Translate this page into:
Cytologic studies of in vivo fallopian tube specimens in patients undergoing salpingo-oophorectomy

*Corresponding author: Sharmila Pramanik, MD Department of Pathology, Santa Clara Valley Medical Center, San Jose, California. sharmila.pramanik@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pramanik S, Yang E, Wu W. Cytologic studies of in vivo fallopian tube specimens in patients undergoing salpingo- oophorectomy. CytoJournal 2020;17:19.
Abstract
Objectives:
Recent research shows that most high grade ovarian cancer (OC) originates from the fallopian tube (FT). Cytologic evaluation of FT cells may enable early detection of OC.
Material and Methods:
This was a prospective study with patients enrolled from 3 centers (October 2016– August 2017). Forty-two women undergoing salpingo-oophorectomy for a pelvic mass suspicious for malignancy or undergoing risk-reducing surgery for BRCA mutations were included in the study. At the time of scheduled surgery, a novel catheter was used to collect FT cells through hysteroscopy. A pathologist blinded to surgical or pathologic findings evaluated FT cytology, and results were compared to pathology.
Results:
Of the 61 samples collected, 72% (44/61) met the adequacy criteria (≥5 clusters of cells with 20 cells in each cluster). Cytology classification criteria were established and applied to adequate samples. Forty-four samples were benign with mixed population of cells with round, oval, and spindled nuclei; 2-dimensional clusters; columnar cell configuration; flat sheets; cilia presence; no/mild nuclear pleomorphism; no nuclear membrane irregularities; and no nucleoli. Five samples had benign features with reactive nuclear and cytoplasmic changes and/or background inflammation, which were categorized as “reactive atypia.” Two malignant samples had features of 3-dimensional (3D) clusters, loss of mixed population of cells; increased nuclear/cytoplasmic ratio; nuclear membrane irregularity and nucleoli presence. Three samples with some but not all of malignant features were categorized as “neoplastic” (anisonucleosis; small nucleoli and features suggestive of 3D clusters). Malignant/ neoplastic samples were labeled as “Positive” (n = 5) while benign/reactive samples were labeled as “Negative” (n = 39). A high concordance rate (95%, 42/44) was observed between cytology results and histology.
Conclusions:
We characterized cytologic features for pathologically distinct FT samples collected in vivo using a novel catheter and demonstrated its value in detecting OC.
Keywords
Ovarian cancer
Hysteroscopy
Cytuity
BRCA
Detection
INTRODUCTION
In the United States alone, it is estimated there will be almost 14,000 deaths from ovarian cancer (OC) in 2019, making OC the fifth leading cause of cancer death in women and the most lethal gynecologic malignancy.[1] Majority of the patients are diagnosed in advanced stages when the survival rate is very low,[2] highlighting the need for effective screening test to detect OC at an early stage.[3-6]
In recent years, multiple lines of evidence have shown that the majority of OCs including high- grade and low-grade serous ovarian carcinoma originate from the distal fallopian tubes (FTs).[7-11] As a result, there has been an increasing interest in cytological evaluation of the FTs to enable early detection of OC. Chen et al. showed that in women undergoing salpingo-oophorectomy, FT samples collected ex vivo yielded encouraging cytology results that highly correlated to histopathology.[12] Benign tubal cytology has been characterized in samples collected through a cytobrush both in vitro[13] and in vivo.[14,15] However, the in vivo sample collection efficiency was not satisfactory partly because the cytobrush was not specifically designed for collecting samples from the FT, hindering further research into utilizing FT cytology for OC detection.
In this study, we characterized both benign and malignant features of FT cytology samples collected in vivo using the Cytuity™ cell collection catheter. In addition, we also evaluated the concordance between cytological results and histopathology of the FTs and ovaries.
MATERIAL AND METHODS
This was a prospective, multicenter study. The aim was to evaluate the ability of the Cytuity cell collection catheter to collect FT cytology samples that are adequate to enable differentiation of normal vs atypical vs malignant cells (ClinicalTrials.gov: NCT02974842). The study was approved by institutional review board of each center. Informed consent was obtained from all patients before patient enrollment.
Study population
Eligible patients were women over age 18 undergoing salpingo-oophorectomy for a pelvic mass suspicious for malignancy or undergoing risk-reducing salpingo- oophorectomy for BRCA1 or BRCA2 mutations. Exclusion criteria were: (1) Contradiction to hysteroscopy; (2) intolerance of anesthesia; (3) pregnancy, delivery or termination of a pregnancy in the past 6 weeks; (4) active or recent lower pelvic infection; (5) acute pelvic inflammatory disease; (6) known tubal obstruction including tubal ligation; and (7) invasive carcinoma of the cervix or endometrium.
Patients were enrolled from three gynecologic oncology centers in the United States (October 2016–August 2017). A total of 42 patients were included in this study, with 81% (34) had pelvic mass suspicious for malignancy. Patients’ age averaged at 56.6 years old.
In vivo FT sample collection
At the time of scheduled salpingo-oophorectomy, an FDA- cleared device (Cytuity™ cell collection catheter, nVision Medical, San Bruno, CA) was used in a hysteroscopic procedure to collect cells from the FT right before the surgical excision of the FT (s) and ovary(ies). Following placement of the hysteroscope, the FT ostium was located, and the catheter was inserted into the working channel of the hysteroscope until the distal tip of the catheter was placed at the tubal ostium. Then, the pressurized linear-everting balloon was deployed into the FT up to 7 cm, making contact with the inside of the FT to capture cells on the surface of the balloon. Once fully deployed, pressure was released and the balloon was then retracted into the device’s sheath. Finally, the catheter was withdrawn from the working channel of the hysteroscope and the distal end of the catheter was cut and placed in a cytopreservative (ThinPrep) for cytology analysis.
Cytology sample processing and evaluation
Cytological analysis was performed by an independent board-certified pathologist blinded to surgical findings and pathology diagnosis. The sample was first poured into a tube containing 30 mL of cytopreservative (CytoLyt). After the sample was vortexed, it was processed using a monolayer cytology sample preparation kit (ThinPrep). Once the cytology slide was prepared, it was fixed in 95% alcohol for 10 min. The Papanicolaou stain method was used to stain the sample for evaluation. The sample was first examined for overall cellularity, background, artifact, and cytological details to determine adequacy. An adequacy criterion of at least five cell groups/clusters of 20 or more cells with well visualized cytological details, clean background with no overlay on cells, and no artifactual changes in a single specimen was applied. Samples were classified into benign, reactive atypia, neoplastic, and malignant categories. Then, samples were further classified as positive (neoplastic/ malignant) or negative (benign or reactive atypia).
Comparing cytological evaluation and pathological findings
During salpingo-oophorectomy, the ovary(ies) and FT (s) were surgically excised and sent to the pathology department of the study site for evaluation, as is standard of care. The site’s redacted histology report on pathology findings in ovary(ies) and FT (s) was then sent to an independent board-certified pathologist, blinded to the cytology results, for entry into the database. These histology results were compared to cytology results for the corresponding samples.
RESULTS
Cytology criteria for FT cytology
Specimen was determined as adequate if it had at least five clusters of cells with 20 cells in each cluster. Individual naked nuclei were ignored. Clusters with marked artifactual changes were not evaluated. An exception to this requirement was made when atypical/malignant cells were present; a minimum number of FT epithelial cells was not required in this case.
A total of four cytology features were assessed including cellularity, architecture, cellular features, and nuclear features. The range of cellularity was similar in all the samples and thus was not included as a feature to categorize the samples. As a result, cytology categorization was based on three features: Architecture, cellular features, and nuclear features.
Benign samples were characterized by the following findings: (1) Architecture: Cells were arranged in flat sheets and/ or angulated sheets with cellular overlap. Notable cellular crowding may be seen, but 3-dimensional (3D) ball-like clusters were not present. (2) Cellular features: Multiple cell types in a cluster were the hallmark of benign cytology. The FT is lined by three basic cell types: Ciliated cells, secretory cells, and intercalated (peg) cells. The relative number of these cells may vary depending on patient age and timing of collection relative to menstrual cycle. The majority of the cells appeared to represent secretory cells. Ciliated cells with terminal bars were helpful when present, but were not always seen and were not usually present in large quantities. Intercalated cells were present interspersed within sheets and clusters of secretory or ciliated cells and might appear to be in a different plane of focus from the other cells. A background of mixed inflammatory cells might be present in association with reactive epithelial changes. (3) Nuclear features. Nuclear: cytoplasmic (N: C) ratio was variable depending on cell type, with ciliated cells having the most abundant cytoplasm and intercalated cells having the least. Nuclear size might vary overall, but significant anisonucleosis was not seen within a sheet or cluster of cells. Nuclear contours were mostly smooth, without significant hyperchromasia. Spindled nuclear shapes could be seen occasionally.
The reactive atypia samples had the same benign features described above, with additional reactive nuclear feature and inflammation. Nuclear crowding and enlargement might be present, along with multiple small nucleoli. Nuclear clearing was also present in reactive samples. However, significant anisonucleosis, nuclear contour irregularities, hyperchromasia, and large prominent nucleoli were not commonly present. The N:C ratio remained low in reactive atypia samples. Both reactive atypia and benign samples were labeled as “Negative” in cytological diagnosis.
Malignant samples were characterized by the following findings: (1) Architecture: The presence of markedly crowded, 3D ball-like clusters was the most important criteria to entertain a malignant diagnosis. Multiple planes of focus were needed to confirm this feature. (2) Cellular features: A uniform population of markedly atypical cells was present without intermixed ciliated cells or intercalated cells. This was another required feature of malignancy. (3) Nuclear features: Enlarged, pleomorphic nuclei with irregular contours, prominent nucleoli, and hyperchromasia were observed. Prominent pinkish-red nucleoli were present in one case. N:C ratios were increased, but some cells might have moderate amounts of delicate cytoplasm. While frankly malignant nuclear features were not expected in a reactive specimen, there might be some overlapping features in the most floridly reactive cases. Therefore, in addition to nuclear atypia, we require a 3D ball-like architecture as well as a lack of ciliated-differentiation for the diagnosis of malignancy.
The neoplastic samples contained cells with cytologic features that were highly suggestive of a malignancy. For example, neoplastic samples could have suggestive features of 3D clustering and partial changes in a cluster ranging from benign to malignant. However, these features might not fulfill all criteria for malignancy, or evaluation might be limited by the scarcity of these cells or preparation artifact. The malignant samples and neoplastic samples were labeled as “Positive” in cytological diagnosis.
Cytological evaluation
A total of 61 FT samples were collected for cytological evaluation. Among these, 72% (44/61) were adequate for cytological evaluation. Seventeen samples were inadequate. Among the 44 adequate samples, 34 were benign, five were reactive atypia, three were neoplastic, and two were malignant based on cytological evaluation. Malignant and neoplastic samples were classified as “Positive” (n = 5) while benign and reactive samples were classified as “Negative” (n = 39).
Comparison between cytological evaluation and histopathology results
A comparison of cytology evaluation to histology findings of the FTs showed a concordance rate of 95% (42/44). All 39 cytology negative results (34 benign and five reactive atypical cytology) corresponded to histological evaluation of the FT [Figure 1]. These cases included three OCs with negative tubal cytology and negative tubal histology: Stage IC cystic teratoma containing invasive squamous carcinoma was seen in the left ovary on histology in one case (left FT had benign cytology and pathology); Stage IC endometrioid OC was seen in the right ovary histology in a second case (right FT had benign cytology and pathology); and Stage IIA high grade serous OC was present in the left ovary in a third case (left FT had benign cytology and pathology).
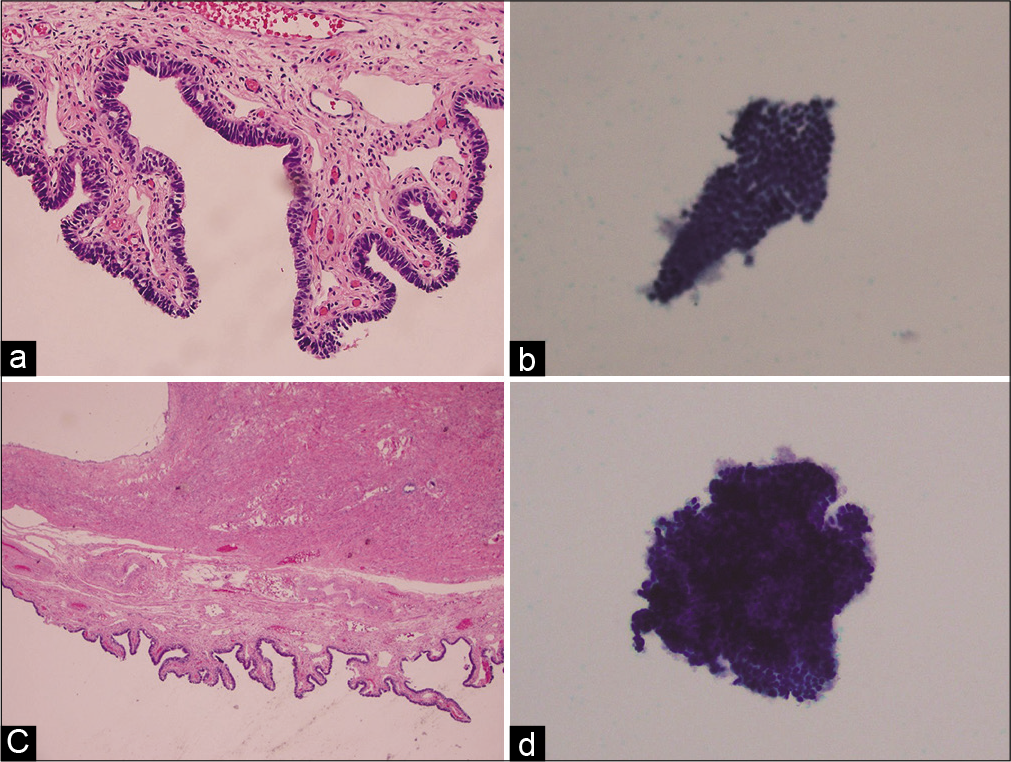
- Two examples of benign samples. Histology images are on the left (a and c), while corresponding cytology images are on the right (b and d). Typical benign samples present as flat sheet of cells with different cell types (b) and some benign samples may have slight overlap of cells with different cell types (d).
The three samples with positive cytology results consistent with positive histology results of the FTs were from two patients. In the Stage IV high grade serous OC case, histology results of ovaries and FTs from both sides revealed malignancy and the cytology samples also showed positive results [Figure 2]. The second patient had Stage IIIC high grade serous OC in the right ovary and tube, and incidental mixed mullerian tumor of the uterus. Histology of the left FT also revealed serous tubal intraepithelial carcinoma [Figure 3]. Consistent with the tubal histology results, the cytology sample was found to be neoplastic [Figure 3].
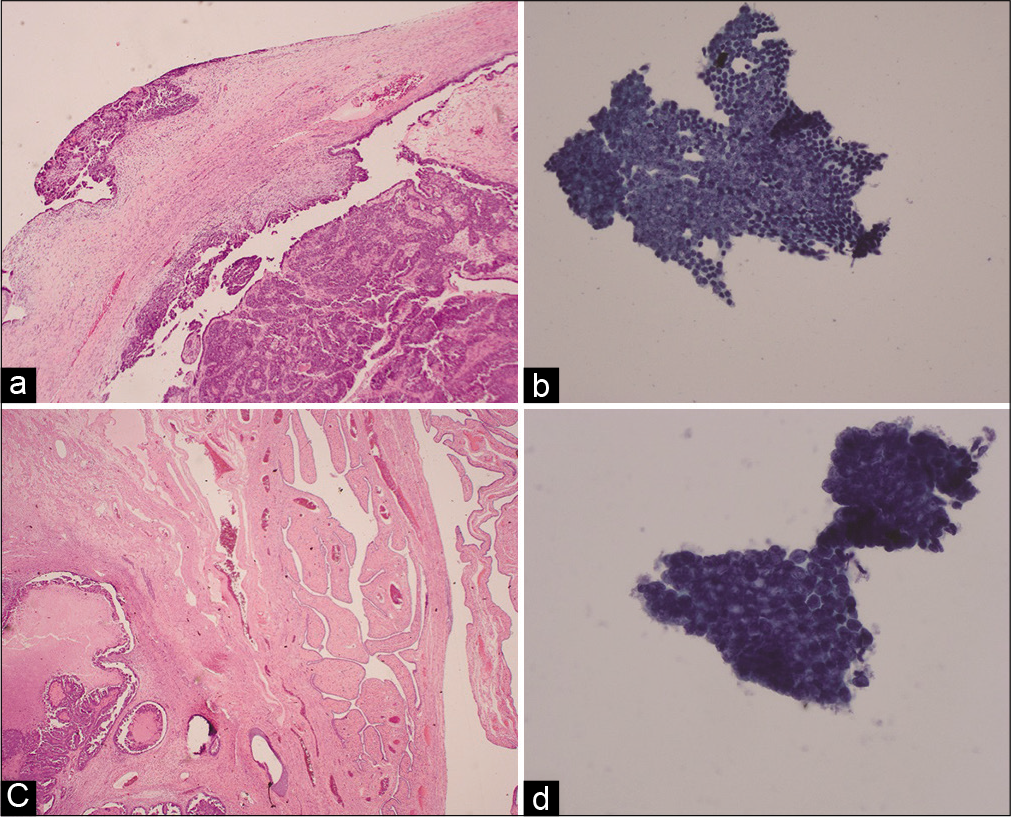
- Histology (a and c) and cytology (b and d) images from a Stage IV high grade serous ovarian cancer case. (a) Histology of the right ovary reveals surface tumor. (b) Right tube cytology image showing a spectrum of cell types ranging from 3-dimensional clustering to flat sheet. (c) Histology of the right fallopian tube reveals serous carcinoma. (d) Right tube cytology image showing 3-dimensional clustering with loss of the normal three cell types and marked nuclear atypia.
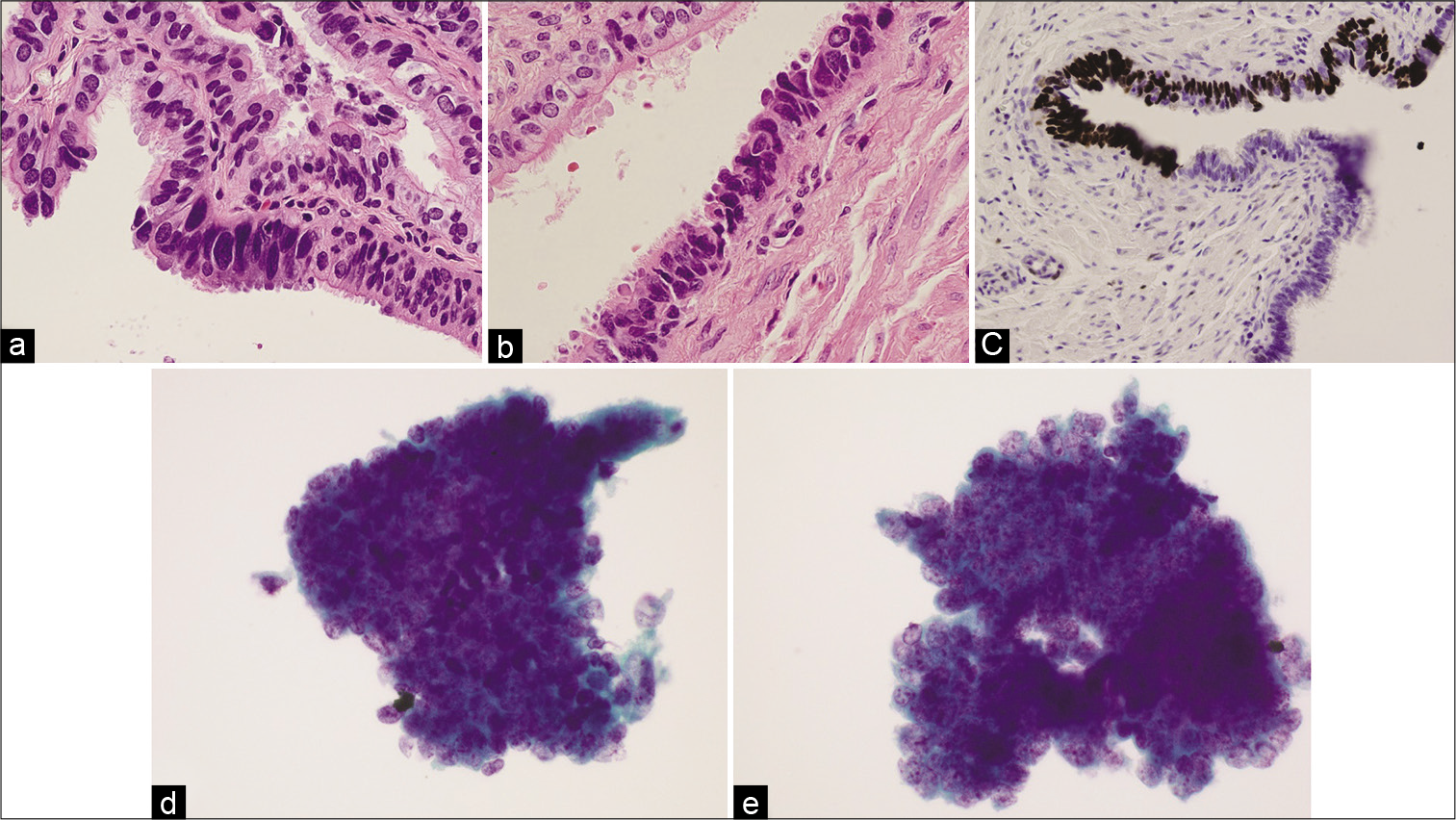
- Histology (a and b), p53 stain (c), and cytology (d and e) images from a patient with serous tubal intraepithelial carcinoma in the left fallopian tube. The cytology images show neoplastic features, with 3-dimensional clusters (d and e) and prominent nucleoli (d).
There were two samples with positive cytology results but histology of the FTs did not show malignancy. Further evaluation revealed that both cases were Stage I OCs with malignant ovary histology (one case of Stage IA clear cell carcinoma on the right ovary and one case of Stage IC endometrioid adenocarcinoma on the left side with disrupted ovarian surface with tumor [Figure 4]. In both cases, tubal cytology was found to be neoplastic on the side of the cancer.
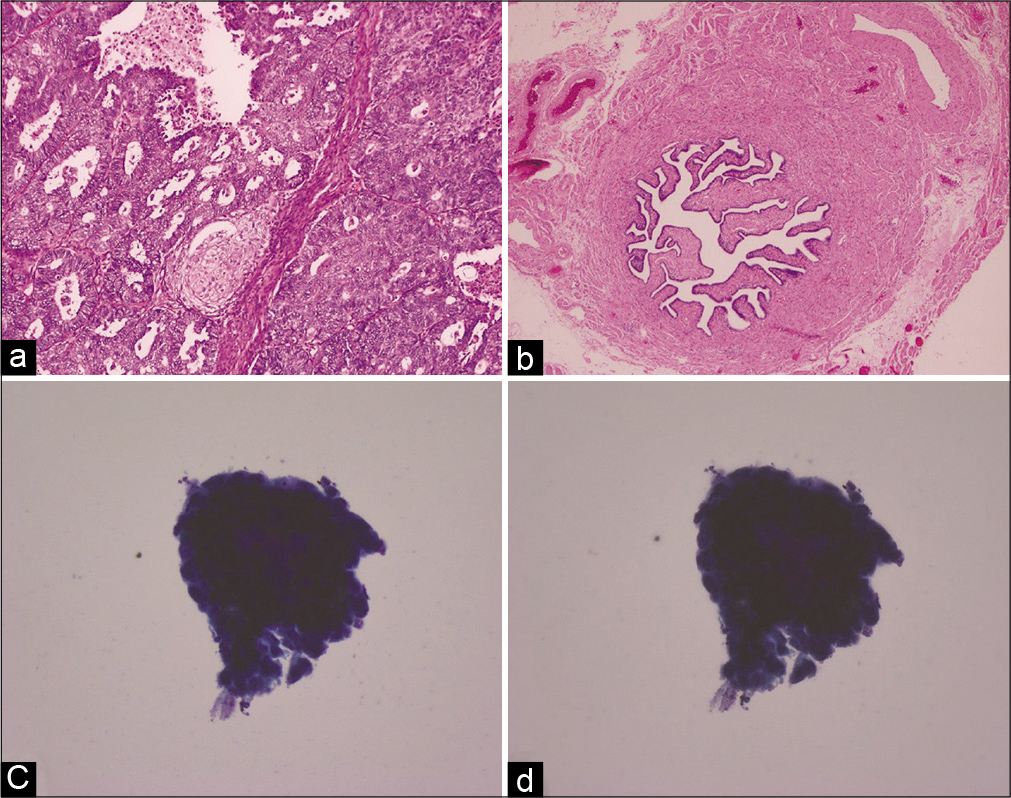
- Histology (a and b) and cytology (c and d) images from a Stage IA clear cell carcinoma (right ovary) case. (a) Histology of the right ovary reveals tumor. (b) Histology of the right fallopian tube does not show malignancy. (c and d) Cytology images of the right fallopian tube at two different focal planes show 3-dimensional cluster with atypia.
DISCUSSION
This study shows that cytology evaluation of the FT samples collected by a novel catheter can differentiate malignant/ neoplastic versus benign/reactive atypia cells. Cytological features of malignant, neoplastic, benign, and reactive atypia samples were characterized in detail. Furthermore, cytological classification was highly concordant with histopathology findings (95%). These results show that cytology evaluation can provide valuable information to aid in the detection of peritoneal cancers involving FTs.
As an increasing body of evidence has revealed that transvaginal ultrasound and serum CA-125 are ineffective for OC screening,[3,4,6] there is an active search and evaluation of other screening modalities. Recently, sampling the FT becomes a promising strategy for developing new methods to detect OC, as the significance of distal FT is better understood in the pathogenesis of the disease.[16,17] There have been few previous studies that characterized nonmalignant cytomorphological and nuclear features of cells collected from FTs.[13-15] Consistent with these previous studies, we found there was a range of variations in cytological features present in nonmalignant samples. The variations could exist in appearance of cellular or nuclear features. In a pilot study by Chen et al., encouraging results showed that cytological evaluation of distal FT samples was highly correlated with histological diagnoses.[12] Our results were consistent with the prior study and revealed a high concordance rate between cytology evaluation and histology diagnosis. The difference between our study and the prior study by Chen et al. lies in the patient population. Chen et al. was limited to women with clinically apparent symptoms, which might skew the study cases to more advanced stages. In contrast, our study included not only patients with clinical symptoms (complex pelvic masses) but also women without symptoms but undergoing risk-reducing salpingo-oophorectomy for BRCA1 or BRCA2 mutations. This study design increased the likelihood that cytology samples from earlier stage OC cases get included and characterized. As expected, cytology results from a few Stage I cases were included in our study. Together with prior findings, our results suggest that cytological evaluation of FT samples could potentially aid in OC detection. In particular, the cytological evaluation of FT samples may be helpful for surveillance of high-risk women (e.g., BRCA mutation carriers) who have elected to delay risk-reducing surgery. In additional, the in vivo cytological sampling of the FTs examined in this study yielded minimal false-positive results, which could potentially urge women found to have malignancy to seek for surgical care earlier by a gynecologic oncologist.
Another strength of this study is the method of cytology sample collection. In the previous studies using a brush technique, researchers reported that it was challenging to sample the distal FTs in vivo through a minimally invasive in-office procedure such as hysteroscopy.[14,15] Rodriguez et al. had to modify the study protocol in the middle of the study switching from hysteroscopy to laparoscopy due to the length limit of the brush.[14] A later study reported successful sample collection through hysteroscopy, but in only 1 of 5 patients.[15] Our study utilized a catheter with a balloon at the tip that could reach up to 7 cm into the FT. This cell collection catheter is specifically designed for sampling the FT during hysteroscopy procedure, which can be performed in an office setting. This advance may lower the barrier for FT sampling to be incorporated into the routine clinical practice. The catheter used in this study does not seem to create ulcerations/erosions or any injury to the FTs. The histology of the tubes did not demonstrate any injury [Figures 1, 3, and 4].
One interesting result from this study is that the two discordant samples that had positive cytology results with benign FT histology belong to two patients with early-stage OC confirmed by malignancy in ovaries. Similar results were also reported in the previous study by Chen et al.[12] A likely explanation could be that small foci of the lesion were washed away when surgically excised specimens were prepared for histology evaluation. Another possibility is that the histology sampling of the FTs was not extensive and missed the small foci of the lesion in the tissue block, as both patients were not BRCA mutation carriers and therefore the sectioning and extensively examining the fimbriated end protocol was not followed. In contrast, the cytology sample was collected by the catheter along the FT for up to 7 cm, possibly increasing the likelihood of the malignancy being sampled.
The limitation of the study mainly lies in the small sample size, which prevented meaningful estimation of the sensitivity and specificity of cytological classification. More malignant cytology samples would help understand the variability across samples, as the only two malignant cytology samples were collected from the same patient. A larger clinical trial is underway to increase the sample size and address these limitations (ClinicalTrials.gov NCT03593681). Another limitation is that the current study utilized binary classification (positive vs. negative) for cytology diagnosis. There were some overlaps in cytologic features between malignant and reactive entities. Therefore, some specimens might contain cells with cytologic atypia or reactive changes that were insufficient to be classified as malignant, but were more significant than what could be confidently attributed to reactive changes. Such samples were labeled as “positive” in this study, but might have different clinical implications from malignant samples which were also labeled as “positive.” As the field evolves and knowledge increases, middle categories can be considered beyond the binary classification in future studies to capture these borderline cases with atypia of unknown significance.
Working toward the ultimate goal of detecting peritoneal cancers involving FTs at an early stage, this study has shown that cytological evaluation of FT samples can provide a minimally invasive tool for physicians. There are future opportunities to utilize well-known markers such as P53.[18-20] These markers may further improve on cytological evaluation by enabling the identification of molecular abnormalities even before cytological changes are visually present. Furthermore, cytological sampling of the FTs may also complement current screening methods such as CA-125 and ultrasound to eventually achieve earlier detection, better prognosis and outcomes for at-risk women.
CONCLUSIONS
In summary, we characterized cytological features for a range of pathologically distinct FT samples collected in vivo using a novel catheter. We found that the cytology results correlated well with histopathology evaluation. These findings suggest that the device may be valuable in enabling OC detection through a hysteroscopic procedure.
Acknowledgments
This study was funded by nVision Medical (a Boston Scientific Company). The authors thank Dr. Gregory J. Rumore and Dr. Elizabeth M. Hosfield from Permanente Medical Group for providing histology images. Drs. Rumore and Hosfield were compensated as Permanente Medical Group employees and received no additional funding for this study. The authors also acknowledge Dr. Elisheva D. Shanes (affiliated with University of Virginia School of Medicine at the time of this study, currently at Northwestern University Feinberg School of Medicine) for providing histology images and performing additional pathological analysis. Dr. Shanes was compensated as an employee at University of Virginia School of Medicine and received no additional funding for this study.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. SP designed the study, analyzed samples, and drafted the manuscript. EY and WW analyzed samples and helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of all the institutions associated with this study.
LIST OF ABBREVIATIONS (In alphabetic order)
3D - 3-dimensional FT - fallopian tube N:C - nuclear:cytoplasmic OC - ovarian cancer.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
References
- SEER Cancer Statistics Review (CSR) 1975-2015. 2018 Available from: https://www.seer.cancer.gov/csr/1975_2015 [Last accessed on 2019 Oct 01]
- [Google Scholar]
- SGO white paper on ovarian cancer: Etiology, screening and surveillance. Gynecol Oncol. 2010;119:7-17.
- [CrossRef] [PubMed] [Google Scholar]
- Committee opinion No. 716 summary: The role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer in women at average risk. Obstet Gynecol. 2017;130:664-5.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for ovarian cancer: US preventive services task force recommendation statement. JAMA. 2018;319:588-94.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic Familial High-Risk Assessment: Breast and Ovarian. 2019. Available from: http://www.nccn.org/professionals/physician_gls/pdf/ovarian_blocks.pdf [Last accessed on 2019 Oct 01]
- [Google Scholar]
- The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-14.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian cancer: The fallopian tube as the site of origin and opportunities for prevention. Front Oncol. 2016;6:108.
- [CrossRef] [PubMed] [Google Scholar]
- Tubal origin of 'ovarian' low-grade serous carcinoma. Mod Pathol. 2011;24:1488-99.
- [CrossRef] [PubMed] [Google Scholar]
- Tubal origin of ovarian low-grade serous carcinoma. Am J Clin Exp Obstet Gynecol. 2013;1:13-36.
- [Google Scholar]
- Fallopian tube precursors of ovarian low-and high-grade serous neoplasms. Histopathology. 2013;62:44-58.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic studies of the fallopian tube in patients undergoing salpingo-oophorectomy. Cancer Cell Int. 2016;16:78.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology of the fallopian tube: A screening model for high-grade serous carcinoma. Cytojournal. 2018;15:28.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic findings in experimental in vivo fallopian tube brush specimens. Acta Cytol. 2013;57:611-8.
- [CrossRef] [PubMed] [Google Scholar]
- Brush cytology of the fallopian tube and implications in ovarian cancer screening. J Minim Invasive Gynecol. 2014;21:851-6.
- [CrossRef] [PubMed] [Google Scholar]
- New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284-93.
- [CrossRef] [PubMed] [Google Scholar]
- High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8:1093.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16:267-82.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular pathogenesis of endometrial and ovarian cancer. Cancer Biomark. 2010;9:287-305.
- [CrossRef] [PubMed] [Google Scholar]
- Histologic, molecular, and cytogenetic features of ovarian cancers: Implications for diagnosis and treatment. Radiographics. 2011;31:625-46.
- [CrossRef] [PubMed] [Google Scholar]








