Translate this page into:
Intraductal papillary mucinous neoplasms of the pancreas: Cytologic-histologic correlation study and evaluation of the cytologic accuracy in identifying high-grade dysplasia/invasive adenocarcinoma

-
Received: ,
Accepted: ,
How to cite this article: Serinelli S, Khurana KK. Intraductal papillary mucinous neoplasms of the pancreas: Cytologic-histologic correlation study and evaluation of the cytologic accuracy in identifying high-grade dysplasia/invasive adenocarcinoma. 2024;21:6. doi: 10.25259/Cytojournal_71_2023
Abstract
Objective:
Intraductal papillary mucinous neoplasms (IPMNs) may be associated with invasive adenocarcinoma, low-grade dysplasia (LGD), or high-grade dysplasia (HGD). We aimed to review the cytologic-histologic correlation of cases with a histologic diagnosis of IPMN.
Material and Methods:
A database search (January 2010–January 2021) was performed for resected IPMNs with preceding endoscopic ultrasound-guided fine-needle aspiration (FNA). Cytology slides were reviewed for the presence of benign, atypical, or malignant cells, and necrosis. Histologically, IPMNs were classified as benign (LGD) or malignant (HGD or adenocarcinoma).
Results:
There were 41 patients with IPMN; 24 malignant and 17 benign. Sixteen of the 24 malignant IPMNs were accurately classified as malignant on cytology. There were eight false negatives and one false positive. Cytology yielded a sensitivity of 67% and a specificity of 94%. Among the 16 true positives with FNA diagnosis of adenocarcinoma, seven were IPMNs with HGD, and nine had invasive adenocarcinomas on histology. Cellular morphology and absence or presence of necrosis did not help distinguish HGD from adenocarcinoma on cytology (P > 0.5). Sampling errors and interpretative errors resulted in false-negative cases. Cytology yielded diagnoses related to IPMN in 73% of cases (30/41) and lack of identification of mucinous cells/mucinous background resulted in interpretative errors (9).
Conclusion:
This study shows that there is a good correlation between cytopathology and surgical pathology diagnoses of IPMNs and that cytology is mostly able to recognize IPMN with HGD/adenocarcinoma. However, heterogeneity in areas of IPMN with HGD/adenocarcinoma may result in sampling errors yielding false-negative cases. Mucinous cells/background should raise the suspicion of IPMN on cytology, even when no neoplastic epithelium is present for the evaluation of dysplasia.
Keywords
Intraductal papillary mucinous neoplasm
Pancreas
Fine-needle aspiration
Adenocarcinoma
High-grade dysplasia
INTRODUCTION
Intraductal papillary mucinous neoplasms (IPMNs) are grossly visible, mucin-producing epithelial neoplasms occurring within the main pancreatic duct and/or its branches.[1,2] They represent 20–50% of resected cystic pancreatic tumors and up to 5% of all pancreatic neoplasms.[3,4] They occur mostly in subjects between 25 and 94 years of age and are slightly more common in males. Radiologically, they are classified as main duct type, branch duct type, and mixed duct-type, depending on the duct(s) involved. These tumors are more common in the pancreatic head but can involve the entire pancreas and the ampulla of Vater. Most patients are asymptomatic but, when symptoms occur, they are usually related to pancreatic duct obstruction or low-grade pancreatitis (abdominal and/or back pain, anorexia, diabetes mellitus, and weight loss). The treatment of choice is surgical resection. Many patients with IPMN can be cured by complete resection, but some IPMNs evolve into invasive adenocarcinoma, usually of the colloid (mucinous) or tubular type.[5,6] Compared with conventional ductal adenocarcinoma, IPMNs with invasive carcinoma have a better prognosis.[7] The prognosis differs between IPMN not associated with invasive carcinoma (5-year survival rates higher than 85%) and IPMN with invasive carcinoma (5-year survival rates as low as 36%). Patients with IPMNs have also an increased incidence of synchronous or metachronous malignancies, particularly stomach and colon.
Preoperative diagnosis of IPMN is currently based on radiologic studies,[8] pancreatic duct brushings/fine-needle aspiration (FNA) biopsy,[9-11] and carcinoembryonic antigen (CEA) level, which is usually elevated. More recently, molecular/genetic/genomic studies have become critical for the diagnosis.[12] On computed tomography (CT) scans, a dilated main pancreatic duct or single/multiple cysts representing dilated branch ducts are detected. Endoscopic retrograde cholangiopancreatography shows dilated ducts and/or filling defects due to papillary projections of neoplasm or mucus plugs. At magnetic resonance cholangiopancreatography, in addition to dilated ducts, mural nodules can be visualized, which may correspond to high-grade dysplasia (HGD)/carcinoma in situ or invasive carcinoma. Cytology shows small clusters and flat sheets of mucin-producing columnar cells. Papillary structures may also be seen. There is abundant, thick, and viscid mucus in the background in nearly all cases.[3,10,13]
In resected specimens, pancreatic ducts appear dilated and filled with mucin and show friable papillary projections or nodules in the mucosa. Sometimes, single or multiple peripheral cysts that are connected to the main duct are detected. Invasive carcinomas usually arise adjacent to cystically dilated ducts; however, some invasive carcinomas arise elsewhere in the gland. Microscopically,[1,2] ducts lined by a proliferation of flat or papillary mucinous epithelial cells are seen, in a background of dense fibrotic stroma. The epithelium can be classified into three subtypes: Gastric (the cells have basally located nuclei, slightly eosinophilic cytoplasm, and abundant apical mucin); intestinal (villous papillae lined by cells with basophilic cytoplasm and enlarged, hyperchromatic pseudostratified nuclei, and by occasional goblet cells); and pancreaticobiliary (complex papillae lined by cells with moderate amphophilic cytoplasm and round, vesicular nuclei with prominent nucleoli). Dysplasia is now classified according to a two-tiered system. Low-grade dysplasia (LGD) is characterized by uniform cells with mild atypia and a lack of architectural complexity. HGD/carcinoma in situ shows marked architectural complexity, with prominent nuclear atypia, loss of polarity, and increased mitoses. About 60% of main duct IPMN and 15% of branch duct lesions are associated with an invasive component.
The preoperative diagnosis of IPMN and the distinction between invasive and non-invasive carcinomas are of critical importance for the subsequent patient management and prognosis. Neoadjuvant therapy may provide survival benefits in patients with invasive adenocarcinoma.[14,15] Consequently in this study, we investigated (a) the cytologichistologic correlation of cases with histologic diagnosis of IPMN at our institution and (b) the cytologic accuracy in identifying HGD/adenocarcinoma.
MATERIAL AND METHODS
The study was approved by the Institutional Review Board. A search of the laboratory information system of the Department of Pathology was performed to identify all cases with a surgical diagnosis of IPMN from January 2010 to January 2021. Among them, the cases with preoperative cytology studies performed within the preceding year were selected. The demographic, clinical (preoperative treatment, type of surgery), laboratory (preoperative CEA levels), radiology, cytopathology, and histopathology findings were collected. We did not have any molecular test in our data as at our institution molecular testing has been only added recently.
Histologic and cytologic slides were retrieved from the files of the hospital. Histology reports were examined, and the histology slides were reviewed for consistency in grading according to the current nomenclature.[1] In cases where a heterogeneous epithelium was seen, the highest degree of atypia was used for classification.
The cytology specimens available for each patient were pancreatic duct brushings of dilated pancreatic duct and/ or endoscopic ultrasound-guided FNAs of pancreatic cysts. Most of them consisted of direct smears stained using Papanicolaou (Pap) stain. Some specimens that were received as cyst fluid were processed as a cytospin or ThinPrep® preparation. All original cytology reports and cytology specimens were reviewed by the Pap Society of Cytopathology system for reporting pancreaticobiliary cytopathology.[13] The features of the epithelial cells were evaluated (benign, atypical, or malignant cells), including the presence/absence of papillary fragments, monolayered sheets, and single mucinous cells. The background was also assessed for extracellular mucin and necrosis. To summarize, if there was thick viscous extracellular mucin with cyst debris and no epithelial cells; then, the findings were considered consistent with neoplastic mucinous cysts. If in addition to thick mucinous material, there was mucinous epithelium showing columnar glandular cells with mucinous cytoplasm and papillary arrangement, then the cytologic findings were considered consistent with IPMN. In addition, the background was evaluated for necrosis and any cells with high-grade atypia.
For the first portion of the study (evaluation of cytologichistologic correlation of cases with histologic diagnosis of IPMN), the original cytology diagnoses were compared with the histologic diagnoses, and the cases were classified as discrepant and not discrepant. Cytology diagnoses of IPMN, neoplastic mucinous cyst, and adenocarcinoma were considered non-discrepant. The remaining cytology diagnoses (benign cyst and “negative for malignancy”) were considered discrepant. For the discrepant cases, the type of error was determined after a review of the cytology features.
For the second portion of the study (cytologic accuracy in identifying HGD/adenocarcinoma in histologically confirmed IPMNs), IPMNs were histologically classified as benign (LGD) or malignant (HGD or adenocarcinoma). Cytologically, cases were classified as benign (negative for malignancy/atypical/IPMN) or malignant (IPMN with HGD/suspicious for adenocarcinoma/adenocarcinoma). The sensitivity and specificity of cytology in identifying malignant IPMNs were evaluated. For calculation purposes:
True positive cases: Malignant on cytology and histology
True-negative cases: Benign on cytology and histology.
In the false-negative and positive cases, the type of error was determined. Cytology specimen features (cellular morphology and necrosis) and histologic grade of the IPMN were compared using the Chi-square test.
RESULTS
41 cases were included in the study. Age ranged from 41 to 86 years at diagnosis, with 61% males (25/41) and 39% females (16/41). CEA levels at the time of cytology were available for 29 cases (range: 1.5–68,240 ng/mL), while, in the remaining cases, a CEA analysis was not requested by the clinicians at the time of the work-up. CEA level >200 ng/mL was detected in 38% (11/29) cases. In 13 cases (32%), radiology reports included IPMN in the differential diagnoses. 19 lesions (46%) were in the pancreatic head, eight (20%) in the body, six (15%) in the tail, three (7%) in the neck, two (5%) in the ampulla, two (5%) in multiple areas of the pancreas, and one (2%) in an unspecified area. The specimens consisted of 32 cyst aspirates, six solid and cystic masses, and three main pancreatic duct brushings. The results of the two portions of the study are as follows.
Evaluation of cytologic-histologic correlation of cases with histologic diagnosis of IPMN
The original cytologic diagnoses compared with the final histologic diagnosis are outlined in Table 1. Cytology yielded diagnoses related to IPMN in 73% of cases (30/41): IPMN in one case, neoplastic mucinous cyst in 12, adenocarcinoma in 15 cases, adenocarcinoma versus IPMN with HGD in one case, and suspicious for adenocarcinoma in one case [Figure 1a-f].
| Histology diagnosis/Original cytology diagnosis | IPMN without dysplasia | IPMN with LGD | IPMN with HGD/carcinoma in situ | Invasive adenocarcinoma | Total |
|---|---|---|---|---|---|
| Negative for malignancy/benign cyst | - | 8 | 3 | - | 11 |
| NMC | 3 | 5 | 2 | 2 | 12 |
| IPMN | - | - | 1 (focal) | - | 1 |
| IPMN with HGD/adenocarcinoma | - | 1 | 7 | 9 | 17 |
IPMN: Intraductal papillary mucinous neoplasm, HGD: High-grade dysplasia, LGD: Low-grade dysplasia, NMC: Neoplastic mucinous cyst
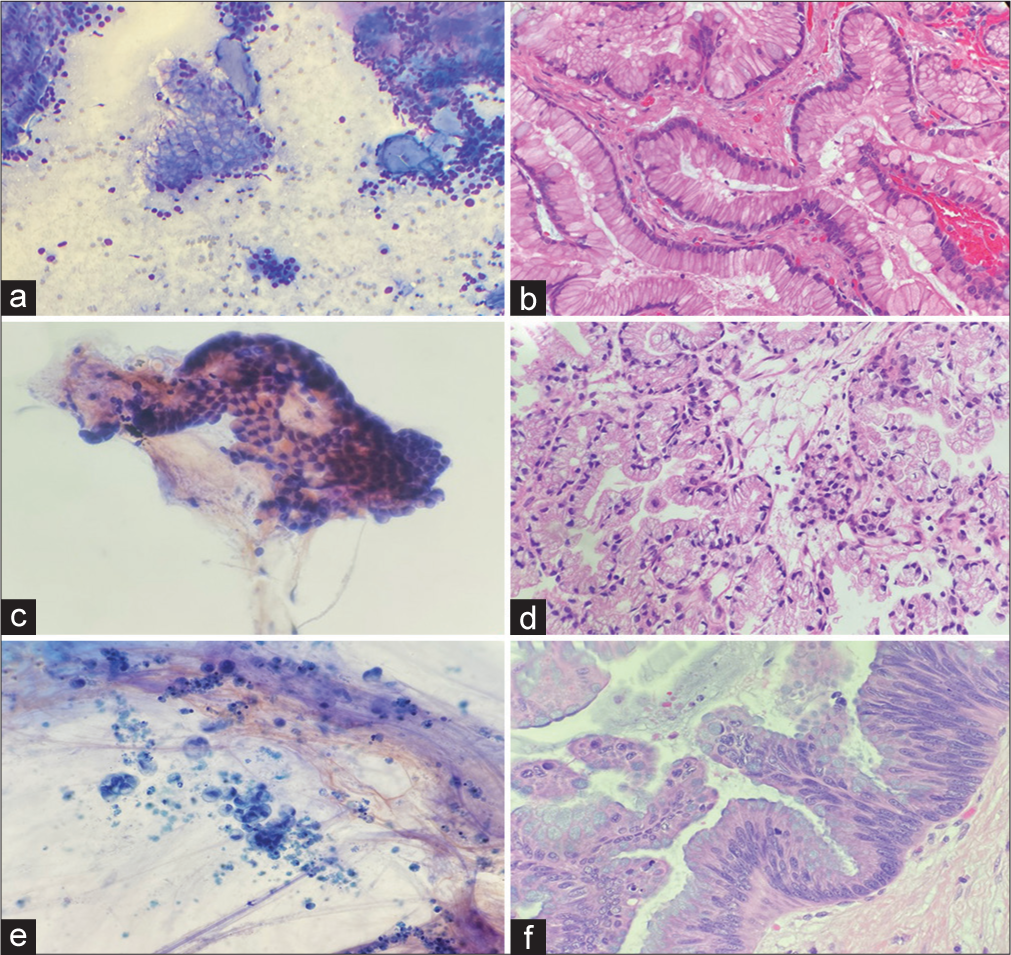
- (a) An intraductal papillary mucinous neoplasm (IPMN) diagnosed on cytology (Diff-Quik stain, 100x). (b) Follow up histology confirmed the diagnosis of IPMN (Hematoxylin & eosin stain, 200x). (c) A case diagnosed as neoplastic mucinous cyst demonstrated mucinous background and rare fragment of gastric epithelium on cytology smears (Papanicoloau stain, 200x). (d) IPMN with low-grade dysplasia was noted in the resection specimen (Hematoxylin & eosin 200x). (e) An adenocarcinoma diagnosis on cytology (Papanicoloau stain, 200x). (f) IPMN with high-grade dysplasia was noted in subsequent histology (Hematoxylin & eosin stain, 200x).
The remaining 11 cases (27%) were signed out as negative for malignancy/benign cysts. A review of these cases [Table 2] revealed ten interpretative errors, with one case of adenocarcinoma possibly arising from IPMN and nine cases showing mucinous cells/mucinous background suggestive of mucinous neoplasm [Figure 2a and b]. The remaining case had no cytologic evidence of IPMN, but the radiologic impression of cystic neoplasm: was classified as a sampling error.
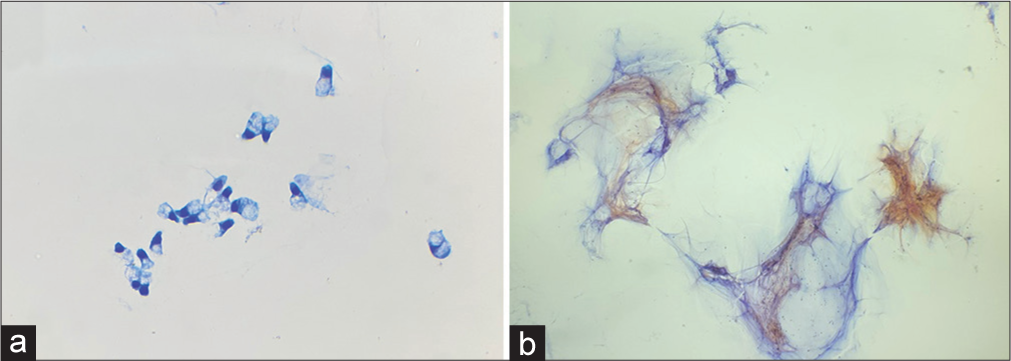
- Findings usually missed in the discrepant cases with interpretative errors: (a) Mucinous epithelium, (Diff-Quik stain 200x) and (b) mucinous background (Papanicoloau stain, 200x).
| Case # | Age/sex | Cytology original diagnosis | Cytology after review | Surgical diagnosis | CEA (ng/mL) | Radiology impression | Site of the lesion | Neoadjuvant therapy | Type of surgery | Type of error |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/65 | Negative for malignancy | IPMN benign mucinous epithelium and, mucin | IPMN, LGD | 9.8 | Cystic neoplasm | Head | No | Partial pancreatectomy | Interpretative |
| 2 | F/82 | Negative for malignancy | c/w IPMN abundant mucin present | IPMN, LGD | 3.5 | Mass | Ampulla | No | Biopsy | Interpretative |
| 3 | M/68 | Negative for malignancy | Adenocarcinoma with mucin (possibly arising from IPMN) | IPMN, HGD | 62.1 | Dilation of the pancreatic duct | Tail | No | Partial pancreatectomy | Interpretative |
| 4 | M/45 | Negative for malignancy | Negative for malignancy | IPMN, LGD | 34.9 | DDx: pseudocyst, cystic neoplasm, mesenteric cyst, abscess and necrotic mesenteric node | Tail | No | Partial pancreatectomy | Sampling |
| 5 | M/75 | Negative for malignancy | IPMN benign mucinous epithelium and abundant; mucin identified | IPMN, LGD | 241.6 | Complex solid and cystic mass. DDx: IPMN, serous cystadenoma | Head | No | Total pancreatectomy | Interpretative |
| 6 | F/70 | Benign cyst | IPMN benign mucinous epithelium and abundant mucin identified | IPMN, LGD | 477.2 | Multiple cystic lesions, highly concerning for IPMN | Head, body, tail | No | Total pancreatectomy | Interpretative |
| 7 | F/68 | Benign cyst | c/w IPMN abundant mucin identified | IPMN, LGD | 72.3 | Complex cyst. DDx: mucinous or serous cystadenoma, IPMN | Tail | No | Partial pancreatectomy | Interpretative |
| 8 | M/61 | Benign cyst | c/w IPMN abundant mucin identified | IPMN, LGD | 41,074 | Cystic lesion | Body | No | Partial pancreatectomy | Interpretative |
| 9 | M/70 | Negative for malignancy | c/w IPMN abundant mucin identified | IPMN, HGD | 475 | Cystic lesion with septations, suggestive of IPMN | Head | No | Partial pancreatectomy | Interpretative |
| 10 | M/85 | Negative for malignancy | c/w IPMN abundant mucin identified | IPMN, HGD | N/A | Mass, suspicious for carcinoma | Ampulla | No | Biopsy | Interpretative |
| 11 | M/69 | Negative for malignancy | IPMN benign mucinous epithelium and abundant mucin identified | IPMN, LGD | 1.5 | Cystic lesions, suggestive of IPMN | Head | No | Partial pancreatectomy | Interpretative |
IPMN: Intraductal papillary mucinous neoplasm, HGD: High-grade dysplasia, LGD: Low-grade dysplasia. c/w consistent with IPMN, CEA: Carcinoembryonic antigen, DDx: Differential diagnoses, N/A: Not available
Cytologic accuracy in identifying HGD/adenocarcinoma in histologically confirmed IPMNs
24 cases were histologically malignant and 17 benign. The original cytologic diagnoses compared to the final histologic diagnoses are outlined in Table 3. 16 of the 24 malignant IPMNs were accurately classified as malignant on cytology. There were eight false negatives and one false positive [Figure 3a-j]. Cytology yielded a sensitivity of 67% and a specificity of 94%. Necrosis was identified on cytology in one case of HGD (1/7) and three adenocarcinomas (3/9). Cellular morphology and absence or presence of necrosis did not help distinguish HGD from adenocarcinoma on cytology (P > 0.5, Chi-square test). A review of the cytology of the eight false-negative cases revealed five sampling errors and three interpretative errors [Table 4].
| Histology diagnosis/Original cytology diagnosis | Benign IPMN | Malignant IPMN | |
|---|---|---|---|
| IPMN with HGD/carcinoma in situ | Invasive adenocarcinoma | ||
| Benign cases | 16 | 6 | 2 |
| Malignant cases | 1 | 7 | 9 |
| Total | 17 | 13 | 11 |
IPMN: Intraductal papillary mucinous neoplasm, HGD: High-grade dysplasia
| Case # | Age/sex | Cytology original diagnosis | Cytology after review | Histology diagnosis | Type of error | CEA (ng/mL) |
Radiology impression | Site of the lesion | Neoadjuvant therapy | Type of surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/75 | Debris; mucin | Consistent with IPMN. No evidence of high dysplasia. | IPMN, HGD | Sampling | N/A | IPMN | Head | No | Total pancreatectomy |
| 2 | M/78 | IPMN | IPMN has no evidence of high dysplasia. | IPMN, low-grade dysplasia, and focal high-grade | Sampling | N/A | Mucinous cystic malignant lesion | Head | No | Partial pancreatectomy |
| 3 | F/79 | Atypical cells; abundant mucin | Adenocarcinoma with mucin (possibly arising from IPMN). Atypical ductal cells represented tumor cells. | Adenocarcinoma arising from IPMN with HGD | Interpretative | 31 | Mass, suspicious for carcinoma | Head | No | Partial pancreatectomy |
| 4 | M/70 | Atypical ductal cells; mucin | Adenocarcinoma versus IPMN with HGD. Atypical ductal cells represented dysplastic cells. | Adenocarcinoma arising from IPMN with HGD | Interpretative | N/A | N/A | Head | No | Partial pancreatectomy |
| 5 | F/67 | Atypical cells | Abundant Mucin present. No evidence of high dysplasia. | IPMN, HGD | Sampling | N/A | Mass | Tail | No | Partial pancreatectomy |
| 6 | M/68 | Negative for malignancy | HGD/mucinous Adenocarcinoma with mucin (possibly arising from IPMN) | IPMN, HGD | Interpretative | 62.1 | Dilation of the pancreatic duct | Tail | No | Partial pancreatectomy |
| 7 | M/70 | Negative for malignancy | Consistent with IPMN. No evidence of high dysplasia. Abundant Mucin present | IPMN, high-grade dysplasia | Sampling | 475 | Cystic lesion with septations, suggestive of IPMN | Head | No | Partial pancreatectomy |
| 8 | M/85 | Negative for malignancy | Consistent with IPMN. No evidence of high dysplasia. Abundant Mucin present | IPMN, high-grade dysplasia | Sampling | N/A | Mass, suspicious for carcinoma | Ampulla | No | Biopsy |
IPMN: Intraductal papillary mucinous neoplasm, HGD: High-grade dysplasia, CEA: Carcinoembryonic antigen, N/A: Not available
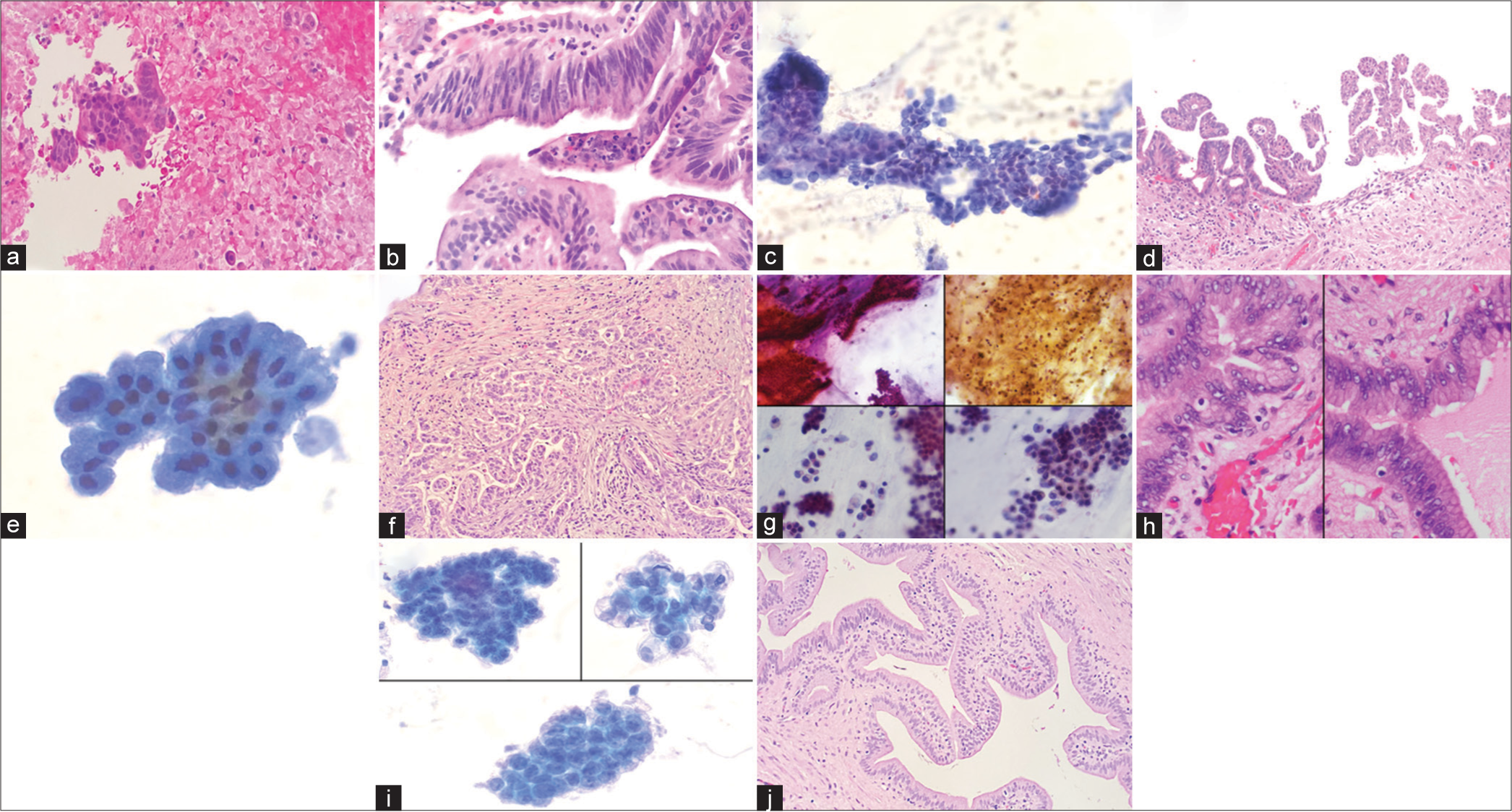
- (a) A case showing necrosis in the cytology cell block, (Hematoxylin and eosin, 100x). (b) Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia (HGD) (markedly atypical cells with loss of polarity) was noted in subsequent resection (Hematoxylin and eosin, 200x). (c) An adenocarcinoma diagnosis on cytology (high N: C ratio, mitoses, marked nuclear atypia, (Papanicoloau stain 200x)) (d) IPMN with HGD was noted on follow up resection. (complex papillary proliferation of tumor cells) (Hematoxylin and eosin, 100x). (e) False-negative case (sampling error): It was diagnosed on cytology as benign (Papanicoloau stain, 400x). (f) An invasive adenocarcinoma was noted in resection (Hematoxylin and eosin, 200x). (g) False-negative case (interpretative error): It was diagnosed on cytology as negative for malignancy. On review, mucin, necrosis, and atypical cells with high a N: C ratio were seen (Papanicoloau stain, 200x). (h) Histology showed IPMN with HGD, (Hematoxylin and eosin, 400x). (i) False-positive case: This case was suspicious for adenocarcinoma on cytology (prominent nucleoli and nuclear overlapping) (Hematoxylin and eosin, 400x) (j) Histology showed IPMN with low-grade dysplasia. The area with high-grade features was presumably not sampled on histology, (Hematoxylin and eosin, 200x).
DISCUSSION
IPMNs are cystic neoplasms of the exocrine pancreas arising in the pancreatic ducts. Pre-operative diagnosis of IPMNs is currently based on imaging, cytology, and CEA level. It is now widely recognized that the findings of cytology should be interpreted in the context of the radiologic and laboratory findings, to avoid misdiagnosis.[16-19] For example, many branch duct IPMNs have a lining that is indistinguishable from that of normal gastric epithelium, so gastric contamination cannot always be excluded from cytology.[20,21] The characteristic ovarian-type stroma of mucinous cystic neoplasms is usually not apparent in FNA samples, and these entities can be cytologically indistinguishable from IPMNs.[22] It is also important to consider that foci of HGD or invasive carcinoma can often be small and may not be sampled, leading to an underdiagnosis of malignancy in IPMNs. On the other hand, acute inflammation can lead to marked cytological atypia and can cause a false-positive diagnosis of malignancy.[23] In cytologic specimens, it is often impossible to distinguish the cells of HGD from those of invasive carcinoma. For these reasons, the results of FNA should always be correlated with the clinical findings.
The present study conducted on 41 cases of histologically confirmed IPMNs showed that the CEA level alone was not a good predictor of these neoplasms (62% of cases showed a CEA level below 200 ng/mL, the threshold value) and radiology evaluation can also be inconclusive. There was a good correlation between cytopathology and surgical pathology diagnoses in IPMNs, with cytology yielding diagnoses related to IPMN in 73% of cases. The previous studies showed a percentage of cytology positivity ranging between 21% and 72%.[10,24,25] The majority of our missed IPMNs were interpretive errors and showed mucin/mucinous cells on review. Layfield and Cramer[26] have found that background mucin, by itself, is not predictive of a diagnosis of IPMN and does not distinguish it from other neoplasms and reactive changes. However, our findings support that a mucinous background on cytology should raise suspicion of IPMN, even when no epithelium is present.
The distinction between invasive and noninvasive carcinomas is of critical importance for patient management and prognosis. In the present study, cytology was able to recognize IPMN with HGD/adenocarcinoma in 67% of cases. Smith et al.[27] found that cytology was able to recognize 52% of malignant IPMNs, while Inoue et al.[28] found that 31% of IPMN patients with intraductal carcinomas were diagnosed as a malignant disease by cytology. As these tumors often show focally variable degrees of dysplasia, heterogeneity in areas of IPMN with HGD/adenocarcinoma may result in sampling errors yielding false-negative cases.
In the present study, HGD/carcinoma in situ and invasive adenocarcinoma were difficult to distinguish on cytology (by cellular morphology and absence/presence of necrosis). Conversely, Michaels et al.[10] found that the presence of necrosis was the only feature that was significantly different between carcinoma in situ and invasive carcinoma (P < 0.01). However, they concluded that necrosis is not unique to IPMN with invasive carcinoma, and in their comparison of original cytology with final histology diagnosis; they had classified 2 of 7 IPMN with carcinoma in situ and only 5 of 8 invasive adenocarcinoma as malignant, while the rest were classified as suspicious/atypical or negative. HGD is difficult to distinguish from invasive on cytology and in cases with HGD, a note for suspicion of invasive adenocarcinoma may be added.[3,10,13] To improve communication of cytopathology results to the clinicians for patient management, we suggest using the terms
“HGD cannot exclude invasive adenocarcinoma” in all IPMNs considered malignant on cytology.
There were some limitations to our study. The small number of cases prevented a more accurate subgroup analysis. Furthermore, we did not analyze the sensitivity and specificity of cytology in conjunction with radiology and laboratory results. Finally, since our study was retrospective, we did not evaluate the impact of cytologic evaluation on clinical management decisions.
CONCLUSIONS
This study shows that there is a good correlation between cytopathology and surgical pathology diagnoses in IPMNs and that cytology is mostly able to recognize IPMN with HGD/adenocarcinoma. Cytologic findings of mucinous background should raise the suspicion of IPMN, even when no epithelium is present, to avoid false-negative diagnoses. Since HGD/carcinoma in situ and invasive adenocarcinoma are difficult to distinguish based on cytology alone, we suggest using the terms “High-grade dysplasia, cannot exclude invasive adenocarcinoma” in all IPMNs considered malignant on cytology.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
CEA – carcinoembryonic antigen
CT – computed tomography
FNA – fine-needle aspiration
HGD – high-grade dysplasia
IPMN – intraductal papillary mucinous neoplasm
LGD – low-grade dysplasia
N:C ratio – nuclear-cytoplasmic ratio
AUTHOR CONTRIBUTIONS
SS and KK designed the research study. SS and KK reviewed the cytology and histology slides. SS wrote the paper and KK reviewed and edited it. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the IRB of SUNY Upstate Medical University (approval number: 1804622-1, Date: 9.24.21). Written informed consent was obtained from all the participants prior to the publication of this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEERREVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through the automatic online system.
FUNDING
None.
References
- WHO classification of tumours In: Digestive system tumours (5th ed). Geneva: World Health Organization; 2019.
- [Google Scholar]
- Intraductal papillary mucinous neoplasm. Available from: https://app.expertpath.com/document/intraductal-papillary-mucinous-neo-/01b476d1-7eb0-4ff2-9a51-fbb4d69675fe [Last accessed on 2022 Aug 21]
- [Google Scholar]
- Intraductal neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:452-66.
- [CrossRef] [Google Scholar]
- Intraductal papillary mucinous neoplasm of the pancreas: Diagnosis and treatment. Pancreas. 2004;28:282-8.
- [CrossRef] [Google Scholar]
- Intraductal papillary-mucinous neoplasms of the pancreas: An analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62-77.
- [CrossRef] [Google Scholar]
- Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26-42.
- [CrossRef] [Google Scholar]
- Intraductal papillary and mucinous pancreatic tumour: A new extracolonic tumour in familial adenomatous polyposis. Gut. 2002;51:446-9.
- [CrossRef] [Google Scholar]
- Radiologic spectrum of intraductal papillary mucinous tumor of the pancreas. Radiographics. 2001;21:323-37.
- [CrossRef] [Google Scholar]
- Background features in the cytology of pancreatic neoplasms. DEN Open. 2022;2:e105.
- [CrossRef] [Google Scholar]
- Intraductal papillary mucinous neoplasm of the pancreas: Cytologic features predict histologic grade. Cancer. 2006;108:163-73.
- [CrossRef] [Google Scholar]
- Single-operator pancreatoscopy is helpful in the evaluation of suspected intraductal papillary mucinous neoplasms (IPMN) Pancreatology. 2014;14:510-4.
- [CrossRef] [Google Scholar]
- A review of mucinous cystic and intraductal neoplasms of the pancreatobiliary tract. Arch Pathol Lab Med. 2022;146:298-311.
- [CrossRef] [Google Scholar]
- The Papanicolaou Society of cytopathology system for reporting pancreaticobiliary cytology. In: Ch 6. Berlin/Heidelberg, Germany: Springer; 2015. p. :45-62.
- [CrossRef] [Google Scholar]
- Resectable invasive IPMN versus sporadic pancreatic adenocarcinoma of the head of the pancreas: Should these two different diseases receive the same treatment? A matched comparison study of the French Surgical Association (AFC) Eur J Surg Oncol. 2017;43:1704-10.
- [CrossRef] [Google Scholar]
- Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. 2023;20:318-37.
- [CrossRef] [Google Scholar]
- Fine needle aspiration biopsy for preoperative workup of pancreatic cystic neoplasms: Report of 4 cases. Acta Cytol. 2007;51:925-33.
- [CrossRef] [Google Scholar]
- Management algorithms for pancreatic cystic neoplasms. Arch Pathol Lab Med. 2022;146:322-9.
- [CrossRef] [Google Scholar]
- Surgical decisions based on a balance between malignancy probability and surgical risk in patients with branch and mixed-type intraductal papillary mucinous neoplasm. J Clin Med. 2020;9:2758.
- [CrossRef] [Google Scholar]
- Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: Recommendations of Verona consensus meeting. Ann Surg. 2016;263:162-77.
- [CrossRef] [Google Scholar]
- Cystic lesions of the pancreas: Differential diagnosis and cytologichistologic correlation. Arch Pathol Lab Med. 2020;144:47-61.
- [CrossRef] [Google Scholar]
- Intraductal neoplasms of the pancreas In: Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO classification of tumors. Lyon: WHO Press; 2010. p. :304-13.
- [Google Scholar]
- Intraductal papillary-mucinous neoplasm of the pancreas. The findings and limitations of cytologic samples obtained by endoscopic ultrasound-guided fine-needle aspiration. Am J Clin Pathol. 2003;120:398-404.
- [CrossRef] [Google Scholar]
- Pancreatic mucinous lesions: A retrospective analysis with cytohistological correlation. Diagn Cytopathol. 2006;34:724-30.
- [CrossRef] [Google Scholar]
- Fine needle aspiration diagnosis of mucinous cystic neoplasms and intraductal papillary mucinous neoplasms of the pancreas: Cytomorphology. CEA level and K-RAS mutation status. 2013. Modern Pathol. Available at: https://www.nature.com/articles/modpathol20135.pdf
- [Google Scholar]
- Intraductal papillary mucinous tumors of the pancreas: The preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58:701-6.
- [CrossRef] [Google Scholar]
- Fine-needle aspiration cytology of intraductal papillary-mucinous tumors: A retrospective analysis. Diagn Cytopathol. 2005;32:16-20.
- [CrossRef] [Google Scholar]
- Cytologic categorization of pancreatic neoplastic mucinous cysts with an assessment of the risk of malignancy: A retrospective study based on the Papanicolaou Society of Cytopathology guidelines. Cancer Cytopathol. 2016;124:285-93.
- [CrossRef] [Google Scholar]
- Preoperative diagnosis of intraductal papillarymucinous tumors of the pancreas with attention to telomerase activity. Cancer. 2001;91:35-41.
- [CrossRef] [Google Scholar]









