Translate this page into:
Cytomorphological findings in confirmed cases of tubercular lymphadenitis

*Corresponding author: Sufian Zaheer, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India. sufianzaheer@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ahuja S, Behera R, Kumari A, Zaheer S. Cytomorphological findings in confirmed cases of tubercular lymphadenitis. CytoJournal. 2024;21:14. doi: 10.25259/Cytojournal_98_2023
Abstract
Objective:
Tuberculosis (TB) remains a major health problem, especially in the developing countries. Fine-needle aspiration cytology is the first line of investigation for tubercular lymphadenitis as it is easy to perform, less invasive, quick, and economical. The typical cytopathological features of tuberculosis TB include epithelioid cell granulomas with Langhans giant cells and caseous necrosis. The present study aimed to evaluate the cytomorphological features of newly diagnosed cases of tubercular lymphadenitis confirmed by GeneXpert.
Material and Methods:
This was a retrospective study in which all fine-needle aspirates from newly diagnosed cases of tubercular lymphadenitis confirmed by GeneXpert over a 1-year period from July 2022 to July 2023 were included in the study. The May–Grunwald–Giemsa stained smears from these aspirates were categorized into three patterns-epithelioid cell granulomas with necrosis, epithelioid granulomas without necrosis, and necrosis only. The granulomas were further categorized into well-formed, ill--formed, and splintered. The background of the aspirate which included a reactive lymphoid background, lymphocytes, eosinophils, and neutrophils was tabulated for all the cases.
Results:
Out of the three cytomorphological patterns, epithelioid granulomas with necrosis were the most predominant (67.5%), followed by necrosis only (20.8%) and granulomas without necrosis (11.6%). An acid-fast bacilli (AFB) positivity of 53.3% (64 cases) was seen on the Ziehl-Neelsen stain. Well-composed, poorly formed, and splintered granulomas were seen in 55 (57.9%), 42 (44.2%), and 21 (22.1%) cases, respectively. Neutrophils were the most common background population (42, 35%) followed by lymphocytes (36, 30%). Reactive lymphoid cells and eosinophils were seen in 12 (10%) and 3 (2.5%) cases, respectively.
Conclusion:
Fine-needle aspiration cytology is a rapid inexpensive minimally invasive test for tubercular lymphadenitis as epithelioid cell granulomas along with caseous necrosis are highly suggestive of TB. However, manual acid-fast AFB detection has a low sensitivity as is illustrated in the present study where only AFBwas demonstrated in only 53.3% of cases.
Keywords
Tubercular
Lymphadenitis
Fine-needle aspiration cytology
Epithelioid granulomas
Necrosis
INTRODUCTION
Tuberculosis (TB) remains a major health problem, especially in developing countries. A total of 1.6 million deaths due to TB were reported worldwide in 2021 and it was the second leading infectious killer after COVID-19. The WHO South-East Asia (SEA) Region is home to 26% of the world’s population with a 43% burden of TB. Six out of the global high TB burden countries are in the SEA Region: Bangladesh, the Democratic People’s Republic of Korea, India, Indonesia, Myanmar, and Thailand. Among extrapulmonary TB, tubercular lymphadenitis is the most prevalent.[1]
Fine-needle aspiration cytology is the first line of investigation for tubercular lymphadenitis as it is easy to perform, less invasive, quick, and economical. The typical cytopathological features of TB include epithelioid cell granulomas with Langhans giant cells and caseous necrosis. However, acid-fast bacilli (AFB) may not be always demonstrated on Ziehl-Neelsen stain on aspirate material. Furthermore, it is a time-consuming procedure with low sensitivity. More sensitive methods such as Lowenstein-Jensen culture and GeneXpert Mycobacterium tuberculosis/rifampicin assay may be required for confirmation where AFB are not demonstrated.[2]
GeneXpert is a sophisticated molecular diagnostic platform developed by Cepheid, a leading player in the field of molecular diagnostics. Utilizing real-time polymerase chain reaction (PCR) technology, GeneXpert enables rapid and precise detection of a diverse range of infectious diseases and genetic disorders. One of the distinctive features of GeneXpert is its automated and on-demand testing capability, delivering results swiftly without compromising accuracy. GeneXpert has garnered significant recognition for its pivotal role in the diagnosis of TB. The platform offers a rapid and reliable method for detecting M. tuberculosis, the causative agent of TB. This proves particularly invaluable in regions with a high prevalence of TB, where timely and accurate diagnosis is paramount for effective disease management. Beyond TB, GeneXpert has demonstrated its versatility in diagnosing other infectious diseases, including HIV, hepatitis, and respiratory infections. Its capacity to deliver quick and precise results has positioned it as an indispensable tool in the realm of molecular diagnostics, contributing to more efficient and targeted healthcare interventions.[2]
The present study aimed to evaluate the cytomorphological features of newly diagnosed cases of tubercular lymphadenitis confirmed by GeneXpert.
MATERIAL AND METHODS
This was a retrospective study in which all fine-needle aspirates from newly diagnosed cases of tubercular lymphadenitis confirmed by GeneXpert over a 1-year period from July 2022 to July 2023 were included in the study. Any previously diagnosed cases already taking ATT or patients with any history of malignancy (primary or metastatic) were excluded.
The May–Grunwald–Giemsa stained smears from these aspirates were categorized into three patterns:
Epithelioid cell granulomas with necrosis
Epithelioid cell granulomas without necrosis
Necrosis only
The cytomorphological pattern of granulomas was, further, classified into:
Well-composed epithelioid cell granuloma
Poorly formed epithelioid cell granuloma
Splintered epithelioid cell granuloma
Well-composed epithelioid cell granuloma was defined as epithelioid cells along with caseous necrosis, lymphocytes, and giant cells. Poorly formed granuloma was defined as a group of epithelioid cells along with caseous necrosis or lymphocytic infiltrate. Splintered epithelioid cell granulomas consisted of dispersed epithelioid cells with or without lymphocytic infiltration. The background of the aspirate which included a reactive lymphoid background, lymphocytes, eosinophils, and neutrophils was tabulated for all the cases.
RESULTS
A total of 120 lymph node aspirates from confirmed cases of tubercular lymphadenitis by GeneXpert were included in the study. The majority of the lymph node aspirates were from the cervical region (84, 70%), followed by axillary (18, 15%), inguinal (12, 10%), and supraclavicular (6, 5%). The age group of cases ranged from 6 years to 74 years with a male-to-female ratio of 1.2:1. Out of the three cytomorphological patterns, epithelioid granulomas with necrosis were the most predominant (67.5%), followed by necrosis only (20.8%) and granulomas without necrosis (11.6%) [Table 1, Figures 1 and 2].
![Cytomorphological patterns in tubercular lymphadenitis. (a and b) May–Grunwald–Giemsa stained smears exhibiting epithelioid cell granulomas against a background of caseous necrosis ([a] x40, [b] x100). (c and d) May–Grunwald–Giemsa stained smears exhibiting well-formed epithelioid cell granulomas against a hemorrhagic background ([c] x40, [d] x100). (e) May–Grunwald–Giemsa stained smears exhibiting caseous necrosis (×40). (f) May–Grunwald–Giemsa stained smears exhibiting caseous necrosis with interspersed lymphocytes (×40).](/content/105/2024/21/1/img/Cytojournal-21-14-g001.png)
- Cytomorphological patterns in tubercular lymphadenitis. (a and b) May–Grunwald–Giemsa stained smears exhibiting epithelioid cell granulomas against a background of caseous necrosis ([a] x40, [b] x100). (c and d) May–Grunwald–Giemsa stained smears exhibiting well-formed epithelioid cell granulomas against a hemorrhagic background ([c] x40, [d] x100). (e) May–Grunwald–Giemsa stained smears exhibiting caseous necrosis (×40). (f) May–Grunwald–Giemsa stained smears exhibiting caseous necrosis with interspersed lymphocytes (×40).
![Cytomorphological findings of type of granuloma with the background population. (a and b) May–Grunwald–Giemsa stained smears exhibiting well-formed epithelioid cell granulomas against a background of reactive lymphoid cells ([a] x40, [b] x100). (c and d) May–Grunwald–Giemsa stained smears exhibiting ill-formed epithelioid cell granulomas against a background of necrosis and neutrophils ([c] x40, [d] x100). (e and f) May–Grunwald–Giemsa stained smears exhibiting well-formed epithelioid cell granulomas against a background of scattered lymphocytes ([e] x40, [f] x100).](/content/105/2024/21/1/img/Cytojournal-21-14-g002.png)
- Cytomorphological findings of type of granuloma with the background population. (a and b) May–Grunwald–Giemsa stained smears exhibiting well-formed epithelioid cell granulomas against a background of reactive lymphoid cells ([a] x40, [b] x100). (c and d) May–Grunwald–Giemsa stained smears exhibiting ill-formed epithelioid cell granulomas against a background of necrosis and neutrophils ([c] x40, [d] x100). (e and f) May–Grunwald–Giemsa stained smears exhibiting well-formed epithelioid cell granulomas against a background of scattered lymphocytes ([e] x40, [f] x100).
| S. No. | Cytomorphological pattern | Total cases n(%) |
Positive for AFB (Ziehl Neelsen stain) n(%) | Negative for AFB (Ziehl Neelsen stain) n(%) |
|---|---|---|---|---|
| 1. | Granulomas with necrosis | 81 (67.5) | 44 (54.3) | 37 (45.7) |
| 2. | Granulomas without necrosis | 14 (11.6) | 5 (35.7) | 9 (64.3) |
| 3. | Necrosis only | 25 (20.8) | 15 (60) | 10 (40) |
AFB: Acid-fast bacilli
An AFB positivity of 53.3% (64 cases) was seen on the Ziehl-Neelsen stain. Amongst these, the proportion of cases with AFB detected on the Ziehl-Neelsen stain was highest for necrosis only (60%) followed by the granulomas with necrosis pattern (54.3%) [Figure 3]. The location of AFB was predominantly extracellular (92%). The remaining cases (8%) exhibited AFB located intracellularly within the macrophages. The AFB were seen predominantly scattered singly (90%) except for very occasional cases (10%) where clumps of bacilli were observed.
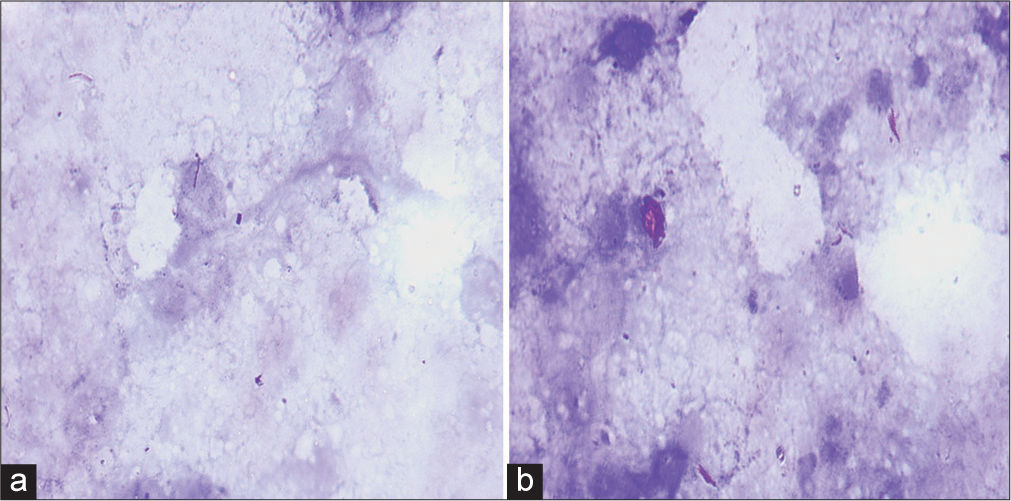
- Acid--fast bacilli on Ziehl-Neelsen stain. (a and b) Acid-fast beaded slender rods lying singly, (a, x1000) as well as in clumps, and (b, x1000) in an extracellular location (x1000).
Well-composed, poorly formed, and splintered granulomas were seen in 55 (57.9%), 42 (44.2%), and 21 (22.1%) cases, respectively. [Table 2] Neutrophils were the most common background population (42, 35%) followed by lymphocytes (36, 30%). Reactive lymphoid cells and eosinophils were seen in 12 (10) and 3 (2.5%) cases, respectively [Table 3].
| S. No. | Type of granuloma (n=95) | Absent n(%) |
Present n(%) |
|---|---|---|---|
| 1. | Well-composed epithelioid cell granuloma | 40 (42.1) | 55 (57.9) |
| 2. | Poorly formed epithelioid cell granuloma | 53 (55.8) | 42 (44.2) |
| 3. | Splintered granuloma | 74 (77.9) | 21 (22.1) |
| S. No. | Background (n=120) | Absent n(%) |
Present n(%) |
|---|---|---|---|
| 1. | Reactive lymphoid cells | 108 (90) | 12 (10) |
| 2. | Lymphocytes | 84 (70) | 36 (30) |
| 3. | Neutrophils | 78 (65) | 42 (35) |
| 4. | Eosinophils | 117 (97.5) | 3 (2.5) |
DISCUSSION
The study illustrated that cases of tubercular lymphadenitis exhibit various patterns on cytopathological examination-granulomas with necrosis, granulomas without necrosis, and necrosis only. The most common pattern observed in the present study included epithelioid cell granulomas with caseous necrosis followed by necrosis only. This was concordant with the findings of most of the previous studies.[2-14] However, few authors found necrosis to be the most prevalent pattern.[15-18] The majority of the lymph node aspirates were from the cervical region (84, 70%), followed by axillary (18, 15%), inguinal (12, 10%), and supraclavicular (6, 5%). These findings were similar to those of previous studies.[2,4,6,10,11]
[Table 4] summarizes the different cytomorphological patterns in previously done studies. This difference could be attributed to the varying immune status of the cases.
| Author | Number of cases | Granuloma with necrosis (%) | Granuloma without necrosis (%) | Necrosis only (%) |
|---|---|---|---|---|
| Helle et al.[2] (2023) | 158 | 59 | 6 | 21 |
| Shetty and Vyas[3] (2022) | 100 | 59 | 22 | 14 |
| Pooja et al.[4] (2020) | 461 | 74.4 | 9.2 | 16.4 |
| Rammeh et al.[5] (2018) | 171 | 83.04 | 8.77 | 7.01 |
| Hasija et al.[6] (2018) | 271 | 45.75 | 39.11 | 15.12 |
| Bhatta et al.[7] (2018) | 126 | 53.17 | 38.09 | 8.73 |
| Umar et al.[17] (2018) | 612 | 30.4 | 16.7 | 78.3 |
| Mitra et al.[14] (2017) | 180 | 41 | 29 | 30 |
| Gupta and Bhake[13] (2017) | 69 | 49 | 26 | 25 |
| Venkatraman[12] (2017) | 132 | 55 | 17 | 28 |
| Dasgupta et al.[18] (2017) | 257 | 25 | 24 | 51 |
| Masilamani et al.[9] (2015) | 212 | 48 | 19 | 33 |
| Hemalatha et al.[10] (2014) | 150 | 56 | 19 | 23 |
| Chand et al.[16] (2014) | 550 | 22 | 28 | 50 |
| Thakur et al.[11] (2013) | 90 | 57.8 | 11.1 | 31.1 |
| Mittal et al.[8] (2010) | 36 | 47 | 17 | 36 |
| Nayak et al.[15] (2004) | 42 | 33 | 17 | 50 |
An AFB positivity of 53.3% (64 cases) was seen on the ZiehlNeelsen stain which was highest for necrosis only (60%) followed by the granulomas with necrosis pattern (54.3%). The AFB were predominantly found extracellularly in the areas of necrosis. This is concordant to the study by Helle et al.[2,4,6,7,10,16,17] Cell--mediated immunity is responsible for mounting immune response against the tubercle bacteria in the form of a granulomatous reaction. Thus, cases with epithelioid granulomas without necrosis exhibit the least acid-fast bacilliAFB positivity. The cases with necrosis o nly exhibit the highest AFB positivity due to a lower cell-mediated immunity due to which they are unable to mount a granulomatous reaction.[7]
SUMMARY
Fine-needle aspiration cytology is a rapid inexpensive minimally invasive test for tubercular lymphadenitis as epithelioid cell granulomas along with caseous necrosis are highly suggestive of TB. However, manual AFB detection has a low sensitivity as is illustrated in the present study where only AFB was demonstrated in only 53.3% of cases. GeneXpert in addition to diagnosing TB can also test for Rifampicin resistance. Among confirmed cases of TB by GeneXpert, epithelioid cell granulomas with caseous necrosis followed by necrosis only are the most frequently observed cytomorphological patterns.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
AFB: Acid fast bacilli
ATT: anti-tubercular therapy
FNAC: fine needle aspiration cytology
PCR: polymerase chain reaction
SEA: South East Asia TB: tuberculosis.
AUTHOR CONTRIBUTIONS
SA: Data curation, formal analysis, writing- original draft ; RB: Data curation, formal analysis; AK: Data curation, formal analysis; SZ: Conceptualization, methodology, validation, writing- review and editing, supervision, resources. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi (approval no. IEC/VMMC/SJH/Thesis/2020-11/CC-236).
Written informed consent was obtained from all the participants prior to the publication of this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest and amongst authors, the authors affiliated in Vardhman Mahavir Medical College and Safdarjung Hospital claims no conflict of interest.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Global tuberculosis report 2021. Geneva: World Health Organization; 2021; :15. Available from: https://www.whoint/publications/i/item/9789240037021 [Last accessed on 2023 Oct 24]
- [Google Scholar]
- Significance of non-granulomatous cytomorphology on fine needle aspirate in lymphadenitis cases classified as tuberculous by using a composite reference standard. Diagn Cytopathol. 2023;51:575-83.
- [CrossRef] [PubMed] [Google Scholar]
- Combination method for the diagnosis of tuberculous lymphadenitis in high burden settings. Surg Exp Pathol. 2022;5:11.
- [CrossRef] [Google Scholar]
- Cytomorphological patterns of tuberculous inflammation and its comparison with positivity for tubercle bacilli by fluorescent staining. Int J Clin Diagn Pathol. 2020;3:475-9.
- [CrossRef] [Google Scholar]
- Efficacy of fine-needle aspiration cytology in the diagnosis of tuberculous cervical lymphadenitis. Acta Cytol. 2018;62:99-103.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphological spectrum of extra pulmonary tuberculosis in rural tertiary care hospital: A study. Int J Biomed Adv Res. 2018;9:59-63.
- [Google Scholar]
- Cytopathological patterns of tuberculous lymphadenitis: An analysis of 126 cases in a tertiary care hospital. Int J Res Med Sci. 2018;6:1898-901.
- [CrossRef] [Google Scholar]
- Comparative evaluation of fine needle aspiration cytology, culture, and PCR in diagnosis of tuberculous lymphadenitis. Diagn Cytopathol. 2011;39:822-6.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of cytomorphological patterns and acid-fast bacilli positivity in tuberculous lymphadenitis in a rural population of southern India. J Nat Sci Biol Med. 2015;6:134-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphological patterns of tubercular lymphadenitis revisited. Ann Med Health Sci Res. 2014;4:393-6.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of various techniques in diagnosis of tuberculous lymphadenitis on fine needle aspiration cytology. Pathol Res Int. 2013;2013:824620.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphological patterns of tuberculous lymphadenitis in correlation with AFB positivity. Ind J Pathol Res Pract. 2017;6:250-4.
- [CrossRef] [Google Scholar]
- Clinical and cytological features in diagnosis of peripheral tubercular lymphadenitis-a hospital-based study from Central India. Indian J Tuberc. 2017;64:309-13.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphological patterns of tubercular lymphadenitis and its comparison with ZiehlNeelsen staining and culture in eastern up (Gorakhpur region): Cytological study of 400 cases. J Cytol. 2017;34:139-43.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology in tuberculous lymphadenitis of patients with and without HIV infection. Diagn Cytopathol. 2004;31:204-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cytopathological pattern of tubercular lymphadenopathy on FNAC: Analysis of 550 consecutive cases. J Clin Diagn Res. 2014;8:16-9.
- [Google Scholar]
- Different cytomorphological patterns in tuberculosis in a tertiary health care centre: 5 year study. IOSR J Dent Med Sci (IOSR-JDMS). 2018;17:53-7.
- [Google Scholar]
- Shifting trend of tubercular lymphadenitis over a decade-a study from eastern region of India. Biomed J. 2017;40:284-9.
- [CrossRef] [PubMed] [Google Scholar]








