Translate this page into:
Cytomorphology of papillary renal neoplasm with reverse polarity

*Corresponding author: Swati Satturwar, Department of Pathology, The Ohio State University, Columbus, Ohio, United States. swati.satturwar@osumc.edu
-
Received: ,
Accepted: ,
How to cite this article: Satturwar S, Parwani AV. Cytomorphology of papillary renal neoplasm with reverse polarity. CytoJournal. 2023;20:43. doi: 10.25259/Cytojournal_9_2023
HTML of this article is available FREE at: https://dx.doi.org/10.25259/Cytojournal_9_2023
Abstract
Papillary renal neoplasm with reverse nuclear polarity (PRNRP) is an emerging oncocytic renal tumor. Cytomorphologic features of this tumor have not been described in the literature before. The objective of this study was to review the cytomorphology of a case PRNRP and compare with cytomorphologic features of papillary renal cell carcinomas (pRCCs) reported in the literature. 1 case of core needle biopsy (CNB) with touch preparation (TP) of a renal mass diagnosed as PRNRP was reviewed retrospectively. Clinical presentation, cytomorphologic features, ancillary tests and histopathology results were analyzed. The touch preparation was cellular and showed tight 3-D clusters of cuboidal epithelial cells with variable presence of fibrovascular cores (FC), granular eosinophilic cytoplasm, round apically located grade 1 nuclei compared to cases of pRCC that consistently showed presence of FCs lined by cuboidal to columnar epithelial cells with variable degree of cytologic atypia. Features characteristic of pRCC like foamy macrophages, hemosiderin laden macrophages, nuclear grooves or psammoma bodies were not present. No necrosis or mitosis were identified. By immunohistochemistry (IHC) the tumor cells were positive for cytokeratin 7, GATA-3 and AMACR (focal) and negative for CA-IX, CD117 and vimentin. Cytomorphologic features of PRNRP are unique and characterized by tight 3-D clusters (with or without FCs) of cuboidal cells with small round apically located nuclei and finely granular oncocytic cytoplasm. Specific diagnosis of PRNRP on cytology or CNB is feasible along with use of ancillary tests IHC and /or molecular tests.
Keywords
Cytology
Immunohistochemistry
Papillary renal neoplasm with reverse polarity
Papillary renal cell carcinoma
Touch preparation
INTRODUCTION
Papillary renal neoplasm with reverse polarity (PRNRP) is an emerging oncocytic renal neoplasm. This entity was first described in 2019 by Al-Obaidy et al.[1] In the current World Health Organization (WHO) 5th edition, this entity is included under the papillary renal cell carcinoma (pRCC) category.[2,3] Subsequent studies have shown that these tumors have specific morphologic, immunohistochemical, and molecular features and propose that this entity be separated from pRCCs as these tumors tend to have indolent clinical behavior.[4-14] These tumors affect adults and show slight male preponderance. These tumors are well-circumscribed and may be partly cystic.[11] Histologically, PRNRPs are characterized by papillary architecture lined by a single layer of cuboidal cells with granular eosinophilic/oncocytic cytoplasm and apically located small round bland nuclei. The most characteristic feature is the positioning of the small round nuclei away from the basement membrane, reflecting the nomenclature given to describe these tumors.[4-14] By immunohistochemistry (IHC), these tumors show diffuse expression of CK7 and GATA-3 and are typically negative for CD117 and vimentin. Molecular analysis has shown that these tumors (80–90%) show alterations of KRAS gene[6-10] and chromosomal abnormalities in 7, 17, and Y chromosomes (30%). Differential diagnosis of these tumors includes pRCCs that are typically managed by surgery.
Fine-needle aspiration and/or core-needle biopsy (CNB) with touch preparation (TP) are increasingly used for diagnosing incidentally detected small renal masses. Specific subtyping of renal neoplasms by cytology is possible in the majority of the cases. The results can guide further management of patients.
Cytomorphologic features of this emerging entity have not been described in the literature before. In this study, we report cytomorphologic features of a case of PRNRP and compare with cytomorphologic features of pRCCs reported in the literature.
MATERIAL AND METHODS
One case of PRNRP was identified from the pathology database at the Ohio State University (OSU). This case was a consult case of renal mass CNB with TP. IHC was performed on formalin-fixed paraffin-embedded tissue blocks at the OSU as well as at the referring hospital. Clinical presentation, cytomorphologic features, ancillary tests, and histopathology results were analyzed. Cytomorphology features were assessed, including cell size, cytoplasm, nuclear grade, nuclear grooves, foamy histiocytes, hemosiderin-laden histiocytes, psammoma bodies, hyaline globules, and inflammation. Nuclear grade was assigned using the World Health Organization/International Society of Urologic Pathologists (ISUP) grading system as grade 1: No nucleoli or inconspicuous nucleoli at ×400, grade 2: Conspicuous and eosinophilic nucleoli at ×400, grade 3: Conspicuous and eosinophilic nucleoli at ×100, and grade 4: Extreme nuclear pleomorphism, multinucleated tumor giant cells, and rhabdoid or sarcomatoid differentiation. Cytomorphologic features were compared to the reported cytomorphologic features of pRCCs.
Clinical presentation
A 65-year-old woman presented with low-back pain. During the work-up, computed tomography (CT) of the abdomen showed a small mass (1.1 cm) in the mid-pole of the left kidney [Figure 1]. The patient underwent CNB with TP of this mass at an outside institution.
Cytomorphology
The TP was cellular [Figure 2] and showed tight 3-D clusters of epithelial cells with variable presence of fibrovascular cores (FCs). The cells of PRNRP were cuboidal with round apically located nuclei reminiscent of the histomorphology of these tumors. The nuclei were small, round with regular nuclear contour and inconspicuous nucleoli (World Health Organization/ISUP grade 1). The background showed singly scattered and loosely cohesive clusters of oncocytic cells with moderate cytoplasm and round eccentric nuclei. Occasional oncocytic cells showed intranuclear pseudoinclusion and binucleation. No necrosis or mitosis was identified. Clear cells, foamy macrophages, hemosiderin-laden macrophages, nuclear grooves, hyaline globules, or psammoma bodies were not present. [Table 1] summarizes the comparison between cytomorphologic features of pRCCs reported in the literature and PRNRP in the present study.
Histomorphology
The biopsy [Figure 3] showed papillae lined by a single layer of cuboidal cells with finely granular eosinophilic cytoplasm, low-grade (World Health Organization/ISUP grade1) nuclei, and inconspicuous nucleoli and characteristic apical nuclear localization away from the basement membrane (reverse polarity) [Figure 2]. Some of the FCs were hyalinized. Foamy macrophages or psammoma bodies were not identified.
Ancillary tests: IHC
The tumor cells showed diffuse expression of cytokeratin 7 and GATA-3 and focal expression of AMACR. The tumor cells were negative for CAIX, CD-117, and vimentin.
Small biopsy size precluded molecular testing in the present case.
Follow-up
The patient is currently under active surveillance. A follow-up CT scan of the abdomen showed stable tumor size after 6 months of initial diagnosis.
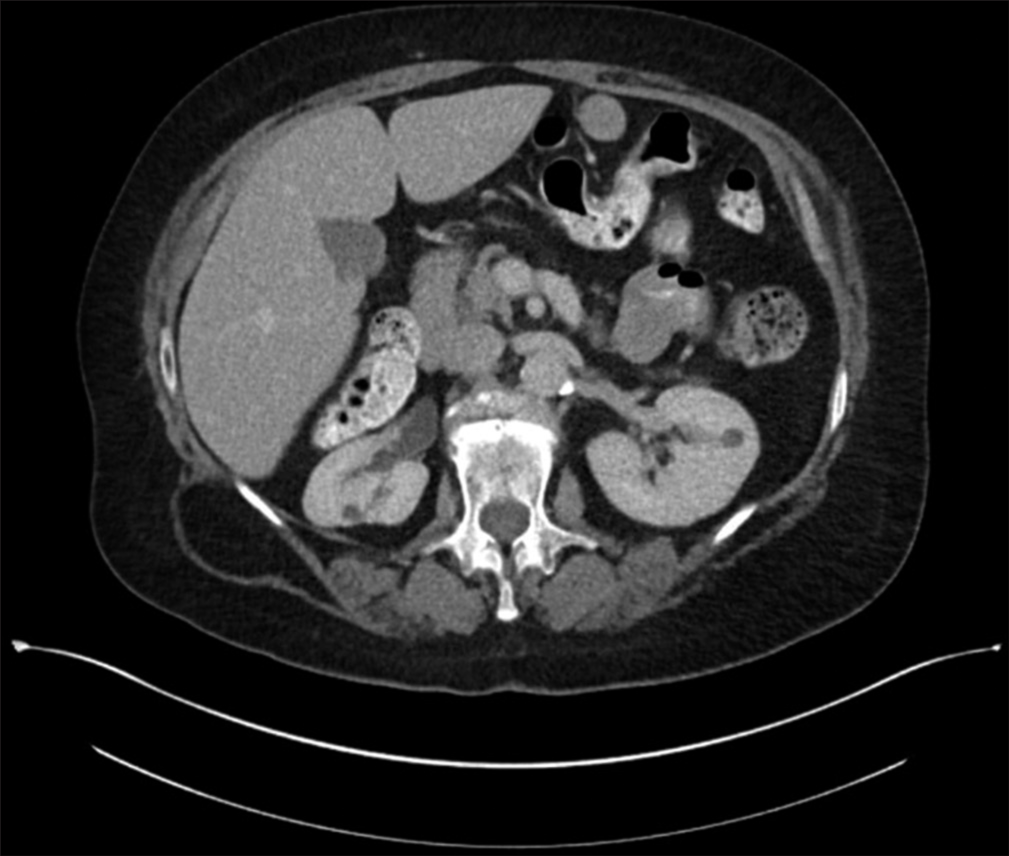
- Computed tomography (CT) abdomen showing small renal mass involving left kidney.
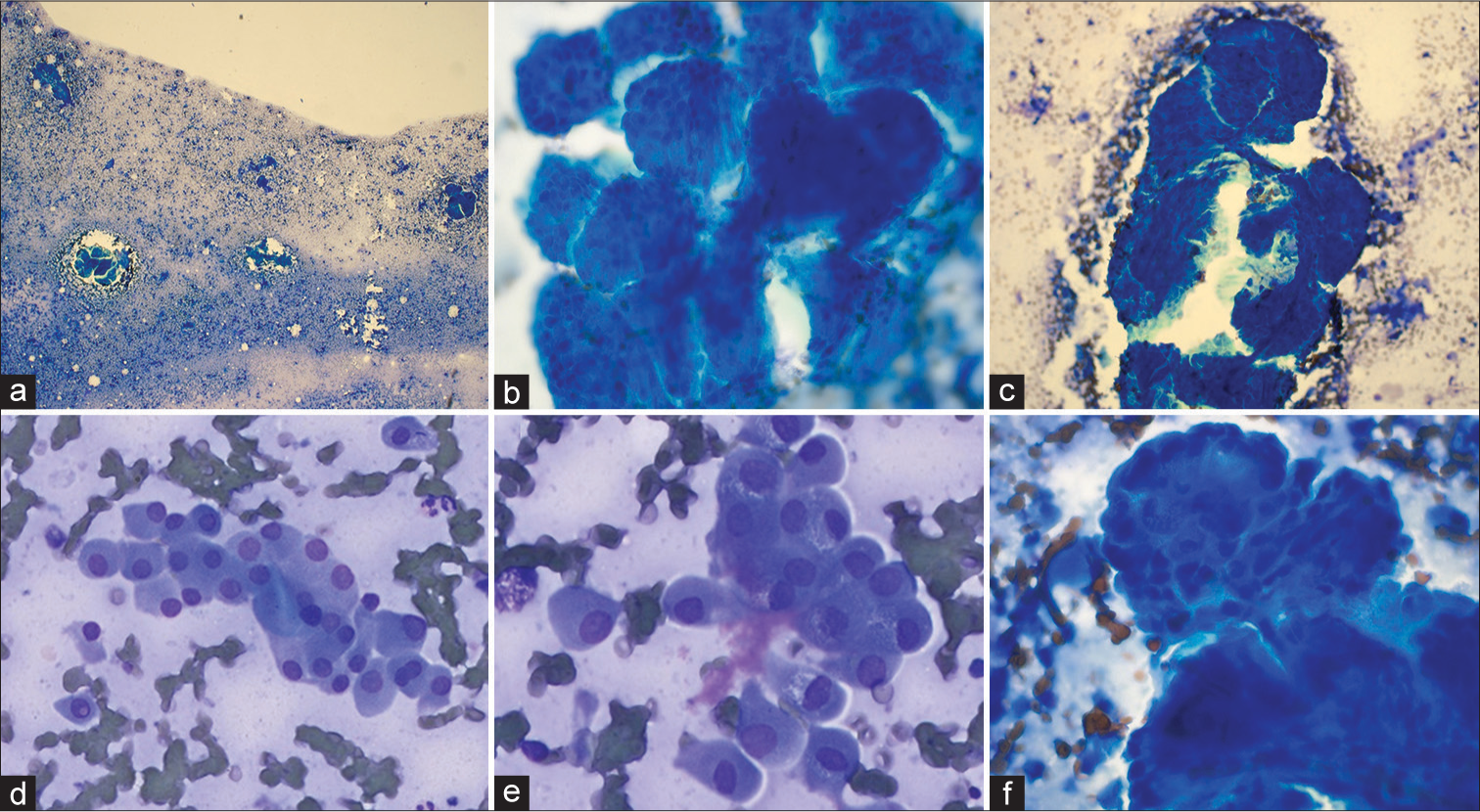
- (a) Highly cellular touch preparation showing tight cohesive 3-D clusters of tumor cells and singly dispersed cells in the background. (b) The cells are arranged in 3-D clusters with variable fibrovascular cores (FCs). (c) Note the presence of characteristic FC transgressing the tumor cell cluster. (d) Background shows singly dispersed and loosely cohesive clusters of oncocytic cells with round eccentric nuclei and abundant eosinophilic granular cytoplasm. (e) Some of the single cells showed intranuclear pseudo-inclusion and bi-nucleation. (f) Note the characteristic apical localization of nuclei away from the FC.
| Cytologic features | PRNRP | pRCC |
|---|---|---|
| 3-D clusters | + | ± |
| Lose clusters | + | ± |
| Singly scattered oncocytic cells | + | ± |
| Papillary structures | + | + |
| Fibrovascular cores | ± | ++ |
| Cell size | Small | Small-medium-large |
| Cell types | Cuboidal | Cuboidal, columnar, polygonal |
| Cytoplasm | Fine granular, oncocytic | Light basophilic, clear or eosinophilic |
| Cytoplasmic volume | Moderate | Variable |
| Nuclear features | Round nuclei with inconspicuous nucleoli | Round to oval, variably prominent nucleoli |
| Nuclear grade | 1 | 1–4 |
| Nuclear grooves | - | ± |
| Mitosis | - | + |
| Necrosis | - | ± |
| Foamy macrophages | - | ± |
| Hemosiderin-laden macrophages | - | ± |
| Psammoma bodies | - | ± |
| Intracytoplasmic hemosiderin | - | + |
| Inflammation | - | + (Warthin like variant of pRCC) |
| Hyaline globules | - | ± |
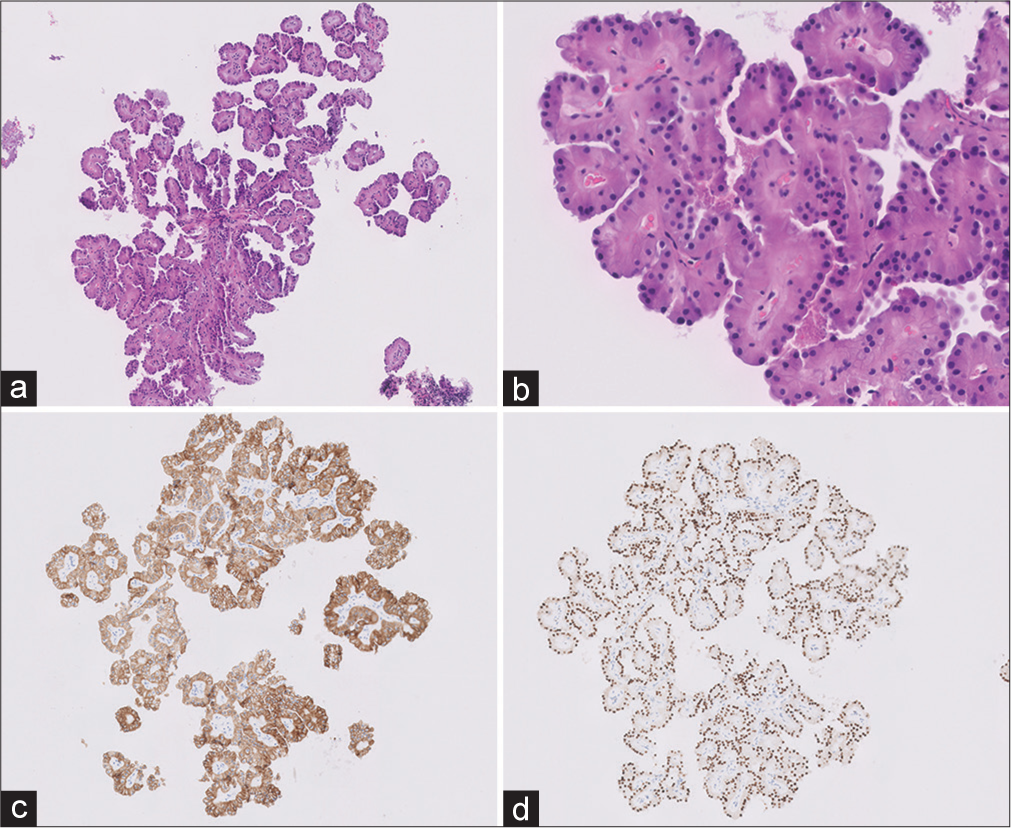
- (a and b) Core-needle biopsy showing a papillary tumor with fibrovascular cores lined by single layer of cuboidal oncocytic cells. The cells show presence of round monomorphic nuclei with inconspicuous nucleoli away from the basement membrane (Hematoxylin and eosin ×10) (c and d). The tumor cells were positive for CK7 and GATA-3, respectively.
DISCUSSION
PRNRP is a distinct entity with unique histomorphology, IHC, and KRAS missense mutation. The diagnosis can be made based on morphology and IHC.[1-14] To the best of our knowledge, this is the first study describing the cytologic features of PRNRP. In this case study, the TP was cellular and showed 3-D tight clusters of epithelial cells with variable presence of FCs. The background showed singly dispersed and loosely cohesive clusters of oncocytic cells. The cells of PRNRP were cuboidal to polygonal with round apically located nuclei reminiscent of the histomorphology of these tumors. The nuclei were small, round with regular nuclear contour and inconspicuous nucleoli (World Health Organization/ISUP grade 1). No necrosis or mitosis was identified. Differential diagnosis includes low-grade pRCCS and other renal tumors with papillary architecture. [Table 1] compares cytologic features PRNRP and pRCCs. As per the new World Health Organization 5th edition, subclassification of pRCCs to type 1 and type 2 is no longer required. Cytologic features of pRCC[15-19] include cellular smears composed of abundant papillary structures with true FCs or 3-D spheres of tumor cells and few single cells. The tumor cells show variable amounts of cytoplasm, small nuclei with mild-to-moderate hyperchromasia with nucleoli and grooves. The background shows the variable presence of histiocytes and psammoma bodies. The tumor cells show intracytoplasmic hemosiderin pigment in a subset of cases. It is feasible to distinguish PRNRP from pRCC based on cytologic features. In challenging cases of low-grade pRCCs, IHC should be used. PRNRP shows a diffuse expression of CK7 and GATA-3. The tumor cells show focal or negative AMACR staining. pRCCs show diffuse expression of CK7 and AMACR and are typically negative for GATA-3. PRNRP shows characteristic KRAS missense mutation. pRCCs show trisomy 7, 17, and loss of Y chromosome and MET gene mutations in low-grade pRCCs.[20]
Other renal tumors with papillary architecture include papillary adenoma, clear cell pRCC, fumarate hydratase (FH) deficient RCC, eosinophilic solid cystic RCC, collecting duct carcinoma, and TFE3 re-arranged RCC. A renal papillary adenoma is morphologically similar to low-grade pRCC and would not demonstrate reverse nuclear polarity as seen in PRNRP. CPRCT[21] shows clear cytoplasm by definition and apical localization of nuclei like PRNRP, but these entities can be effectively distinguished using IHC. CPRCTs are positive for CK7, which show characteristic cup-like positivity of CA-IX and variable positivity for CD10 and AMACR whereas PRNRPs are typically negative for CAIX. FH-deficient RCC, eosinophilic solid cystic RCC, and collecting duct carcinoma are high-grade renal carcinomas and, therefore, are less likely to be considered in the differential diagnosis of PRNRP. Cytological features of some of these tumors are described in the literature. FH-deficient RCC shows cell clusters, voluminous cytoplasm, macro-nucleoli, and nuclear pleomorphism. TFE3 re-arranged RCC shows papillary architecture with epithelioid clear cells and abundant psammoma bodies. Due to the presence of oncocytic cells, other differential diagnoses include oncocytoma, chromophobe RCC, and low-grade oncocytic tumor.[22] Morphologic features helpful for differentiation include a lack of papillary architecture in these tumors. By IHC, oncocytoma and chromophobe RCC show diffuse expression of CD117 as opposed to PRNRP that is typically negative for CD117.
Simple excision is curative for this tumor. However, given the indolent nature of these tumors, active surveillance remains the best choice for managing these patients. Whether these tumors are precursors to the conventional pRCC remains to be explored.
SUMMARY
Cytomorphologic features of PRNRP are unique and characterized by tight 3-D clusters (with or without FCs) of cuboidal cells with small round apically located nuclei and finely granular oncocytic cytoplasm. Specific diagnosis of PRNRP on cytology or CNB is feasible along with the use of ancillary tests such as IHC and/or molecular. Knowledge of the cytomorphologic features of this emerging entity is essential to avoid unnecessary surgical intervention.
DATA AVAILABILITY STATEMENT
Data will be available on request to the corresponding author Dr. Swati Satturwar. swati.satturwar@osumc.edu
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
SS and AVP: Concept; SS: Data collection, analysis and writing draft and editing the draft; AVP: Data interpretation, supervision, review and editing of the manuscript.
All authors were equally involved in the conceptualization, review, writing, and editing of this manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This is an original case report. This work has not been submitted to any other article. Either the approval from the local ethic review board or an informed consent statement. This study was approved by the institutional review board of The Ohio State University on July 1, 2022.
LIST OF ABBREVIATIONS (IN ALPHABETIC ORDER)
CNB - Core needle biopsy
CPRCT - Clear cell papillary renal cell tumor
FC - Fibrovascular core
FFPE - Formalin fixed paraffin embedded
FH= Fumarate hydratase
IHC – Immunohistochemistry
ISUP - International Society of Urologic pathologists
OSU - Ohio State University
PRNRP - Papillary renal neoplasm with reverse polarity
pRCC - Papillary renal cell carcinoma
RCC - Renal cell carcinoma
TFE3 - Transcription Factor Binding to IGHM Enhancer 3.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of cytojournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
References
- Papillary renal neoplasm with reverse polarity: A morphologic, immunohistochemical, and molecular study. Am J Surg Pathol. 2019;43:1099-111.
- [CrossRef] [PubMed] [Google Scholar]
- WHO 2022 landscape of papillary and chromophobe renal cell carcinoma. Histopathology. 2022;81:426-38.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent KRAS mutations identified in papillary renal neoplasm with reverse polarity-a comparative study with papillary renal cell carcinoma. Mod Pathol. 2020;33:690-9.
- [CrossRef] [PubMed] [Google Scholar]
- New developments in existing WHO entities and evolving molecular concepts: The genitourinary pathology society (GUPS) update on renal neoplasia. Mod Pathol. 2021;34:1392-424.
- [CrossRef] [Google Scholar]
- Papillary renal neoplasm with reverse polarity: A clinical, pathologic, and molecular study of 8 renal tumors from a single institution. Arch Pathol Lab Med. 2023;147:692-700.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent KRAS mutations in papillary renal neoplasm with reverse polarity. Mod Pathol. 2020;33:1157-64.
- [CrossRef] [PubMed] [Google Scholar]
- Morphological, immunohistochemical, and genomic analyses of papillary renal neoplasm with reverse polarity. Hum Pathol. 2021;112:48-58.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent KRAS mutations are early events in the development of papillary renal neoplasm with reverse polarity. Mod Pathol. 2022;35:1279-86.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary renal neoplasm with reverse polarity: A clinicopathological and molecular genetic characterization of 16 cases with expanding the morphologic spectrum and further support for a novel entity. Front Oncol. 2022;12:930296.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological and molecular characterisation of papillary renal neoplasm with reverse polarity and its renal papillary adenoma analogue. Histopathology. 2021;78:1019-31.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary renal neoplasm with reverse polarity is often cystic: Report of 7 cases and review of 93 cases in the literature. Am J Surg Pathol. 2022;46:336-43.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary renal neoplasm with reverse polarity with a favorable prognosis should be separated from papillary renal cell carcinoma. Hum Pathol. 2022;127:78-85.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary renal cell neoplasm with reverse polarity: A new subtype of renal tumour with favorable prognosis. Actas Urol Esp (Engl Ed). 2022;46:600-5.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary renal neoplasm with reverse polarity-a comparative study with CCPRCC, OPRCC, and PRCC1. Hum Pathol. 2022;129:60-70.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary renal-cell carcinoma: Fine-needle aspiration of 15 cases. Diagn Cytopathol. 1991;7:198-203.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic diagnosis of the papillary variant of renal-cell carcinoma. Acta Cytol. 1987;31:325-9.
- [Google Scholar]
- Abundant intracytoplasmic hemosiderin in both histiocytes and neoplastic cells: A diagnostic pitfall in fine-needle aspiration of cystic papillary renal-cell carcinoma. Diagn Cytopathol. 2001;24:82-5.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of a renal cell adenocarcinoma with massive intracellular hemosiderin accumulation. Diagn Cytopathol. 1991;7:147-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphologic comparison of Type 1 and Type 2 papillary renal cell carcinoma: A retrospective analysis of 28 cases. Cancer Cytopathol. 2019;127:370-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374:135-45.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphologic analysis of clear cell papillary renal cell carcinoma: Distinguishing diagnostic features. Cancer Cytopathol. 2021;129:192-203.
- [CrossRef] [PubMed] [Google Scholar]
- Algorithm-based approach to the histological routine diagnosis of renal oncocytic tumors in core biopsy specimens. Curr Urol Rep. 2022;23:327-33.
- [CrossRef] [PubMed] [Google Scholar]








