cellular composition of PA raises diagnostic difficulty especially in FNAC. A cellular PA, on FNAC, needs to be differentiated from monomorphic adenoma, myoepithelioma, and ACC.[815] Cases with prominence of plasmacytoid myoepithelial cells may be mistaken for malignant lymphoma or plasmacytoma. Rare cases may display nuclear atypia and need to be differentiated from carcinoma ex PA.[14] In addition, secondary changes like fibrosis, hyalinization, cystic degeneration may lead to sampling error. In our study, two cases of PA showed cystic change in histology where the cytologic material was interpreted as inadequate. In four cases, presence of hyaline globules and lack of chondromyxoid stroma led to a cytologic diagnosis of ACC with a differential diagnosis of PA. However, histology showed these tumors to be PA. Hyaline globules, seen most commonly in ACC, are also reported in basal cell adenoma, epithelial myoepithelial carcinoma and occasionally PA.[8] Previous cytologic reports have emphasized the sharp smooth borders of hyaline globules in ACC.[16] In our cases, the hyaline globules showed sharp smooth borders. However, some of the hyaline globules also showed blending of epithelial cells, a feature that assists in diagnosis of PA. The diagnosis of Warthin's tumor requires the presence of large polygonal oncocytes with abundant granular cytoplasm and lymphocytes/lymphoid stroma in a fluid background. Rarely, metaplastic squamous cells and sebaceous cells may be seen. The differential diagnoses include oncocytoma, squamous cell carcinoma, mucoepidermoid carcinoma (in presence of atypical metaplastic squamous cells) and acinic cell carcinoma.[14] Oncocytoma shows sheets of oncocytes with small regular nuclei and absence of fluid, debris or lymphoid cells.[17] Myoepithelioma, a tumor composed of myoepithelial cells and devoid of duct-like structures, can be differentiated from PA by the absence of chondromyxoid stroma in myoepithelioma, though stroma-poor cases of PA may be difficult to distinguish from myoepithelioma.[18] Immunocytochemistry in myoepithelioma shows positivity for myoepithelial markers (calponin, S-100 protein, glial fibrillary acidic protein [GFAP]) while epithelial antigens like cytokeratin 7 and 8/18 are negative. The latter are positive in glandular cells of PA.[19]
Among the benign tumors, two mesenchymal tumors were also included. In both these cases, FNAC smears were interpreted as benign neoplasm. One case showed a lesion composed of fragments of spindle cells without nuclear pleomorphism, or mitoses and a diagnosis of spindle cell neoplasm, possibly benign was rendered. Histology of excised specimen showed features of an intraparotid schwannoma. The other case showed adipose tissue fragments along with few salivary gland acini and a cytologic diagnosis of lipoma was made. Histologic examination showed an intimate mixture of adipose tissue and salivary gland parenchyma leading to a final diagnosis of sialolipoma. The difficulty in cytologic diagnosis of these uncommon lesions is documented in literature.[2021]
Among the malignant salivary gland neoplasms, mucoepidermoid carcinoma shows mucus producing and intermediate cells in a dirty mucoid background with varying degree of atypia depending on the grade of the tumor.[22] The differential diagnoses include squamous cell carcinoma, both primary as well as metastatic or contiguous involvement from cutaneous or intra-oral location. The distinction from metastatic carcinoma requires clinical and imaging data suggesting the involvement of intraparotid or submandibular lymph node.[14] Acinic cell carcinoma, on cytology, shows acinic cells with vacuolated cytoplasm and anisonucleosis and bare nuclei in the background.[23] Various studies have reported the characteristic cytologic features of ACC as tight clusters of hyperchromatic epithelial cells with hyaline globules having a smooth sharp border.[24]
The rate of false-negative diagnosis on cytology reported in literature ranges from 0% to 37%.[9] The false-negative rate in our study was 7.1%, due to one case of acinic cell carcinoma misdiagnosed as oncocytoma on FNAC. A review of the smear showed predominantly oncocyte-like cells with dense granular cytoplasm. The classical acinic cells with vacuolated cytoplasm were not seen in the smears leading to an erroneous diagnosis. The final diagnosis was rendered on histologic examination of the resected tumor. Acinic cell carcinoma, especially well-differentiated, shows cells closely resembling normal acinar cells. However, unlike normal salivary gland cells, there is usually no representation of ductal epithelial cells or adipose tissue fragments. Furthermore, the cell clusters in carcinoma are larger and more irregular than normal acini.[14] Immunocytochemistry (on smears or cell blocks) helps in differentiating acinic cell carcinoma from other salivary gland neoplasms. Absence of myoepithelial antigens differentiates this tumor from PA and myoepithelioma. Other tumors like mucoepidermoid carcinoma can be differentiated on the basis of cytologic features like mucin-secreting, intermediate and keratinizing cells.
The false-positive rate has been reported to be low with greater accuracy and less sampling error in FNAC. This rate ranges from 0% to 10% in the published literature.[1] In our series, the false-positive rate was 6.06%, and this was due to four cases of PA being diagnosed as ACC on FNAC. These cases showed presence of hyaline globules in the cytologic smears with only occasional globule demonstrating blending with epithelial cells. The cytologic smears were interpreted as ACC while histologic examination showed features of PA.
FNAC as a screening modality for malignancy
Considering histologic diagnosis as the gold standard, the sensitivity of FNAC for diagnosis of malignant lesions was 92.8% in our study. The specificity of malignant diagnosis was 93.9%. The positive predictive value of FNAC for malignant diagnosis was 81.2% and negative predictive value was 98.4% [Table 2]. Further statistical analysis showed a good cyto-histologic agreement with a kappa value of 0.6. These results denote that FNAC can serve as a good pre-operative screening modality for malignant salivary gland lesions. In the study by Mihashi et al., the sensitivity of FNAC for diagnosis of malignant tumors was 88.2% with a low histological type agreement rate of 30%.[5] A pre-operative diagnosis of malignant lesion allows the surgeon and the patient to discuss and plan better for further course of action, while a benign diagnosis spares the patient of several days of anxiety for a surgical biopsy diagnosis.[25]

A recently published study analyzed cost-efficiency of FNAC in work-up of parotid and submandibular gland masses. This study showed an approximate 33% savings over surgical management due to cytologic diagnoses such as chronic radiation-induced sialadenitis and intraparotid lymph node resulting in non-surgical management.[26] We did not perform cost analysis in our study, since the health-care costs are borne by the state by virtue of working in a government hospital. However, even in our setting, FNAC appears to be a cost-effective initial diagnostic modality for salivary gland masses.
CONCLUSION
FNAC is a highly reliable technique for pre-operative diagnosis of salivary gland tumors in hands of experienced pathologists. An accurate cytologic diagnosis can avoid unwarranted surgery. Due to the minimally invasive nature of this technique, FNAC offers valuable information for planning of subsequent therapeutic management. However, there still remain few cases that may be inaccurately diagnosed on cytology due to overlapping features and in these cases histopathology is the only modality for final diagnosis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no conflict of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
-
RJ collected cases, performed FNACs, assisted in sign-outs of cases, reviewed the literature and wrote the manuscript.
-
RG helped in literature review and writing of the manuscript and critically reviewed the manuscript.
-
MK and SS reviewed the cytology and histology slides and critically reviewed the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
The authors declare that institutional ethics committee approval was sought for the study.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/5/109547
REFERENCES
- Diagnostic difficulties in the interpretation of fine needle aspirate samples in salivary lesions: Diagnostic pitfalls revisited. Acta Cytol. 2009;53:53-70.
- [Google Scholar]
- Fine needle aspiration cytology of salivary gland lesions: Advantages and pitfalls. Cytopathology. 1990;1:269-75.
- [Google Scholar]
- Fine needle aspiration biopsy cytology of major salivary glands. Acta Cytol. 1985;29:503-12.
- [Google Scholar]
- Comparison of preoperative fine-needle aspiration cytology diagnosis and histopathological diagnosis of salivary gland tumors. Kurume Med J. 2006;53:23-7.
- [Google Scholar]
- Fine needle aspiration cytology of salivary gland lesions. J Oral Pathol Med. 1994;23:256-61.
- [Google Scholar]
- Study of salivary gland lesions with fine needle aspiration cytology and histopothology along with immunohistochemistry. J Indian Med Assoc. 2010;108:833-6.
- [Google Scholar]
- Fine-needle aspiration cytology of salivary glands: Diagnostic pitfalls – Revisited. Diagn Cytopathol. 2006;34:580-4.
- [Google Scholar]
- Diagnostic reliability of FNAC for salivary gland swellings: A comparative study. Diagn Cytopathol. 2010;38:499-504.
- [Google Scholar]
- Fine needle aspiration cytology of salivary gland. J Jpn Soc Clin Cytol. 2004;43:155-60.
- [Google Scholar]
- Fine needle aspiration cytology (FNAC) of salivary gland tumours: Repeat aspiration provides further information in cases with an unclear initial cytological diagnosis. Br J Oral Maxillofac Surg. 2010;48:26-9.
- [Google Scholar]
- Fine-needle aspiration of normal tissue from enlarged salivary glands: Sialosis or missed target? Diagn Cytopathol. 1995;13:300-3.
- [Google Scholar]
- Role of fine needle aspiration cytology in diagnosis of pleomorphic adenomas. Cytopathology. 2002;13:121-7.
- [Google Scholar]
- Review of fine-needle aspiration cytology of salivary gland neoplasms, with emphasis on differential diagnosis. Am J Clin Pathol. 2002;118:S100-15.
- [Google Scholar]
- Adenoid cystic carcinoma of salivary gland: diagnosis and pitfalls. J Cytol. 2003;20:85-8.
- [Google Scholar]
- Sources of diagnostic error in the fine-needle aspiration diagnosis of Warthin's tumor and clues to a correct diagnosis. Diagn Cytopathol. 1997;17:230-4.
- [Google Scholar]
- Myoepithelioma of the parotid. Report of a case initially examined by fine needle aspiration biopsy. Acta Cytol. 1994;38:417-21.
- [Google Scholar]
- Fine-needle aspiration of spindle cell and mesenchymal lesions of the salivary glands. Diagn Cytopathol. 2000;23:253-9.
- [Google Scholar]
- Fine-needle sampling of salivary gland lesions. IV. Review of 50 cases of mucoepidermoid carcinoma with histologic correlation. Diagn Cytopathol. 1997;17:92-8.
- [Google Scholar]
- Fine-needle sample of salivary gland lesions. V: Cytology of 22 cases of acinic cell carcinoma with histologic correlation. Diagn Cytopathol. 1997;17:347-52.
- [Google Scholar]
- Fine-needle sampling of salivary gland lesions. III. Cytologic and histologic correlation of 75 cases of adenoid cystic carcinoma: Review and experience at the Institut Curie with emphasis on cytologic pitfalls. Diagn Cytopathol. 1997;17:36-41.
- [Google Scholar]
- Value of fine needle aspiration biopsy of salivary gland masses in clinical decision-making. Am J Surg. 1992;164:667-70.
- [Google Scholar]
- Cost efficiency analysis for fine-needle aspiration in the workup of parotid and submandibular gland nodules. Diagn Cytopathol. 2006;34:734-8.
- [Google Scholar]








