Translate this page into:
Cytologic diagnosis and differential diagnosis of histiocytic signet ring cells in effusion specimens

*Corresponding author: Qing Kay Li, MD PhD Department of Pathology, The Johns Hopkins Hospital, Baltimore, United States. qli23@jhmi.edu
-
Received: ,
Accepted: ,
How to cite this article: Elahi M, Lam H, Adams C, Li QK. Cytologic diagnosis and differential diagnosis of histiocytic signet ring cells in effusion specimens. CytoJournal. 2024;21:30. doi: 10.25259/Cytojournal_14_2024
Abstract
Objective:
Benign histiocytic proliferation in effusion specimens can be found in a variety of diseases such as rheumatoid arthritis, systemic lupus erythematosus, microorganism infections, trauma, reactive eosinophilic pleuritis, and others. In addition, nodular histiocytic/mesothelial hyperplasia is another well-recognized rare cause. The previous studies have shown that proliferative histiocytes have raisinoid nuclei and abundant eosinophilic granular cytoplasm and can be confused with malignant lesions, especially metastatic carcinomas. In this study, we evaluated the cytomorphology of benign histiocytes, discussed the diagnosis and differential diagnosis, and the clinical significance of histiocytic signet ring cells in effusion cytology.
Material and Methods:
Seven hundred and fifty-five benign effusion cases (433 pleural effusions and 322 abdominal fluids) were found over 1 year. Among benign cases, 35 cases (28 pleural effusions and seven abdominal fluids) were included with findings of dominantly histiocytic signet ring cell morphology as well as immunohistochemical (IHC) stains. The clinical findings were also correlated.
Results:
In contrast to the well-documented cytomorphology of raisinoid nuclei and eosinophilic cytoplasm of proliferative histiocytes in previous studies, we find that these cells predominately presented as signet ring cell morphology with clear cytoplasm. The most characteristic findings of benign histiocytes in pleural effusions are: (1) cells are arranged in sheets and/or scattered individual cells, but no two- or three-dimensional cell clusters; (2) cells are intermediate in size and with normal N/C ratio; (3) cells have eccentric located nuclei and abundant clear cytoplasm, giving signet ring cell appearance; (4) nuclei have fine granular chromatin pattern, no hyperchromia or coarse chromatin pattern, no nuclear atypia; and (5) immunohistochemical (IHC) stains demonstrate a strongly positivity for macrophage-histiocyte lineage marker CD68, but negativity for epithelial markers and mesothelial markers. Clinically, these patients do not demonstrate nodularity or lesions in the mesothelial lining of serous cavities.
Conclusion:
Our study provides a detailed characterization of benign histiocytic signet ring cells in effusion cytology. The differential diagnosis of histiocytic signet ring cells is broad. The most important differential diagnoses are metastatic adenocarcinoma and epithelioid signet ring cell mesothelioma. The accurate diagnosis is critical for the appropriate clinical management of the patient. Cytopathologists should be aware of the diagnostic pitfalls of benign histiocytic signet ring cells in effusion samples in daily practice.
Keywords
Histiocytic signet ring cells
Benign histiocytic proliferation in effusion specimen
Differential diagnosis of signet ring cell carcinoma in effusion
Immunohistochemical stain of histiocyte
INTRODUCTION
A variety of malignant tumors and benign diseases can involve the pleural and/or peritoneal cavity, causing the development of effusions. In the United States, malignant pleural effusion alone affects more than 150,000 patients each year.[1-3] In cancer patients, the development of pleural and/or peritoneal effusion indicates an advanced stage of the tumor and is associated with a poor prognosis. However, pleural and peritoneal effusions can also be found in many benign conditions, such as microorganism infections, rheumatoid arthritis, systemic lupus erythematosus, macrophage activation syndrome, nodular histiocytic/mesothelial hyperplasia (NHMH), and others.[2,4-7] There are certain overlapping cytomorphology between malignant and benign effusions such as the finding of so-called signet ring cells.[5,7-10] These cells have characteristic cytomorphologic features of eccentrically located nuclei and abundant cytoplasmic mucinous material. The signet ring cells can be found in both benign reactive cellular changes and malignant diseases.[11-14] Therefore, the separation of benign proliferative histiocytes from malignant signet ring cells in effusion specimens plays a critical role in the clinical management of patients.
Studies have shown that benign proliferative histiocytes can be confused with metastatic carcinomas.[5,7,10-12] In benign conditions such as NHMH, histiocytes demonstrate eccentric located raisinoid nuclei and abundant eosinophilic cytoplasm, giving the impression of signet ring cells.[5-8] These cells can be misdiagnosed as metastatic carcinomas, especially in patients with a history of malignancy. The most common metastatic carcinomas with signet ring cell features are gastric carcinomas in both men and women and breast carcinomas in women.[1,2,5] In addition, metastatic lung, pancreaticobiliary, colorectal, genitourinary tract, and gynecologic tract carcinomas as well as melanoma can also demonstrate signet ring cell morphology.[1] Signet ring cells mesothelioma also reveals predominately malignant signet ring cells.[8,13,14]
In this study, we have included a series of effusion cases with the finding of signet ring cells and characterized benign and malignant signet ring cell morphology. Ancillary studies of immunocytochemistry (IHC) are also included in the assessment and discussion of differential diagnosis. The purpose of our study is to discuss the diagnosis, differential diagnosis, and clinical significance of histiocytic signet ring cells in effusion cytology, particularly in a patient with a benign pleural effusion.
MATERIAL AND METHODS
The files of the cytopathology were searched for effusion specimens, including both benign pleural effusions and abdominal fluids over 1 year. A total of 755 cases were found, including 433 pleural effusions and 322 abdominal fluids, respectively. Among benign effusion cases, 35 cases, including 28 pleural effusions and seven abdominal fluids with findings of dominantly histiocytic signet ring cell morphology as well as immunocytochemical (IHC) stains were included in the study. All effusions were processed at the cytopathology laboratory. Briefly, two cytospin slides and a cell block were prepared from each effusion specimen. The cytospin slides were fixed in 95% ethanol and stained by the Papanicolaou method. Cell blocks were fixed in formalin, embedded in paraffin, and processed in the histology laboratory according to standard protocols. The 5 µm section of cell blocks was cut and stained with hematoxylin and eosin method. The cytospin slides and corresponding cell block slides were reviewed.
For IHC studies, cell blocks were cut at 5 µm thickness and deparaffinized before incubation with primary antibodies. Heat antigen retrieval at 70°C for 40 min was used to enhance the signal detection. The IHC was performed at a clinical IHC laboratory using an autostainer. The detailed information on primary antibodies is summarized in Table 1. Briefly, primary antibodies were incubated with unstained cell block slides based on the manufacturer’s suggestions. After washings, secondary antibodies conjugated with a detection agent were applied to detect and visualize the specific antigen-antibody complexes. Both positive and negative controls were included in procedures to ensure an appropriate quality control.
| Antibody | Clone | Dilution | Source | Catalog number |
|---|---|---|---|---|
| CK7 | Mouse, mAb, OV-TL | 1:500 | Dako | M7018 |
| CK20 | Mouse, mAb, Ks20.8 | Pre-diluted | DaKo | GA777 |
| Calretinin | Rabbit, mAb, SP13 | Pre-diluted | Cell Marque | 232R |
| Claudin-4 | Mouse, mAb, 3E2C1 | 1:250 | Invitrogen | 32–9400 |
| CD68 | Mouse, mAb, KP-1 | Pre-diluted | Ventana | 790–2931 |
| GATA3 | Mouse, mAb, L50–823 | 1:100 | Biocare | CM405B |
| PAX8 | Rabbit, pAb | 1:800 | Invitrogen | PA1–112 |
| P40 | Mouse, mAb, BC28 | 1:100 | Biocare | P40(M) |
| Napsin-A | Mouse, mAb, IP64 | 1:800 | Leica | NCL-L-Napsin A |
| TTF-1 | Mouse, mAb, 8G7G3/1 | Pre-diluted | Ventana | 790–4398 |
TTF-1: Thyroid transcription factor-1, mAb: monoclonal antibody, pAb: polyclonal antibody.
The study and the review of cytopathology material were approved by the Institutional Review Board.
RESULTS
Clinical information
Among 35 benign effusion cases, patients’ age ranged from 36 to 75 years with a median age of 64 years. The female-to-male ratio was 1:0.63. Fourteen of 35 patients had a malignant history, three patients had liver diseases, 12 patients had cardiovascular diseases, four patients had chronic kidney diseases, one patient had endometriosis, and one patient had ulcerative colitis, respectively. In our study, all 35 patients did not demonstrate nodularity or lesions in the mesothelial lining of serous cavities by image studies.
Cytological findings
In benign effusion specimens, the cytological examination revealed sheets and scattered epithelioid cells in a background of blood and mixed inflammatory cells, but no three-dimensional groups were seen [Figure 1]. These histiocytes had epithelioid appearances with eccentrically located nuclei [Figure 1a]. The N/C ratio of cells was in the normal range. The chromatin pattern was finely granular and evenly distributed. No prominent nucleoli were identified. The cytoplasm was with a clear appearance [Figure 1b]. The most characteristic findings of proliferative histiocytes in effusions are as follows: (1) cells are arranged in sheets and/or scattered individual cells, but no two- or three-dimensional cell clusters; (2) cells are intermediate in size and with normal N/C ratio; (3) cells have eccentric located nuclei and abundant clear cytoplasm, giving signet ring cell features; and (4) nuclei have fine granular chromatin pattern, no hyperchromia or coarse chromatin pattern, no nuclear atypia. The immuno profile of these cells was descripted in immunohistochemical studies [Figure 1c to 1e].
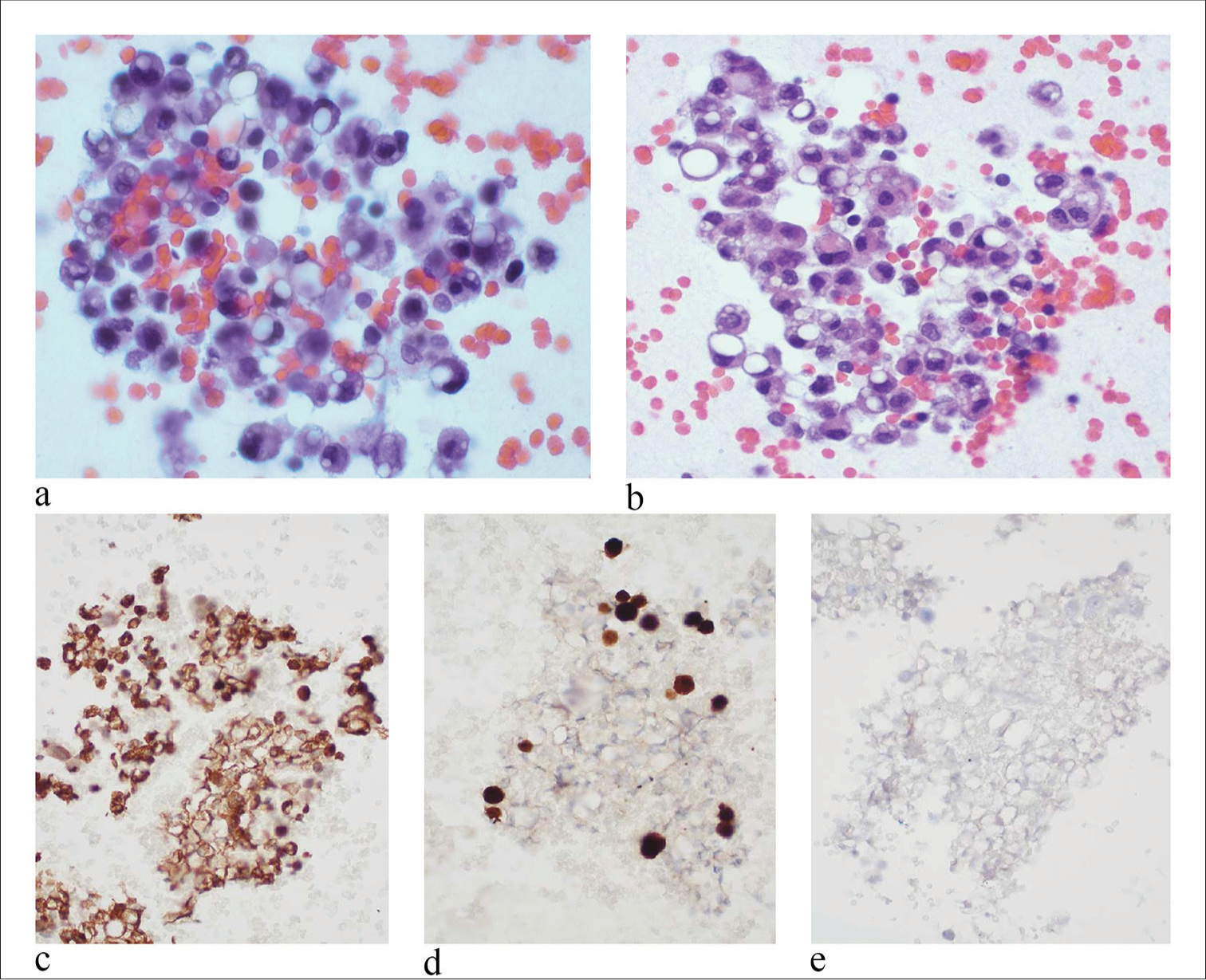
- Pleural effusion specimen with sheets of benign histiocytic signet ring cells. (a) Proliferative histiocytes were arranged in sheets and scattered individual cells in a background of blood and mixed inflammatory cells. No three-dimensional groups were identified (papanicolaou stain, 20×) (b) Cells had an epithelial appearance with eccentrically located nuclei and clear cytoplasm, giving a signet ring cell appearance. The N/C ratio of cells was in the normal range. No prominent nucleoli were identified. (hematoxylin and eosin stain, 20×). (c) IHC stain of CD68 is diffusely positive. (20×) (d) IHC stain of calretinin highlights a few mesothelial cells (20×) (e) IHC stain of claudin-4 is negative (20×), (N/C: nuclear/cytoplasmic, IHC: immunohistochemical.)
For the differential diagnostic purpose, we also included a description of epithelioid signet ring cell mesothelioma [Figure 2]. In epithelioid signet ring cell mesothelioma, tumor cells were arranged as numerous three-dimensional clusters [Figure 2a]. However, no true papillary fragments with fibrovascular cores were identified [Figure 2b]. Tumor cells had an epithelial appearance and distinct cell border. The N/C ratio of tumor cells was high. The nuclei were centrically located with irregular nuclear membranes. In summary, the epithelioid signet ring cell mesothelioma has characteristic single or several prominent nucleoli, hyperchromatic nuclei with coarse granular pattern, and two-tone appearance of cytoplasm. The immuno profile of these cells are descripted in immunohistochemical studies [Figure 2c to 2e].
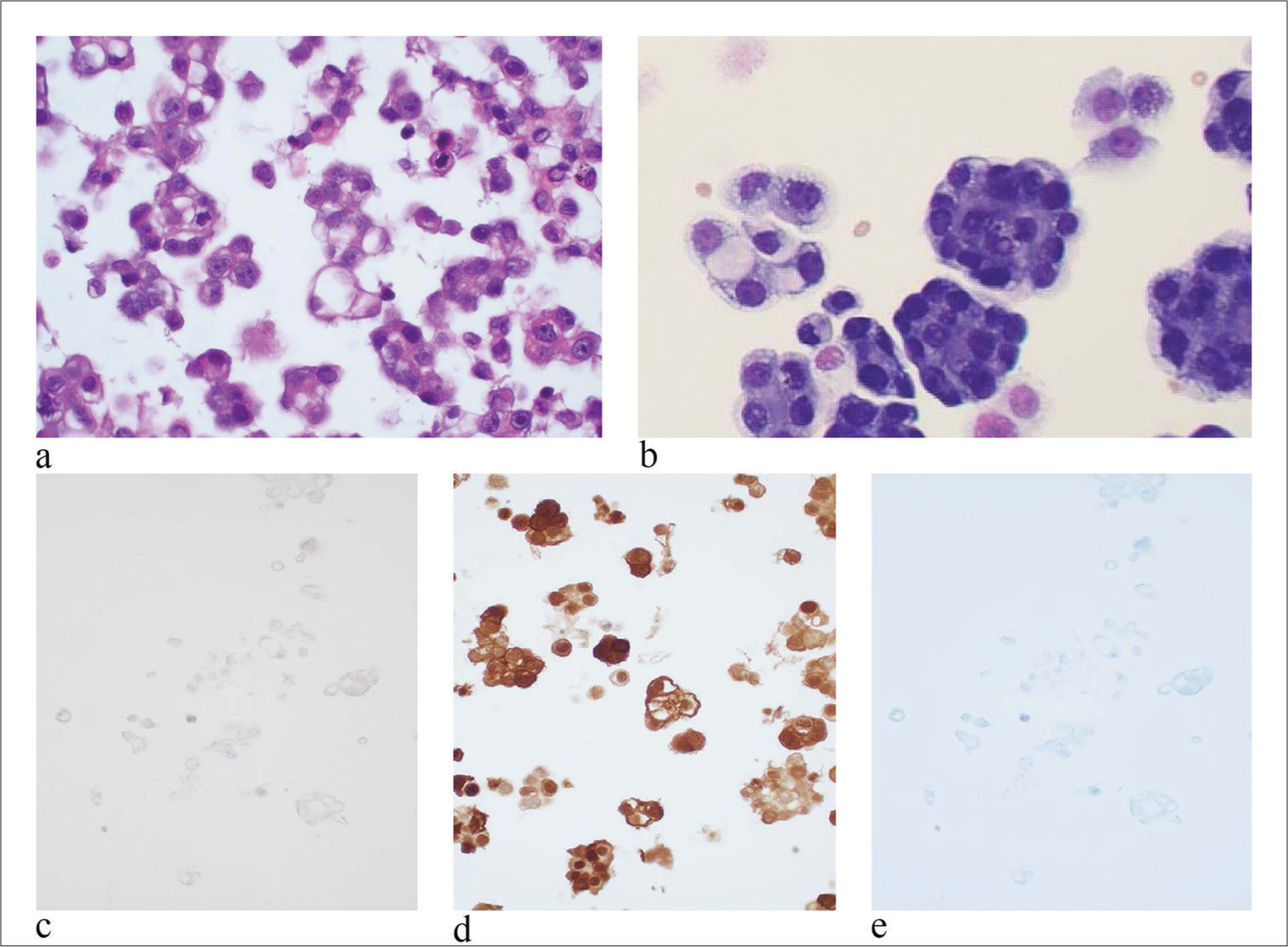
- Epithelioid signet ring cell mesothelioma. (a) Tumor cells were arranged as numerous three-dimensional clusters. However, no true papillary fragments with fibrovascular cores were identified. (hematoxylin and eosin stain, 20×). (b) Tumor cells have an epithelial appearance and distinct cell borders. The nuclei are eccentrically located with irregular nuclear membranes. Single or several prominent nucleoli were seen. The cytoplasm of the tumor cells is vacuolated and has two-tone appearance. The N/C ratio of tumo r cells is high (papanicolaou stain, 40×). (c) IHC stain of CD68 is negative. 20× magnification. (d) IHC stain of calretinin is diffusely positive for mesothelial cells (20×). (e) IHC stain of claudin-4 is negative (20×), (N/C: nuclear/cytoplasmic, IHC: immunohistochemical.)
In malignant effusion of metastatic carcinomas, tumor cells were arranged predominantly in three-dimensional clusters [Figure 3a and 3b]. The tumor could show sheet growth patterns with numerous individual cells [Figure 3c]. Tumor cells had a high N/C ratio, large hyperchromatic nuclei with irregular nuclear membranes, coarse granular chromatin, and single or multiple prominent nucleoli. Mitosis was frequent. Depending on the primary site of the tumor, the metastasis could reveal certain distinctive features. For example, a metastatic colorectal and pancreatic adenocarcinoma (ADC) had a “dirty” background of slides due to the presence of mucinous material and necrotic tumor debris. In a metastatic breast carcinoma, tumor cells could form two-dimensional sheets or arrange as individual cells. They might have eccentrically located nuclei with cytoplasmic mucin, giving a signet ring cell morphology [Figure 3c]. In the metastatic carcinoma of the gynecologic primary, the present of psammoma bodies could be seen [Figure 3d]. In summary, depending on the primary site of the tumor, a metastatic carcinoma can demonstrate certain distinctive cytomorphology, such as the formation of papillary clusters as seen in thyroid, lung, gynecologic, genitourinary, and gastrointestinal metastatic carcinomas, accumulation of abundant mucin in the cytoplasm as seen in gastrointestinal metastatic carcinomas with the positive stain of mucicarmine in the cytoplasm.
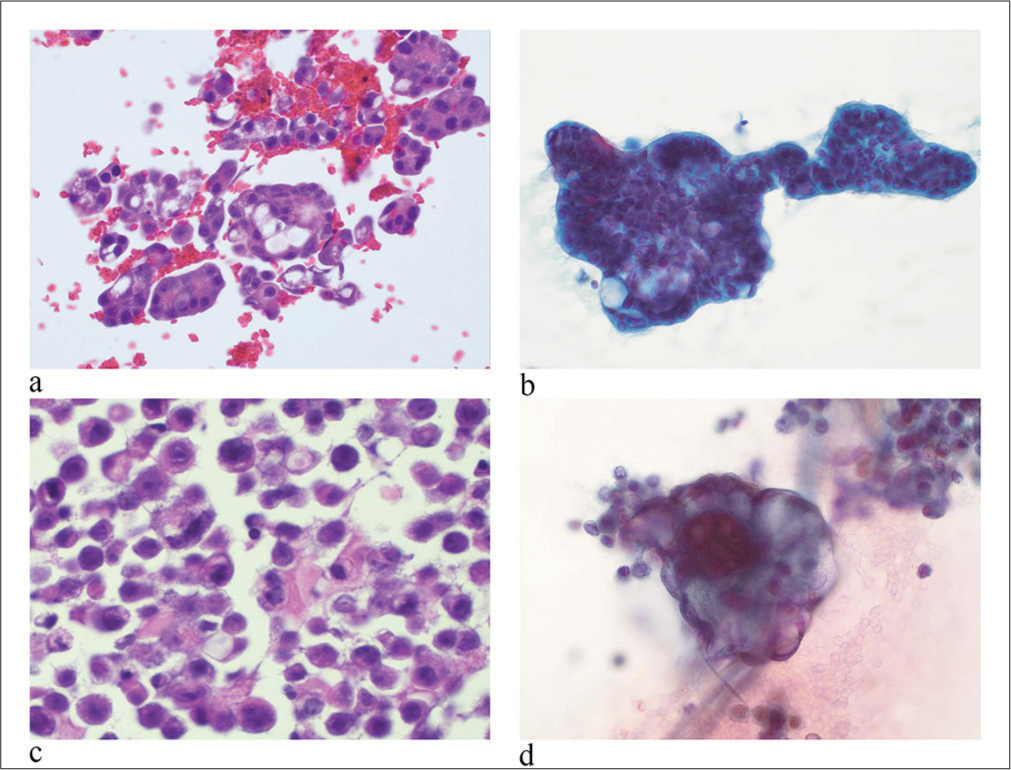
- Malignant pleural effusion of metastatic carcinomas. (a) Tumor cells are arranged pre dominantly in three-dimensional clusters. Tumor cells have large hyperchromatic nuclei, irregular nuclear membranes, fine to coarse chromatin, and single or multiple prominent nucleoli. (hematoxylin and eosin stain, 20×) (b) A metastatic pancreatic adenocarcinoma contains cytoplasmic mucinous material. (papanicolaou stain, 20×). (c) A metastatic breast carcinoma contains discohesive individual tumor cells. Tumor cells have eccentrically located nuclei and cytoplasmic mucin, giving the signet ring cell morphology. (hematoxylin and eosin stain at 20×). (d) A metastatic carcinoma of the gynecologic primary. Tumor cells contain psammoma bodies. (papanicolaou stain at 40×).
Immunohistochemical (IHC) studies
IHC studies were performed on cell blocks. In benign effusions, the epithelioid histiocytes were diffusely positive for macrophage-histiocyte lineage marker CD68 [Figure 1c], but were negative for mesothelial cell marker (calretinin, [Figure 1d]) and epithelial membrane antigen (EMA) (claudin 4, [Figure 1e]). In malignant epithelial signet ring cell mesothelioma, tumor cells were diffusely positive for calretinin [Figure 2d], but negative for CD68 [Figure 2c] and claudin 4 [Figure 2e]. In metastatic carcinomas, tumor cells were positive for claudin 4, but negative for CD68 and calretinin. Depending on the site of the primary tumor, metastasis may express characteristic tumor-site markers, such as positive for thyroid transcription factor-1 (TTF-1) and napsin A in the lung primary, positive for P40 in squamous cell carcinoma (SqCC), positive for estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (Her2) in the breast primary, positive for CK20 and CDX2 in the gastrointestinal tract primary, and positive for PAX8 and GATA3 in the genitourinary tract primary. The differential diagnosis of tumor site-related IHC markers is summarized in Tables 2 and 3.
| Primary carcinoma | Selective IHC markers |
|---|---|
| Lung | TTF-1, Napsin A |
| Breast | GATA3, ER, PR, Her2 |
| Esophageal ADC | CK7, CK20, CDX2 |
| Stomach ADC | CK7, CK20, CDX2 |
| Colorectal ADC | CK20, CDX2, SATB2 |
| Pancreatic ADC | CK7, CK20, CDX2, DPC4 |
| Gynecologic tract | PAX8, P16, WT-1, ER |
| Prostate | PSA, NKX3 |
| Kidney | CK7, CAXI |
| Mesothelioma | Calretinin, D2–40, Mesothelin, WT-1, BAP1 |
ADC: adenocarcinoma, TTF-1: Thyroid transcription factor-1, ER: Estrogen receptor, PR: Progesterone receptor, Her2: Human epidermal growth factor receptor 2, PSA: prostate specific antigen, CAXI: Carbonic anhydrase IX, BAP1: BRCA1 associated protein-1, WT-1: Wilms tumor protein-1, NKX3: NK3 transcription factor homolog A, DPC4: SMAD4 family protein 4, SATB2: Special AT-rich sequence-binding protein 2, CDX2: Caudal-related homeobox gene protein 2, GATA: GATA binding protein 3, PAX8: Paired box gene protein 8.
| Cytology features | Benign histiocytes | Metastatic signet ring cell carcinoma | Epithelioid signet ring cell mesothelioma |
|---|---|---|---|
| Cellular arrangement | Sheets and scattered individual cells | Three-and two-dimensional clusters | Three dimensional clusters |
| Size of nuclei | Small, similar to mature lymphocyte Eccentrically located |
Enlarged, at least 3-–5-fold of mature lymphocytes Eccentrically located |
Enlarged, at least 3-–5-fold of mature lymphocytes Eccentrically located |
| Nuclear/cytoplasmic | In normal range | Increased | Increased |
| Nuclear pleomorphism | Absent | Present | Present |
| Nuclear membrane | Smooth | Irregular | Moderately to markedly irregular |
| Chromatin pattern | Fine granular | Coarse granular | Coarse granular |
| Nucleoli | Absent | Present | Present |
| Cytoplasm | Clear | Vacuolated with mucin | Vacuolated with mucin |
| Cytoplasmic membrane | Smooth | Smooth | Micro villi Intercellular window |
| Immunohistochemical markers | CD68 positive Claudin-4 negative Calretinin negative |
CD68 negative Claudin-4 positive Calretinin negative |
CD68 negative Claudin-4 negative Calretinin positive |
DISCUSSION
Benign histiocytic proliferation has been reported to occur in the pleura, pericardium, peritoneum, and hernia sac.[5-9] The previous studies have shown that proliferative histiocytes demonstrated raisinoid nuclei and abundant eosinophilic granular cytoplasm, especially in NHMH.[5-8,15] The benign proliferation of histiocytes is correlated with chronic irritation of the pleura.[6,16-18] In addition to these well-documented morphologies, we present the morphologic characteristics of reactive histiocytes identified in effusions. These cells show eccentrically located nuclei and clear cytoplasm, giving a signet ring cell appearance. The most important diagnostic features of benign reactive histiocytes are (1) sheets of cells and scattered individual cells, no two- or three-dimensional clusters; (2) normal fine granular chromatin pattern, no hyperchromia or coarse chromatin pattern; and (3) cell is intermediate in size, with a normal N/C ratio, and without nuclear atypia. Mitosis can be seen. Histiocytic signet ring cells should not be confused with metastatic carcinomas or signet ring cell mesothelioma. The strong positive stain of macrophage-histiocyte lineage marker CD68 and negative stains of epithelial marker claudin-4 and mesothelial marker calretinin confirm that signet ring cells in this lesion are histiocytes, rather than mesothelial cells or signet ring cell carcinoma. In our series, we did not find any nodularity or lesions in the mesothelial lining of serous cavities by image reports. About 40.0% (14 of 35) of patients had a history of malignancy, 34.3% (12 of 35) of patients had a history of cardiovascular diseases,11.4% (4 of 35) of patients had chronic kidney diseases, 8.6% (3 of 35) patients had chronic liver diseases, and 5.7% (2 of 35) of patients had other medical conditions. Finally, in our study, we only found one benign pleural effusion demonstrating raisinoid nuclei and abundant eosinophilic granular cytoplasm in histiocytes. In this case, we could not find any signification clinical history of the patient. Therefore, it is difficult to compare the clinical features of the case with the current series.
The main differential diagnosis of histiocytic signet ring cells is metastatic signet ring cell carcinoma and epithelioid signet ring cell mesothelioma [Table 3]. One of the important ways to identify malignancy is to search for a “second population” of cells that appear different than benign histiocytes and mesothelial cells; and the evaluation of the N/C ratio of cells.[1,5,7-9] Increased N/C ratio is an important feature of tumors in the differential diagnosis. It is always a good practice to review patients’ clinical history and previous pathology material and compare tumor cells with the patient’s known primary. In metastatic adenocarcinomas regardless of the primary sites, tumor cells are commonly arranged in three-dimensional clusters, papillary groups, and/or as discohesive individual tumor cells.[1,5,7-9] Depending on the primary site of the tumor, a metastatic carcinoma can demonstrate certain distinctive cytomorphology, such as the formation of papillary clusters as seen in thyroid, lung, gynecologic, genitourinary, and gastrointestinal metastatic carcinomas, accumulation of abundant mucin in the cytoplasm as seen in gastrointestinal metastatic carcinomas with the positive stain of mucicarmine in the cytoplasm.[1,11,18] In signet ring cell mesothelioma, tumor cells form three-dimensional clusters with signet ring cell features.[13,14] The awareness of the morphological and ancillary study pitfalls of signet ring cell mesothelioma is the key to avoiding diagnostic errors. These cells have overlapping features with adenocarcinomas, such as IHC stain positive for cytokeratin and special stain positive for mucicarmine. However, tumor cells are positive for calretinin and negative for claudin-4. Other mesothelial cell markers can be used in the differential diagnosis [Table 2].
For the differential diagnosis, other metastatic malignant tumors should also be considered. In metastatic SqCC, tumor cells can mimic a metastatic adenocarcinoma, since exfoliated squamous cells may be rounded up due to the surface tension in the effusion fluids.[1,18] Features that can help with the differential diagnosis of metastatic SqCC include that tumor cells predominantly form loosely cohesive two-dimensional clusters and have relatively dense cytoplasm and a sharply defined cytoplasmic border.[1] In a metastatic small cell carcinoma, the tumor cells are usually smaller in size, have scant cytoplasm, and reveal nuclear molding and crowding. The chromatin pattern demonstrates a fine granular (salt and pepper) appearance.[1] Mitosis and single-cell necrosis are also common findings. These tumor cells are fragile and easy to break down, giving an appearance of blue stripes in the background of the slide. In metastatic malignant melanoma, tumor cells demonstrate a variety of cytologic appearances ranging from epithelioid to large bizarre cells to atypical spindle cells.[3,13,14] Nuclei are highly variable in size with single or multiple prominent cherry-red nucleoli. The cytoplasm tends to be abundant and may or may not contain melanin pigment. The melanin pigments are black or dark brown in color on the Papanicolaou stain. Other characteristic features include plasmacytoid cells, binucleation, and intranuclear inclusions. Occasionally, certain types of epithelioid sarcomas may mimic metastatic signet ring cell carcinomas, causing diagnostic challenges, such as epithelioid angiosarcoma, epithelioid synovial sarcoma, pleomorphic liposarcoma, and epithelioid angiosarcoma.[1] Epithelioid synovial sarcoma is a tumor of children and young adults and needs to be differentiated from poorly differentiated carcinoma and epithelioid mesothelioma, both of later entities occur in elderly patients. Tumor cells of epithelioid synovial sarcoma are large in size with round to ovoid nuclei and scant cytoplasm. The nuclei are hyperchromatic with small nucleoli. In pleomorphic liposarcoma, tumor cells are markedly enlarged with single or multiple cytoplasmic fat vacuoles. In epithelioid angiosarcoma, tumor cells may resemble cytoplasmic lumen with entrapped red blood cells.
Immunocytochemistry is a critical tool in distinguishing malignant cells from benign histiocytes and mesothelial cell, and further classification of the origin of metastatic carcinoma.[1,3,18] Thus, the preparation of cell blocks should be routinely performed during the process of effusion samples. Furthermore, cell blocks can be used for several ancillary studies such as next-generation sequencing, fluorescence in situ hybridization (FISH), and other molecular analyses. These ancillary studies not only improve diagnostic accuracy but also identify therapeutic targets. Immunohistochemistry is particularly important in resolving equivocal cases. We summarize the most commonly used IHC markers for the differential diagnosis in Table 2. The cell block preparation of effusion samples usually contains scant material. In daily practice, a selective panel of IHC markers, including at least an epithelial marker and a mesothelial marker, as well as CD68, should be performed as an initial step in the differential diagnosis. In addition to CD68, CD163 is an alternative choice for the histiocytes. CD68 and CD163 have 30–100% and <10–100% immunoreactivity for macrophage/histiocytic lineage, respectively.[19]
Among IHC markers, commonly used epithelial markers are claudin-4, BerEP4, and B72.3. Claudin-4 is a tight junction-associated protein and is expressed in most epithelial cells, but has not been reported to express in mesothelial cells, and it has a 99% and 100% sensitivity and specificity for adenocarcinomas in this study.[20] BerEP4 is a cell membrane glycoprotein and is expressed in carcinomas. B72.3 is a tumor-associated glycoprotein and is expressed in most adenocarcinomas. However, both BerEP4 and B72.3 are also expressed in mesothelial cells with weak and patchy pattern.[1,3] CK7 is positive in mesothelioma and adenocarcinoma of the lung, breast, stomach, urinary bladder, and ovary. Thus, CK7 is a non-specific IHC marker for differentiating mesothelioma from carcinoma. GATA3 is a common marker used in the diagnosis of breast and urothelial carcinoma.[21] IHC markers of melanoma include SOX10, S-100, melanin A, and HMB45.[1] In epithelioid synovial sarcoma, immunostaining of cytokeratin, EMA, and CD99 (O13) are positive for tumor cells. Molecular analysis of t (X; 18) (p11.21; q11)-SYT/SSX1 fusion gene can be performed on the cell block and is detected in most of the tumors. In pleomorphic liposarcoma, the immunostain and FISH test of murine double-minute 2 (MDM2) may help with the diagnosis. In epithelioid angiosarcoma, immunostain of erythroblost transformation-specific (ETS)-related gene (ERG) and CD31 is positive in tumors.[1]
Commonly used mesothelial markers are calretinin, WT-1, D2–40 (podoplanin), and CK5/6.[1] Calretinin is a very useful mesothelial marker and is expressed in almost all epithelioid mesotheliomas.[22] However, it has across reactivity with a subset of adenocarcinomas such as breast and lung adenocarcinomas.[3,22] WT-1, CK5/6, and D2-40 are positive in most of mesothelioma.[3,18] However, these markers are also detected in carcinomas.[3] Recently, a tumor suppressor gene BRCA1-associated protein (BAP1) has been identified in mesothelioma. BAP1 is located at 3p21 and regulates the calcium-mediated apoptosis and the development of cancers.[23,24] Both germline and sporadic BAP1mutation can be found in mesotheliomas.[23,24] In mesotheliomas, the loss of nuclear staining of BAP1 has been reported in approximately 50% of tumors.[3,23,24] Therefore, the negative nuclear stain of BAP1 can be used for the differential diagnosis of mesothelioma.
Finally, there are certain limitations of our study such as a limited number of cases and lack of long-term follow-ups in patients with malignant histories. Future study is still necessary to characterize the molecular features of benign histiocytes in those patients.
SUMMARY
Our study presents a clinically useful cytomorphological characterization of benign histiocytic signet ring cells; and the differential diagnosis of these cells in effusion cytology. Benign histiocytic signet ring cells may have cytomorphologic features overlapping with metastatic carcinoma and primary signet ring cell mesothelioma. In contrast to the well-documented cytomorphology of raisinoid nuclei and eosinophilic cytoplasm, we find these cells predominately presented as signet ring cell with clear cytoplasm. The most characteristic findings of proliferative histiocytes in effusions are: (1) cells are arranged in sheets and/or scattered individual cells, but no two- or three-dimensional cell clusters; (2) cells are intermediate in size and with normal N/C ratio; (3) cells have eccentrically located nuclei and abundant clear cytoplasm, giving signet ring cell features; (4) nuclei have fine granular chromatin pattern, no hyperchromia or coarse chromatin pattern, no nuclear atypia; and (5) IHC stains demonstrate a strong positivity for macrophage-histiocyte lineage marker CD68, but negativity for epithelial markers and mesothelial markers. Clinically, these patients do not demonstrate nodularity or lesions in the mesothelial lining of serous cavities. The most important differential diagnoses are metastatic adenocarcinoma and epithelioid signet ring cell mesothelioma. The accurate diagnosis is critical for an appropriately clinical management of the patient. Cytopathologists should be aware of the diagnostic pitfalls of benign histiocytic signet ring cells in effusion.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
ABBREVIATIONS
ADC - Adenocarcinoma
BAP1 – BRCA1-associated protein 1
EMA - Epithelial membrane antigen
ER - Estrogen receptor
ERG - Erythroblost transformation-specific (ETS)-related gene
FISH - Fluorescence in situ hybridization
Her2 - Human epidermal growth factor receptor 2
IHC – Immunohistochemical
mAb - Monoclonal antibody
MDM2 - Murine double-minute 2
NHMH - Nodular histiocytic/mesothelial hyperplasia
N/C - Nuclear/cytoplasmic
pAb - Polyclonal antibody.
PR - Progesterone receptor,
SqCC - Squamous cell carcinoma
TTF-1 - Thyroid transcription factor-1
AUTHOR CONTRIBUTIONS
ME,HL: Literature search, data acquisition, clinical studies, manuscript drafting and review; CA: Data acquisition, clinical studies and manuscript review; QKL: Concepts, design, definition of intellectual content, literature search, and manuscript preparation, manuscript editing and review. All authors read and approved the final manuscript. Each author has participated sufficiently in the work and takes responsibility for appropriate portions of the content of this article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The work described was carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki). The work was also approved by the Institutional Research Board of the Johns Hopkins Hospital (IRB00262408). As it was a retrospective cytomorphologic study, the informed consents were obtained during the procedure of sample collection.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Cytology: Diagnostic principles and clinical correlates (4th ed). Philadelphia, PA: Elsevier; 2015.
- [Google Scholar]
- Pleural effusion: Diagnosis, treatment, and management. Open Access Emerg Med. 2012;4:31-52.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med. 2018;142:89-108.
- [CrossRef] [PubMed] [Google Scholar]
- KikuchiFujimoto disease as the initial manifestation of systemic lupus erythematosus complicated with macrophage activation syndrome: two case reports and a review of literature. BMC Pediatr. 2022;22:673.
- [CrossRef] [PubMed] [Google Scholar]
- Reactive histiocytic proliferation in the pleural fluid mimicking metastatic signet ring adenocarcinoma. Diagn Cytopathol. 2018;46:525-7.
- [CrossRef] [PubMed] [Google Scholar]
- Lesions described as nodular mesothelial hyperplasia are primarily composed of histiocytes. Am J Surg Pathol. 1998;22:285-92.
- [CrossRef] [PubMed] [Google Scholar]
- Nodular histiocytic/mesothelial hyperplasia mimicking mesenteric metastasis. Cureus. 2022;14:e24971.
- [CrossRef] [Google Scholar]
- "Signet-ring" cells--a caveat in the diagnosis of a diffuse peritoneal mesothelioma occurring in a lady presenting with recurrent ascites: An unusual case report. Diagn Cytopathol. 2010;38:435-9.
- [CrossRef] [PubMed] [Google Scholar]
- A unique case of diffuse histiocytic proliferations mimicking metastatic clear cell carcinoma in the hydrocele sac. J Lab Physicians. 2014;6:43-5.
- [CrossRef] [PubMed] [Google Scholar]
- Mucophagocytizing histiocytes in a low-grade appendiceal mucinous neoplasm mimicking signet-ring mucosecreting adenocarcinoma cells. Int J Surg Pathol. 2014;22:241.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of Lung Adenocarcinoma with Clear Cell Features in Pleural Effusion: Cytomorphologic Features, Immunocytochemical Studies, and Differential Diagnosis. J Cytol 2022:134-6.
- [CrossRef] [PubMed] [Google Scholar]
- Histiocytic/mesothelial hyperplasia. Am J Surg Pathol. 1998;22:1036-7.
- [CrossRef] [PubMed] [Google Scholar]
- Signet ring cell mesothelioma; A diagnostic challenge. Pathol Res Pract. 2019;215:152462.
- [CrossRef] [PubMed] [Google Scholar]
- Mesothelioma with signet-ring cell features: Report of 23 cases. Mod Pathol. 2012;26:370-84.
- [CrossRef] [PubMed] [Google Scholar]
- Histiocytosis with Raisinoid nuclei: A unifying concept for lesions reported under different names as nodular mesothelial/histiocytic hyperplasia, mesothelial/ monocytic incidental cardiac excrescences, intralymphatic histiocytosis, and others: A report of 50 cases. Am J Surg Pathol. 2016;40:1507-16.
- [CrossRef] [PubMed] [Google Scholar]
- Nodular histiocytic/mesothelial hyperplasia as consequence of chronic mesothelium irritation by subphrenic abscess. Future Oncol. 2015;11(24 Suppl):51-5.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue fragments recovered at cardiac surgery masquerading as tumoral proliferations. Evidence suggesting iatrogenic or artefactual origin and common occurrence. Am J Surg Pathol. 1994;18:167-74.
- [CrossRef] [PubMed] [Google Scholar]
- Ancillary studies in pleural, pericardial, and peritoneal effusion cytology. Cancer Cytopathol. 2018;126(Suppl 8):590-8.
- [CrossRef] [PubMed] [Google Scholar]
- CD163: A specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794-801.
- [CrossRef] [PubMed] [Google Scholar]
- Claudin-4 immunohistochemistry is highly effective in distinguishing adenocarcinoma from malignant mesothelioma in effusion cytology. Cancer Cytopathol. 2014;122:299-306.
- [CrossRef] [PubMed] [Google Scholar]
- Utility and pitfalls of GATA3 immunocytochemistry for diagnosis of metastatic breast carcinoma and urothelial carcinoma on cytology specimens. J Am Soc Cytopathol. 2017;6:73-9.
- [CrossRef] [PubMed] [Google Scholar]
- Calretinin but not caveolin-1 correlates with tumour histology and survival in malignant mesothelioma. Pathology. 2016;48:660-5.
- [CrossRef] [PubMed] [Google Scholar]
- Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet. 2013;206:206-10.
- [CrossRef] [PubMed] [Google Scholar]
- Loss of expression of BAP1 is a useful adjunct, which strongly supports the diagnosis of mesothelioma in effusion cytology. Mod Pathol. 2015;28:1360-8.
- [CrossRef] [PubMed] [Google Scholar]









