Translate this page into:
Cytomorphology of unusual primary tumors in the Pap test
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Rare entities in the Pap test, which include neoplastic and non-neoplastic conditions, pose challenges due to the infrequent occurrence of many of these entities in the daily practice of cytology. Furthermore, these conditions give rise to important diagnostic pitfalls to be aware of in the Pap test. For example, cases with adenoma malignum (AM) have been called benign. Recognition of these conditions can help correctly interpret Pap tests as abnormal and thereby ensure that patients get appropriately diagnosed. In this paper, we illustrate and discuss selected uncommon primary neoplastic lesions of the cervix and the vagina that may be seen in Pap test, with a focus on cytomorphology, differential diagnosis and the role of possible ancillary studies. These cases include high-grade squamous intraepithelial lesion cells with small cell morphology; small cell carcinoma; large neuroendocrine carcinoma; glassy cell carcinoma; AM; malignant mixed Müllerian tumor; clear cell carcinoma and primary malignant melanoma. Recognition of these rare variants/neoplasms is important so that involved Pap tests are not diagnosed as benign and that patients with these conditions get additional follow-up.
Keywords
Cytology
cytopathology
gynecological cytology
malignant tumors
Pap test
INTRODUCTION
For more than half a century, the Pap test has been an effective way to screen women for the precursors of cancer of the uterine cervix. Rare entities in the Pap test, which include neoplastic [Table 1] and non-neoplastic conditions, pose challenges due to the infrequent occurrence of many of these entities in the daily practice of cytology. Furthermore, these conditions give rise to important diagnostic pitfalls to be aware of in the Pap test. For example, cases with adenoma malignum (AM) have been called benign in Pap tests, as well as in surgical specimens. Recognition of these conditions can help to improve the accuracy and precision of Pap test diagnoses and decrease the potential for misdiagnosis and litigation. In addition, it will help achieve more timely management of patients with such conditions. These rare entities can be divided into three categories: (1) Rare or unusual inflammatory or infectious conditions that affect the cervix or vagina, (2) rare types and special variants of primary cervical carcinoma and (3) secondary or extrauterine neoplasms of the cervix or vagina. Although it is helpful to think of these entities in those three categories, some entities fall into more than one of these categories. For instance, some malignancies (e.g. lymphoma, melanoma and small cell carcinoma) can be primary or secondary to the female genital tract. We have recently reported[1] the cytomorphology of selected rare infections and we focused on their cytomorphology, differential diagnosis and role of ancillary diagnostic studies. In this paper, we illustrate and discuss selected uncommon primary neoplastic lesions of the cervix and the vagina that may be seen in Pap test, with a focus on cytomorphology, differential diagnosis and the role of ancillary studies. The cases include high-grade squamous intraepithelial lesion cells (HSIL) with small cell morphology; small cell carcinoma; large neuroendocrine carcinoma; glassy cell carcinoma; AM; malignant mixed Müllerian tumor (MMMT); clear cell carcinoma; and primary malignant melanoma. Recognition of these rare variants/neoplasms will decrease the potential for misdiagnosis and expedite appropriate management of patients with these conditions.

A brief discussion on the role of ancillary tests is also included.
HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION OF SMALL CELL TYPE
HSIL small cell type or small cell carcinoma in situ (CIS)[234] is derived from reserve or basal cells of the endocervical epithelium. Small malignant cells replace the epithelium lining the endocervical canal. The lesion frequently extends and involves the endocervical glands and is difficult to diagnose and to identify by colposcopy unless it involves the transformation zone.[3] Endocervical curettage may show tiny fragments of tumor that are sometimes difficult to interpret or are overlooked. Pap tests show single and clusters of small undifferentiated dysplastic cells with scant, often barely visible basophilic, or rarely eosinophilic cytoplasm[234] [Figure 1]. The cells have relatively hyperchromatic, coarsely granular nuclei that usually show irregularity of nucleus contour.[4] Some cases may show prominent nucleoli and rarely even cytoplasmic vacuoles may be seen. There is no necrosis or apoptosis as occurs with small cell carcinoma. However, some large dysplastic squamous or glandular cells may also be seen. This lesion is a frequent cause of false-negative diagnoses as these cells may be mistaken for follicular cervicitis, benign metaplastic cells or endometrial cells. The differential diagnosis also includes lymphoma and small cell carcinoma.[45]
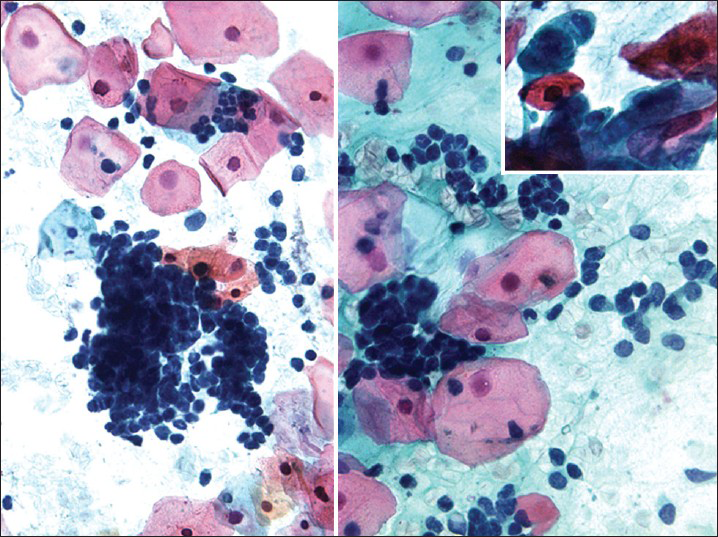
- High-grade squamous intraepithelial lesion of small cell type seen in a conventional Pap test. The hyperchromatic dysplastic cells are seen singly and in clusters (syncytial-like) (Pap stain, ×400). The insert shows the presence of a few accompanying dysplastic keratinizing squamous cells
SMALL CELL CARCINOMA
Small cell or neuroendocrine tumors of the uterine cervix are uncommon, accounting for 1-5% of cervical malignancies.[6] These tumors occur in women with a wide age range (21-94 years). As in other body locations, they are classified as typical carcinoid, atypical carcinoid, small cell carcinoma and large cell neuroendocrine carcinoma.[78] These tumors mostly occur in a pure form; however, they may coexist with squamous cell carcinoma or adenocarcinoma of the cervix. They are associated with human papillomavirus (HPV) type 16 or 18.[9] Most patients present with vaginal bleeding, discharge or post-coital spotting. However, paraneoplastic syndromes such as Cushing's syndrome, syndrome of inappropriate antidiuretic hormone, hypercalcemia, or hypoglycemia may be seen. Coloposcopy shows a white-yellow polypoid (exophytic), infiltrative or ulcerated mass (1-6 cm). Pap tests contain small cell carcinoma tumor cells dispersed as single cells or arranged as loosely cohesive sheets or gland-like aggregates. Tumor cells range from being small to large with pleomorphic, angulated nuclei that are hyperchromatic, show nuclear molding and smear artifact. Mitotic figures are common and karyorrhectic debris can be identified [Figures 2 and 3]. Small cell carcinoma should be differentiated from squamous cell carcinoma with small cell morphology [Figure 4]. Searching for a squamous component or ancillary studies can confirm this possibility. Ancillary studies for small cell carcinoma include positive immunostaining for CD 56, chromogranin, synaptophysin, cytokeratin, carcinoembryonic antigen (CEA) and variable immunoreactivity for serotonin, intestinal polypeptide or somatostatin.[10] Thyroid transcription factor-1 immunopositivity has been reported in rare small and large cell neuroendocrine carcinomas of the cervix. The prognosis is generally poor and is related to the degree of differentiation. Surgery followed by chemotherapy and radiation is the mainstay of therapy. The differential diagnoses include follicular cervicitis, HSIL of small cell type, benign small blue cell dilemma associated with tamoxifen therapy[5] and non-Hodgkin lymphoma.[11] In malignant lymphoma, the cells are individually scattered and loosely arranged in a dirty background with inflammatory cells. Nuclear pseudomolding may infrequently be seen with lymphoma. In squamous cell CIS, the tumor cells are arranged singly or in syncytial aggregates with smooth cell borders. Nuclei are hyperchromatic with coarsely granular and irregularly distributed chromatin. The N/C ratio is high. Cytoplasm is more appreciable when compared with small cell carcinoma. Neither nuclear molding nor a necrotic background is seen. High-grade lymphomas can have some dirty background and pseudomolding can also be difficult to distinguish from real molding of small cell carcinoma. In follicular cervicitis, the lymphoid cells consist of a reactive polymorphous population including lymphocytes in every stage of maturation as well as germinal center macrophages containing phagocytosed cellular debris. Abundant reactive mature and immature lymphoid cells might be misinterpreted as small cell carcinoma cells. The chromatin of lymphocytes is more granular and delicate with more abundant cytoplasm compared to small cell carcinoma.[6] Other entities in the differential diagnosis include endometrial cells, adenocarcinoma (primary or metastatic with microacinar architecture as well as metastatic lobular carcinoma of the breast) and other unusual malignant neoplasms including small cell non-keratinizing squamous cell carcinoma, primitive neuroectodermal tumor, myeloid sarcoma, melanoma and undifferentiated sarcoma or undifferentiated carcinoma.

- Small cell carcinoma in a ThinPrep Pap test. The malignant cells are dispersed and loosely cohesive. They are pleomorphic and show nuclear molding (Pap stain, ThinPrep, ×400)
![Cervical biopsy with small cell carcinoma (corresponding to Pap test of case shown in Figure 2. H and E stain, ×100 [left] and ×400 [right])](/content/105/2013/10/1/img/CJ-10-17-g004.png)
- Cervical biopsy with small cell carcinoma (corresponding to Pap test of case shown in Figure 2. H and E stain, ×100 [left] and ×400 [right])
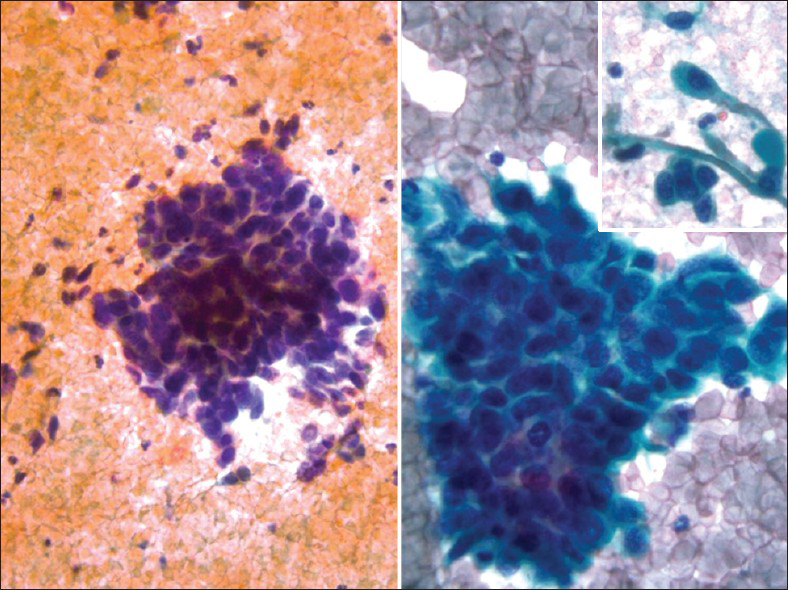
- Squamous cell carcinoma with small cell morphology in a conventional Pap test. The malignant cells are single and in clusters showing scant cytoplasm and small cell morphology with nuclear molding. However, few clusters and single cells showing characteristic squamous cell morphology with dense cytoplasm (right). The follow-up biopsy confirms squamous cell carcinoma (Pap stain, ×400)
LARGE CELL NEUROENDOCRINE CARCINOMA
Large cell neuroendocrine cervical carcinoma is an extremely rare and aggressive poorly differentiated cancer.[121314] It may occur during pregnancy and may also arise from a cervical polyp.[14] The cytomorphology can be mistaken for squamous or adenocarcinoma. Pap tests contain large cells dispersed as single cells or arranged as loosely cohesive sheets or hyperchromatic crowded groups or gland-like aggregates [Figure 5]. Tumor cells have moderately abundant cytoplasm with small to large angulated hyperchromatic nuclei. The nuclei are mildly pleomorphic with coarse chromatin and prominent nucleoli.[1213] Mitotic figures are common and karyorrhectic debris can be identified with no keratinization seen [Figure 5]. Ancillary studies can be performed on cell block material and show positive immunostaining for neuroendocrine markers.
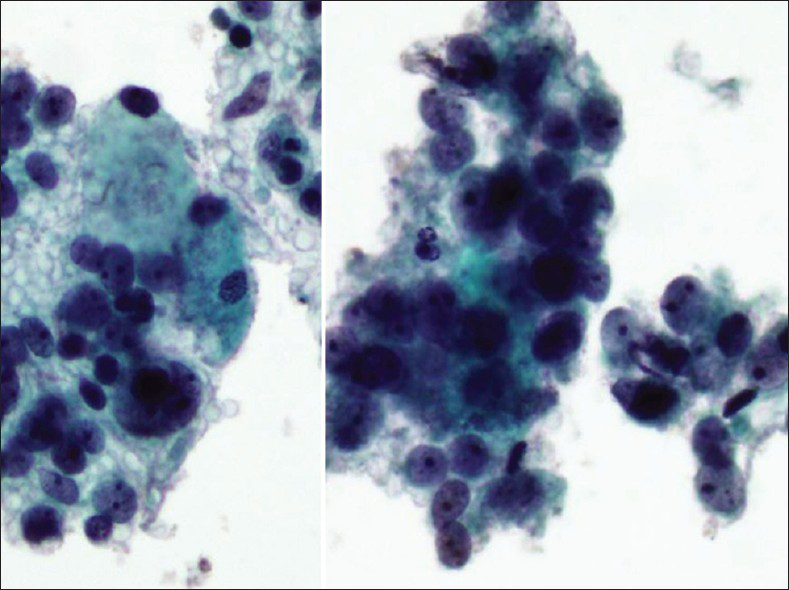
- Large neuroendocrine carcinoma in a ThinPrep Pap test from a 24-year-old female. The malignant cells are loosely cohesive. The nuclei are only mildly pleomorphic with one or more prominent nucleoli (Pap stain, ThinPrep, ×400)
GLASSY CELL CARCINOMA
Glassy cell carcinoma of the cervix is a rare variant of poorly differentiated adenosquamous carcinoma. It typically affects younger patients compared to other invasive cervical carcinomas, with a peak incidence in the 3rd to 4th decade.[1516] Some studies have noted an association with pregnancy. These tumors present as rapidly growing bulky exophytic masses or with a barrel shaped cervix. Histologically, they are characterized by sheets of large polygonal cells with finely granular ground glass type cytoplasm. These cells have distinct cell borders and vesicular nuclei with prominent nucleoli. The majority of the tumor cells lack intracellular bridges, dyskeratosis and intracellular glycogen. However, focal abortive keratin production, squamous or glandular differentiation may be present. There may be focal clear cell differentiation. Mitotic figures are frequently seen. The stroma is heavily infiltrated by lymphocytes, plasma cells and eosinophils.[151617] Glassy cells can show focal mucin and CEA positivity. Ultrastuctural findings show evidence of both squamous and glandular differentiation, including well-developed desmosomal complexes and microvilli. The ultrastructural correlate of the glassy cytoplasm is unclear. Deoxyribonucleic acid (DNA) of HPV types 18 and 16 have been detected in the tumor cells of glassy cell carcinoma.[18] In Pap tests [Figure 6], tumor cells are arranged in sheets and clusters with large with abundant granular (ground-glass-like) cytoplasm and large pleomorphic nuclei. The nuclei have coarse irregular chromatin and prominent nucleoli.[1920] The presence of large nucleoli is characteristic and may be mistaken for inclusions of herpes virus or Reed-Stenberg cells in Hodgkin's disease. Cytoplasmic vacuolization and bizarre cells with multinucleation can be seen. An inflammatory cell infiltrate, including lymphocytes, plasma cells and eosinophils may also be seen. Glassy cell carcinomas have also been reported in other organs such as colon,[21] the endometrium, vagina and urethra. The differential diagnosis is with other poorly differentiated neoplasms involving the cervix including non-keratinizing squamous cell carcinoma, poorly differentiated adenocarcinoma and clear cell carcinoma. The clinical course is similar to the other poorly differentiated carcinomas, but it distinguishes itself by affecting younger patients, making early recognition extremely important.
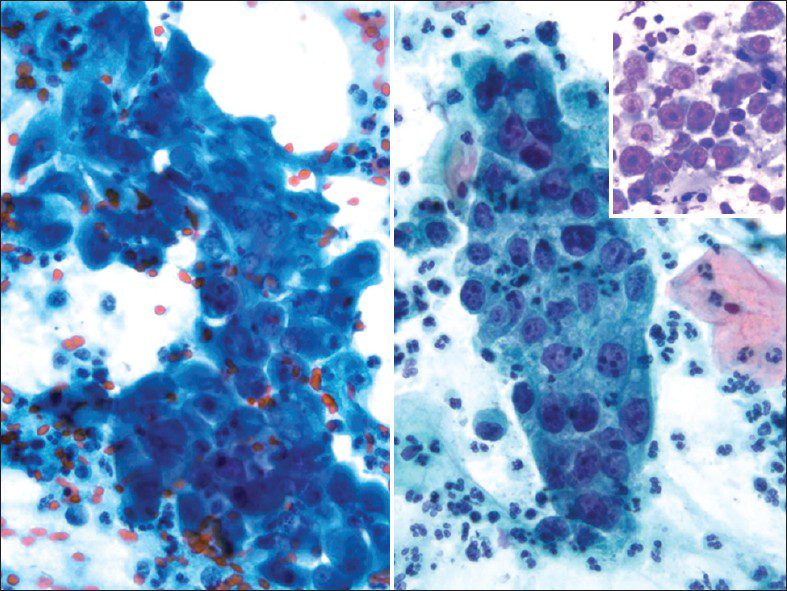
- Glassy cell carcinoma. The tumor cells are arranged in sheets with abundant granular (ground-glass-like) cytoplasm, large pleomorphic nuclei, coarse irregular chromatin and prominent nucleoli. An inflammatory cell infiltrate is present (Pap stain, ×400). Insert: Corresponding cervical biopsy showing prominent/large nucleoli (H and E stain, ×400)
ADENOMA MALIGNUM
Adenoma Malignum (AM) is a rare variant of endocervical adenocarcinoma. It is also known as minimal deviation adenocarcinoma of the cervix. It occurs in women of reproductive age (age ranges from 29-76 years).[22] The most common symptoms at presentation are abnormal vaginal bleeding (menometrorrhagia), mucoid and viscous vaginal discharge and abnormal vaginal bleeding. AM comprises only 1% of endocervical adenocarcinoma and the majority of these tumors are HPV negative.
The cervix in AM is grossly indurated and barrel-shaped (diffusely enlarged).[222324] The cervicovaginal cytology in AM shows a large number of abnormal glandular cells that closely resemble benign endocervical cells. They are arranged in clusters, strips and isolated cells [Figure 7]. The abnormal clusters exhibit loss of polarity with a disorganized “drunken” honeycomb sheet arrangement and the glandular strips have pseudostratification. There is also a spectrum of atypical nuclear changes. The individual cells are cuboidal to columnar and have abundant lacy, golden yellow vacuolated cytoplasm. Although the number of abnormal glandular clusters is large, frankly malignant cells are only seen in a minority of these cell groups. Their nuclei are enlarged (2-3 times the size of intermediate squamous nuclei) and pleomorphic with visible nucleoli [Figure 7]. The presence of “golden yellow” intracytoplasmic mucin reflects the production of neutral, gastric/pyloric-type phenotypic mucin. This unique feature is shared by AM and the pyloric gland pseudoneoplastic lesion known as lobular endocervical glandular hyperplasia (LEGH).[2526] However, LEGH does not show nuclear atypia or isolated/single cells in Pap test specimens. Furthermore, endocervical lobular hyperplasia may also show intranuclear inclusions, a feature that has not been described in AM or in other endocervical adenocarcinomas. AM has been shown to have a worse prognosis than other endocervical adenocarcinomas, due to the difficulty of establishing the diagnosis, which can lead to a late diagnosis at a higher stage where nodal involvement is more common. AM may be sporadic or associated with Peutz-Jeghers syndrome (PJS) in up to 15% of cases. The prognosis of patients associated with PJS is generally poor. The genetic locus of this syndrome has been mapped to chromosome 19p.[27] Ancillary studies can be performed on cell block material or on biopsy. AM exclusively produces neutral mucin and this differs from normal endocervical glands or the other endocervical adenocarcinomas that usually produce an equal amount of neutral and acidic mucin. Neutral mucin can be seen as red stained material by periodic acid schiff-Alcian blue pH 2.5 stains.[2328] NHIK-1083, an antibody that recognizes the pyloric phenotype of gastric mucous cells, is positive in AM; however, it may also be positive in LEGH. AM is also positive for CEA, Ki67 (>50% of tumor nuclei) and p53 while it is negative for estrogen receptor, progesterone receptor and in the majority of cases also negative for high risk HPV DNA.
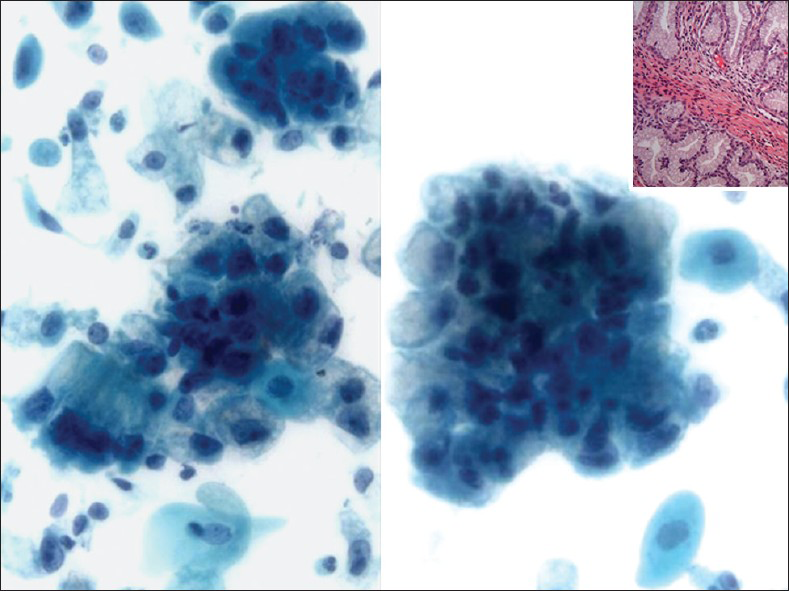
- Adenoma malignum in a liquid based preparation (SurePath). The specimen shows large numbers of atypical glandular clusters associated with some single endocervical-like cells with yellow/golden vacuolated cytoplasm. The cells have abnormal nuclei (enlargement, pleomorphism, crowding, visible nucleoli and loss of polarity) (Pap stain, ×400). Insert: Corresponding cervical biopsy (H and E stain, ×400). Images courtesy of Dr. David C. Wilbur, Massachusetts General Hospital, Boston, MA, USA
The differential diagnosis includes benign endocervical glands, atypical glandular cells, adenocarcinoma in situ (AIS) and endometrial adenocarcinoma.[242526] AIS shows loss of mucin and lacks abnormal single cells, which are both present in AM. The main architectural features that are helpful in recognizing AIS are tightly crowded sheets of glandular cells with overlapping nuclei and “ragged edged” borders. The honeycomb pattern and palisaded edge seen in clusters of normal endocervical cells are absent in AIS. In AIS, the clusters of endocervical cells may also show pseudostratification with nuclei seen at different levels within the groups. However, polarity of cells in AIS is maintained. Cells in AIS clusters may arrange in a circular pattern with peripheral location of nuclei. Feathering is another distinctive feature of AIS. Cell size does not necessarily increase with AIS. The nuclei of AIS have granular chromatin, which is evenly distributed. Nucleoli may be present, but are usually small and inconspicuous. Furthermore, there is no “golden yellow” mucin and no tumor diathesis in AIS. Endometrial adenocarcinoma shows more 3D balls or papillary configurations, has nuclei that typically show greater pleomorphism (higher grade of malignancy), loss of polarity, moderate hyperchromasia, irregular chromatin distribution and parachromatin clearing. The cytoplasm in endometrial adenocarcinoma is also usually scant, cyanophilic and vacuolated with characteristic intracytoplasmic neutrophils.
MALIGNANT MIXED MÜLLERIAN TUMOR
MMMT is an uncommon, but highly aggressive tumor. It is also called carcinosarcoma, sarcomatoid carcinoma, malignant mesodermal mixed tumor and metaplastic carcinoma. They are usually seen in postmenopausal women with a history of bleeding. They are more commonly seen in the endometrium or ovaries.[29] However, they can also rarely originate from the cervix, vagina, peritoneum or extragenital sites.[3031] Pre-disposing factors include a history of radiation therapy in younger patients and chronic estrogen stimulation. As with all endometrial adenocarcinomas, other predisposing factors include nulliparity, diabetes and obesity. MMMT is a biphasic, high-grade tumor with malignant epithelial and stromal components. The most common epithelial component is glandular (endometrioid, clear cell, serous) and these carcinoma areas are usually poorly differentiated. The sarcomatous components may be homologous (endometrial stromal sarcoma, leiomyosarcoma) or heterologous (muscle, cartilage, osteoid, fat). About 61% of uterine MMMT cases may exhibit malignant or abnormal findings on Pap test,[29] and an accurate diagnosis is based on identification of the two malignant components, i.e., carcinoma and sarcoma [Figures 8 and 9]. However, most cases usually lack the sarcoma component on Pap tests. Therefore, a poorly differentiated carcinoma or malignant neoplasm is the most rendered diagnosis for these cases. The Pap test in MMMT is usually hypercellular and shows high-grade malignant tumor cells composed of malignant epithelial or glandular cells admixed with malignant spindle cells [Figure 8]. The malignant spindle cell may show a variety of features[293031] including stromal sarcoma, leiomyosarcoma (homologous element) or rhabdomyosarcoma [Figures 8 and 9], chondrosarcoma or liposarcoma (heterologous element). The differential diagnosis includes botryoid rhabdomyosarcoma (seen in children/adolescents) and other poorly or undifferentiated tumors.
- Malignant mixed Müllerian tumor in a conventional Pap test from a 60-year-old women. The smear shows poorly differentiated carcinoma (left) mixed with malignant spindle cells (right). The malignant spindle cells show cross striations, a feature suggestive of rhabdomyosarcoma (i.e., heterologous element) (Pap stain, ×400)
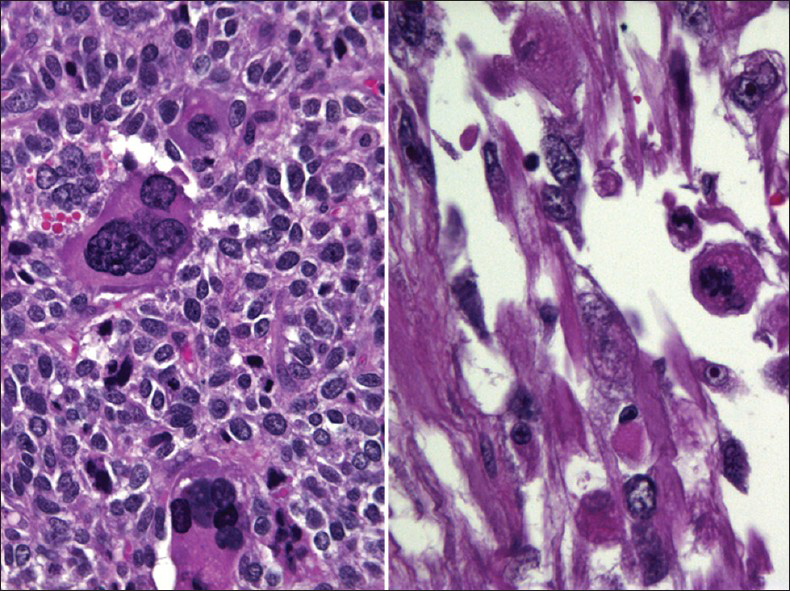
- Histopathology of malignant mixed Müllerian tumor corresponding to the case shown in Figure 8. The tumor shows combined adenocarcinoma and rhabdoid components (heterologous element) (H and E stain, ×400)
CLEAR CELL CARCINOMA
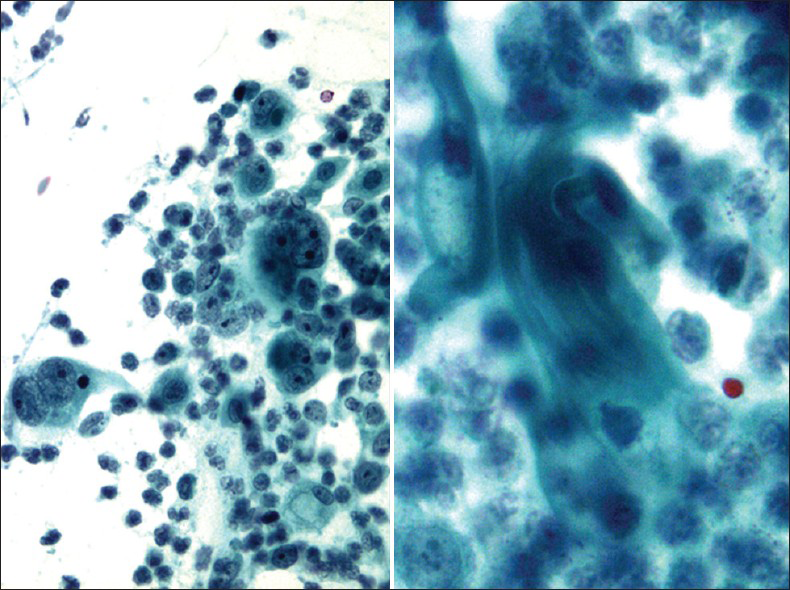
Clear cell carcinoma of the cervix or the vagina is a rare adenocarcinoma of Muüllerian origin that has a slightly worse prognosis than cervical adenocarcinoma.[3233] It occurs more in daughters of women who took diethylstilbestrol (DES), a non-steroidal estrogen, during their pregnancies. The peak age for DES-associated clear cell carcinoma is between 14 and 22 years and for non-DES-associated between 13 and 80 years. Due to the ban on usage of DES, DES-associated clear cell carcinoma currently is extremely rare and generally affects older women.[32] Histologically, they are characterized by three growth patterns: solid, tubulocystic and papillary. The cells have clear cytoplasm, apical hobnail nuclei and prominent nucleoli. In a Pap test,[3435] the cells are arranged in sheets, clusters or papillae. The cells have delicate vacuolated glycogen-rich cytoplasm that may show naked nuclei and a tigroid-background [Figure 10] similar to other glycogen-containing tumor cells such as seminoma and Ewing sarcoma. The nuclei are large, pale, and round with prominent nucleoli. HPV has been detected in only 40% of clear cell carcinoma in both DES- and non-DES-associated tumors.[36]
![Clear cell carcinoma in a conventional Pap test. The smear shows tumor cells arranged in sheets and papillae with abnormal pleomorphic nuclei and a tigroid-background (left) (Pap stain, ×200 [left], and ×400 [right])](/content/105/2013/10/1/img/CJ-10-17-g011.png)
- Clear cell carcinoma in a conventional Pap test. The smear shows tumor cells arranged in sheets and papillae with abnormal pleomorphic nuclei and a tigroid-background (left) (Pap stain, ×200 [left], and ×400 [right])
MALIGNANT MELANOMA
Primary cervicovaginal melanoma is a rare malignancy with only approximately 60 cases reported in the uterine cervix.[3738] They occur predominantly in postmenopausal women and present in most cases with abnormal vaginal bleeding. They have a poor prognosis with high mortality rate. Primary melanomas of the cervix or vagina likely originates from melanocytes known to be present in the cervix. An abnormal Pap test may be the initial presentation of a vaginal or cervical melanoma with most cases presenting as poorly differentiated malignant neoplasms.[39404142] Therefore, they should always be included in the differential diagnosis of a poorly differentiated tumor. The differential diagnosis of poorly differentiated malignant neoplasms in Pap tests includes poorly differentiated squamous or adenocarcinoma, malignant mixed mesodermal tumor, rhabdomyosarcoma, stromal sarcoma and anaplastic lymphoma.
Malignant melanoma in a Pap test[39404142] tends to be highly cellular with predominantly of isolated cells as in other poorly differentiated neoplasms. The cells vary in size and show epithelioid [Figure 11] or spindle cell [Figure 12] morphology. The epithelioid morphology is more common and shows relatively well-defined cytoplasm with a granular or dusty appearance and with round nuclei and often show prominent or giant nucleoli. Melanin pigment within tumor cells or melanophages may be seen, but may not always be present. Binucleation or multinucleation and tumor diathesis are common. The cells may show abundant cytoplasm and eccentric nuclei. There is intranuclear inclusions and central nuclear grooving, the latter are more common in spindle cell melanoma [Figure 12]. Ancillary studies can be performed on cell block material and includes melanocytic markers such as S100, HMB-45, Mart1, tyrosinase MiTF.[43]

- Malignant epithelioid melanoma in SurePath preparation. Numerous tumor cells can be seen dispersed among mature squamous cells. The tumor cells show pleomorphism and have round nuclei with prominent nucleoli. Note the binucleated tumor cell present in the bottom of the left image (Pap stain, ×400). The follow-up in this case showed primary malignant melanoma that presented as an endocervical polyp (insert, H and E stain, ×400)
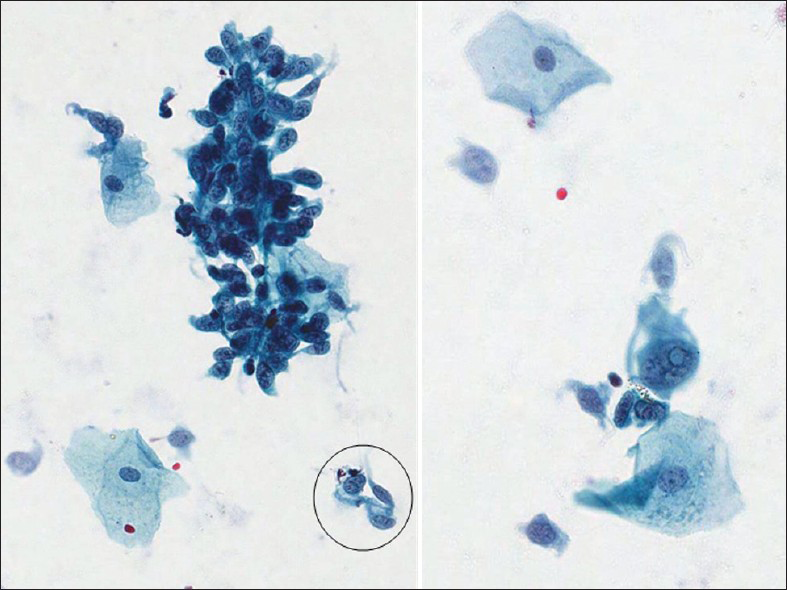
- Malignant spindle cell melanoma from a 73-year-old patient in a conventional cervicovaginal smear. There are numerous single and clusters of spindle cells, among mature squamous cells, showing pleomorphic abnormal nuclei with central grooving (circle) and intranuclear psudoinclusion (right) (Pap stain, ×400). The follow-up in this case showed primary malignant melanoma of the vagina
CONCLUSION
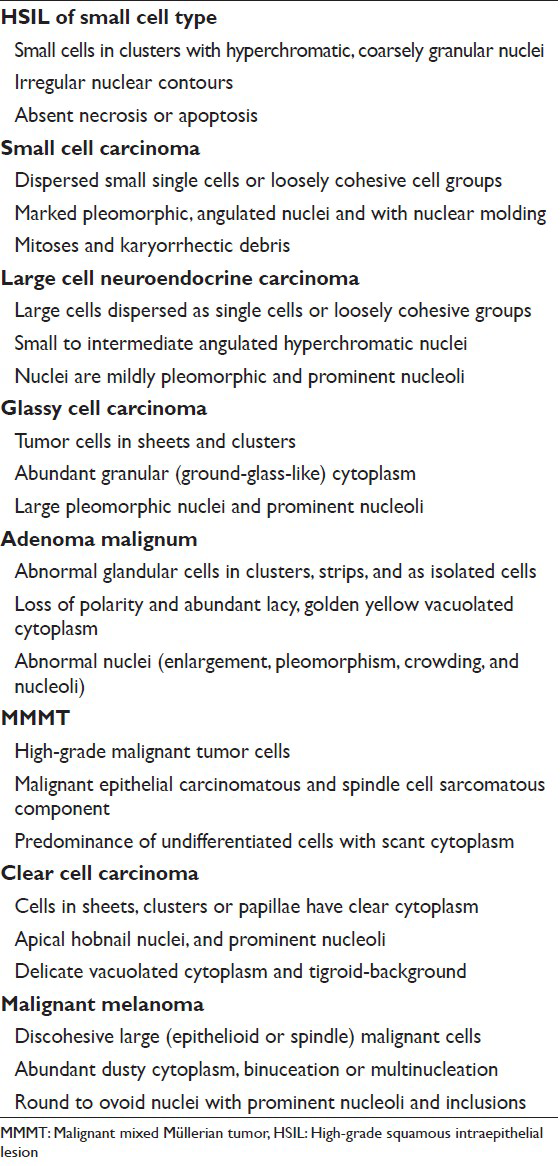
Rare neoplastic entities in a Pap test pose challenges due to the infrequent occurrence in the daily practice of cytology. Furthermore, these conditions give rise to important diagnostic pitfalls to be aware of in the Pap test. Recognition of these conditions can help correctly interpret Pap tests as being abnormal and thereby ensure that patients with these conditions receive expedited and appropriate management. A summary of the cytomorphology of selected entities are shown in Table 2.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All author(s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board (IRB) and granted exempt status. We take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/17/117356
REFERENCES
- Cytomorphology of unusual infectious entities in the pap test. Cytojournal. 2012;9:15.
- [Google Scholar]
- Diagnostic cytopathology of the uterine cervix. In: Wied GL, ed. Monograph in Clinical Cytology Vol 3. (2nd ed). Basel: S Karger; 1978. p. :1-209.
- [Google Scholar]
- The Pap test. In: DeMay RM, ed. The Art and Science of Cytopathology (2nd ed). ASCP Press: Chicago, IL; 2012. p. :70.
- [Google Scholar]
- The small blue cell dilemma associated with tamoxifen therapy. Arch Pathol Lab Med. 2001;125:1047-50.
- [Google Scholar]
- Pap smear screening for small cell carcinoma of the uterine cervix: A case series and review of the literature. J Gynecol Oncol. 2011;22:39-43.
- [Google Scholar]
- Small cell neuroendocrine carcinoma of the cervix: Outcome and patterns of recurrence. Gynecol Oncol. 2004;93:27-33.
- [Google Scholar]
- Exfoliative cytology of invasive neuroendocrine small cell carcinoma in a cervical cytologic smear. A case report. Acta Cytol. 1996;40:980-4.
- [Google Scholar]
- Small-cell neuroendocrine carcinoma of the cervix. A human papillomavirus type 18-associated cancer. Am J Surg Pathol. 1991;15:28-32.
- [Google Scholar]
- Immunocytochemical diagnosis of small cell undifferentiated carcinoma of the cervix. Acta Cytol. 1993;37:131-4.
- [Google Scholar]
- Lymphoma of the female genital tract: Current status. Int J Gynecol Pathol. 2006;25:1-21.
- [Google Scholar]
- Smear cytology findings of large cell neuroendocrine carcinoma of the uterine cervix. Diagn Cytopathol 2011 Epub ahead of print
- [Google Scholar]
- Cervical large cell neuroendocrine carcinoma with cytologic presentation: A case report. Acta Cytol. 2010;54:977-80.
- [Google Scholar]
- Large-cell neuroendocrine carcinoma of the uterine cervix complicating pregnancy. Hong Kong Med J. 2009;15:69-72.
- [Google Scholar]
- Glassy cell features in adenosquamous carcinoma of the uterine cervix. Histologic, ultrastructural, immunohistochemical, and clinical findings. Am J Clin Pathol. 1991;96:520-8.
- [Google Scholar]
- Human papillomavirus typing of rare cervical carcinomas. Arch Pathol Lab Med. 2004;128:553-6.
- [Google Scholar]
- Glassy cell carcinoma of the uterine cervix. Report of a case with cytohistologic and immunohistochemical study. Acta Cytol. 2001;45:407-10.
- [Google Scholar]
- Glassy cell carcinoma of the cervix: Cytologic features and expression of estrogen receptor, progesterone receptor and Her2/neu protein. Acta Cytol. 2006;50:418-22.
- [Google Scholar]
- Glassy cell carcinoma of the colon with human chorionic gonadotropin-production. A case report with immunohistochemical and ultrastructural analysis. Am J Surg Pathol. 1996;20:187-92.
- [Google Scholar]
- Adenoma malignum of the uterine cervix: Clinicopathologic analysis of 18 cases. Kaohsiung J Med Sci. 2012;28:161-4.
- [Google Scholar]
- Adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix. A clinicopathological and immunohistochemical analysis of 26 cases. Am J Surg Pathol. 1989;13:717-29.
- [Google Scholar]
- Cytologic and cytochemical features of adenoma malignum of the uterine cervix. Cancer. 1999;87:245-53.
- [Google Scholar]
- Diagnostic significance of endocervical glandular cells with “golden-yellow” mucin on pap smear. Diagn Cytopathol. 2002;27:80-4.
- [Google Scholar]
- Cytologic findings in minimal deviation adenocarcinoma (adenoma malignum) of the cervix. A report of seven cases. Am J Clin Pathol. 1996;105:327-33.
- [Google Scholar]
- A distinct region of chromosome 19p13.3 associated with the sporadic form of adenoma malignum of the uterine cervix. Cancer Res. 1998;58:1140-3.
- [Google Scholar]
- Utility of the monoclonal antibody HIK1083 in the diagnosis of adenoma malignum of the uterine cervix. Gynecol Oncol. 1999;75:345-8.
- [Google Scholar]
- Cervicovaginal cytology in carcinosarcoma malignant mixed mullerian (mesodermal) tumor of the uterus. Diagn Cytopathol. 1992;8:33-40.
- [Google Scholar]
- Malignant mixed mullerian tumor (MMMT) of the cervix. Gynecol Oncol. 2005;97:442-5.
- [Google Scholar]
- Cervicovaginal (Papanicolaou) smear findings in patients with malignant mixed müllerian tumors. Diagn Cytopathol. 2003;28:245-9.
- [Google Scholar]
- Clear cell carcinoma of the cervix: A multi-institutional review in the post-DES era. Gynecol Oncol. 2008;109:335-9.
- [Google Scholar]
- Clear cell carcinoma of the uterine cervix: Pathology and prognosis in surgically treated stage IB-IIB disease in women not exposed in utero to diethylstilbestrol. Gynecol Oncol. 2000;76:331-5.
- [Google Scholar]
- Cytologic examination to detect clear cell adenocarcinoma of the vagina or cervix. Gynecol Oncol. 1999;75:338-44.
- [Google Scholar]
- Clear-cell endocervical adenocarcinoma in a 19-year-old woman. Diagn Cytopathol. 2006;34:839-42.
- [Google Scholar]
- Human papillomavirus detection and p53 expression in clear-cell adenocarcinoma of the vagina and cervix. Obstet Gynecol. 1994;84:404-8.
- [Google Scholar]
- Primary malignant melanoma of the uterine cervix – Case report and review. Eur J Gynaecol Oncol. 2011;32:448-51.
- [Google Scholar]
- Mucosal melanoma of the female genitalia: A clinicopathologic study of forty-three cases at Duke University Medical Center. Surgery. 1998;124:38-4.
- [Google Scholar]
- Primary malignant melanoma of the uterine cervix: Report of a case diagnosed by cervical scrape cytology and review of the literature. Diagn Cytopathol. 2001;25:108-11.
- [Google Scholar]
- Malignant melanoma of the uterine cervix diagnosed on a cervical cytologic smear. Acta Cytol. 1998;42:1043-5.
- [Google Scholar]
- Cytomorphology of cervicovaginal melanoma: ThinPrep versus conventional Papanicolaou tests. Cytojournal. 2010;7:25.
- [Google Scholar]
- HMB-45 staining for cytology of primary melanoma of the vagina. A case report. Acta Cytol. 2000;44:1077-80.
- [Google Scholar]








