Translate this page into:
Cytomorphology of skin adnexal tumors and its correlation with histopathology in a tertiary care center

-
Received: ,
Accepted: ,
How to cite this article: Shahab J, Sharan S, Singh M, Ahuja S, Ahluwalia C, Agrawal M, et al. Cytomorphology of skin adnexal tumors and its correlation with histopathology in a tertiary care center. CytoJournal 2023;20:37.
Abstract
Objectives:
Even though fine-needle aspiration cytology (FNAC) can be successfully used for describing the cytomorphology of skin adnexal tumors and can help in identification of them as benign or malignant, histopathology remains the gold standard in confirmation of diagnosis. Skin adnexal tumors are a large and diverse group and are relatively less commonly encountered in routine practice; hence, knowledge of cytological features of these lesions is crucial for their appropriate management. The present study aims to evaluate the cytomorphological features of skin adnexal tumors on FNAC smears and their correlation with the histopathology.
Material and Methods:
This is a retrospective study of FNAC of 60 cases of subcutaneous and dermal swellings over a period of 4 years from August 2018 to August 2022 in a tertiary care center.
Results:
In the 60 cases of skin adnexal tumors evaluated, most cases were within the 2nd to 4th decade with male predominance. The FNA smears were helpful in picking up the nature of skin adnexal tumors, as in our case series, malignant lesions were 8.3% and benign were 91.7%. Majority adnexal lesions were of follicular or sebaceous differentiation. Histological diagnosis was available in 38 cases. Cytological diagnosis in 34 cases was concordant with histopathology. There was discrepancy observed in two cases which were reported as benign skin adnexal tumor but later turned out to be sebaceous carcinoma on histopathology.
Conclusion:
Even though histopathology being the gold standard for confirmation of diagnosis, in the current era of minimally invasive procedures, FNAC stands out as a valuable modality and can be a promising alternative to diagnose skin adnexal tumors and understand their cytomorphology as the data are limited about it.
Keywords
Skin adnexal tumors
Basaloid cells
Fine-needle aspiration cytology
Sebaceous
Follicular
INTRODUCTION
Skin adnexal tumors are a large and diverse group and are relatively rare in routine practice. Therefore, knowledge of cytological features of these lesions is crucial so that they can be easily diagnosed with minimally invasive techniques. Fine-needle aspiration cytology (FNAC) can be successfully used for describing the cytomorphology of skin adnexal tumors and for identification of them as benign or malignant. However, histopathology remains the gold standard in confirmation of diagnosis. Skin adnexal tumors are usually diagnosed by excisional biopsy. It is rarely advised to proceed by FNAC since they are easily accessible, and it is feasible to just excise them and diagnose by histopathology. However, FNAC can be of great help in differentiating these from metastatic carcinomas and sarcomas that form a close differential diagnosis of skin adnexal tumors. Recognition of a skin lesion as a skin adnexal tumor on FNAC, and if possible, its subtyping allows correct management and follow-up of the patient.
The study was done to evaluate the cytomorphological features of skin adnexal tumors on FNAC smears and its correlation with histopathology.
MATERIAL AND METHODS
This was a retrospective study design in which all cases of subcutaneous and dermal swellings reported from August 2018 to August 2022 were evaluated. Giemsa-stained slides obtained after FNAC were used for analysis. The reports were then correlated with histopathology if the patient had proceeded with excision of swelling following FNAC.
RESULTS
A total of 60 cases were included in the study. There was a male predominance with 39 (65%) males and 21 (35%) females and a male-to-female ratio of 1.8:1. Majority cases were between the ages of 20–40 with a mean age of 36 years. The youngest and oldest patient were 16 and 72 years, respectively [Table 1].
| Age (years) | Benign lesions | Malignant lesions | Total |
|---|---|---|---|
| 11–20 | 9 | 0 | 9 |
| 21–30 | 13 | 1 | 14 |
| 31–40 | 14 | 0 | 14 |
| 41–50 | 8 | 1 | 9 |
| 51–60 | 6 | 1 | 7 |
| 61–70 | 3 | 1 | 4 |
| 71–80 | 2 | 1 | 3 |
| Total | 55 | 5 | 60 |
Head and neck were found to be the most common site (65%) followed by the arm (21%) [Table 2].
| Site | Benign lesions | Malignant lesions | Total |
|---|---|---|---|
| Head and neck | 34 | 5 | 39 |
| Trunk | 2 | 0 | 2 |
| Upper extremities | 13 | 0 | 13 |
| Lower extremities | 6 | 0 | 6 |
| Total | 55 | 5 | 60 |
All 60 FNAC smears were adequate for opinion. Out of the 60 cases reported, 55 cases (91.7%) were reported to be benign while only five cases (8.3%) were reported as malignant.
Nodular hidradenoma comprised five cases and were located as cystic swellings in the upper extremities. The aspiration cytology smears were cellular comprising of papillaroid fragments and nests of round to oval basaloid cells with scant cytoplasm and bland nuclear chromatin admixed with few cells with vacuolated cytoplasm.
There were nine cases of sebaceous adenoma located in the face and scalp. The FNAC smears showed nests of round cells with eccentric nucleus and abundant foamy cytoplasm. Pilomatricoma comprised seven cases located as cystic swelling in the scalp region. Aspiration smears showed nests of basaloid cells and sheets of ghost cells with foreign body giant cell reaction admixed with inflammatory cells.
There were six cases of chondroid syringoma which presented as dermal nodules in the head and neck, trunk, and lower extremities. FNAC smears showed nests and sheets of benign epithelial cells with moderate cytoplasm and bland chromatin embedded in a chondromyxoid stroma.
Twenty-eight cases were reported as benign skin adnexal tumors with basaloid cells with bland chromatin in basement membrane matrix which could not be characterized further on cytology [Table 3].
| Type of skin adnexal lesion | Cytodiagnosis |
|---|---|
| Nodular hidradenoma | 5 |
| Benign skin adnexal tumor with basaloid cells in basement membrane matrix |
28 |
| Pilomatrixoma | 7 |
| Sebaceous adenoma | 9 |
| Chondroid syringoma | 6 |
| Malignant skin adnexal tumor | 5 |
| Total cases | 60 |
Five cases were reported as malignant adnexal tumors based on moderate to severe degree of pleomorphism, high nucleocytoplasmic ratio, and increased mitotic activity. However, diagnosis of a specific malignant tumor could not be made on cytology.
Histopathological diagnosis was available in 38 cases. The FNAC findings were confirmed and correlated on histopathology [Tables 4 and 5].
| S. No. | Diagnosis on cytology | Diagnosis on histology | No. of cases |
|---|---|---|---|
| 1. | Nodular hidradenoma | Nodular hidradenoma | 5 |
| 2. | Sebaceous adenoma | Sebaceous adenoma | 9 |
| 3. | Pilomatrixoma | Pilomatrixoma | 7 |
| 4. | Chondroid syringoma | Chondroid syringoma | 6 |
| 5. | Benign skin adnexal tumor with basaloid cells in basement membrane matrix | Pilomatrixoma | 3 |
| 6. | Benign skin adnexal tumor with basaloid cells in basement membrane matrix | Cylindroma | 2 |
| 7. | Benign skin adnexal tumor with basaloid cells in basement membrane matrix | Hidradenoma | 2 |
| Total cases | 34 |
| S. No. | Diagnosis on cytology | Diagnosis on histology | No. of cases |
|---|---|---|---|
| 1. | Malignant skin adnexal tumor | Sebaceous carcinoma | 1 |
| 2. | Malignant skin adnexal tumor | Porocarcinoma | 1 |
| 3. | Benign skin adnexal tumor with basaloid cells in basement membrane matrix | Sebaceous carcinoma | 2 |
| Total cases | 4 |
Out of the 55 benign skin adnexal tumors [Figures 1 and 2], histopathology was available for 36 cases. Cytological diagnosis in 34 cases was concordant with histopathology. Most turned out to be sebaceous adenoma and pilomatrixoma. Seven out of the benign skin adnexal tumors turned out to be pilomatricoma, cylindroma, and hidradenoma, respectively. There was discrepancy observed in two cases which were reported as benign skin adnexal tumor but later turned out to be sebaceous carcinoma on histopathology, possibly due to paucicellular smears. Out of the five cases diagnosed as malignant skin adnexal tumors on cytology [Figure 3]; histology was available only for two cases, which were diagnosed as porocarcinoma, and sebaceous carcinoma, respectively.
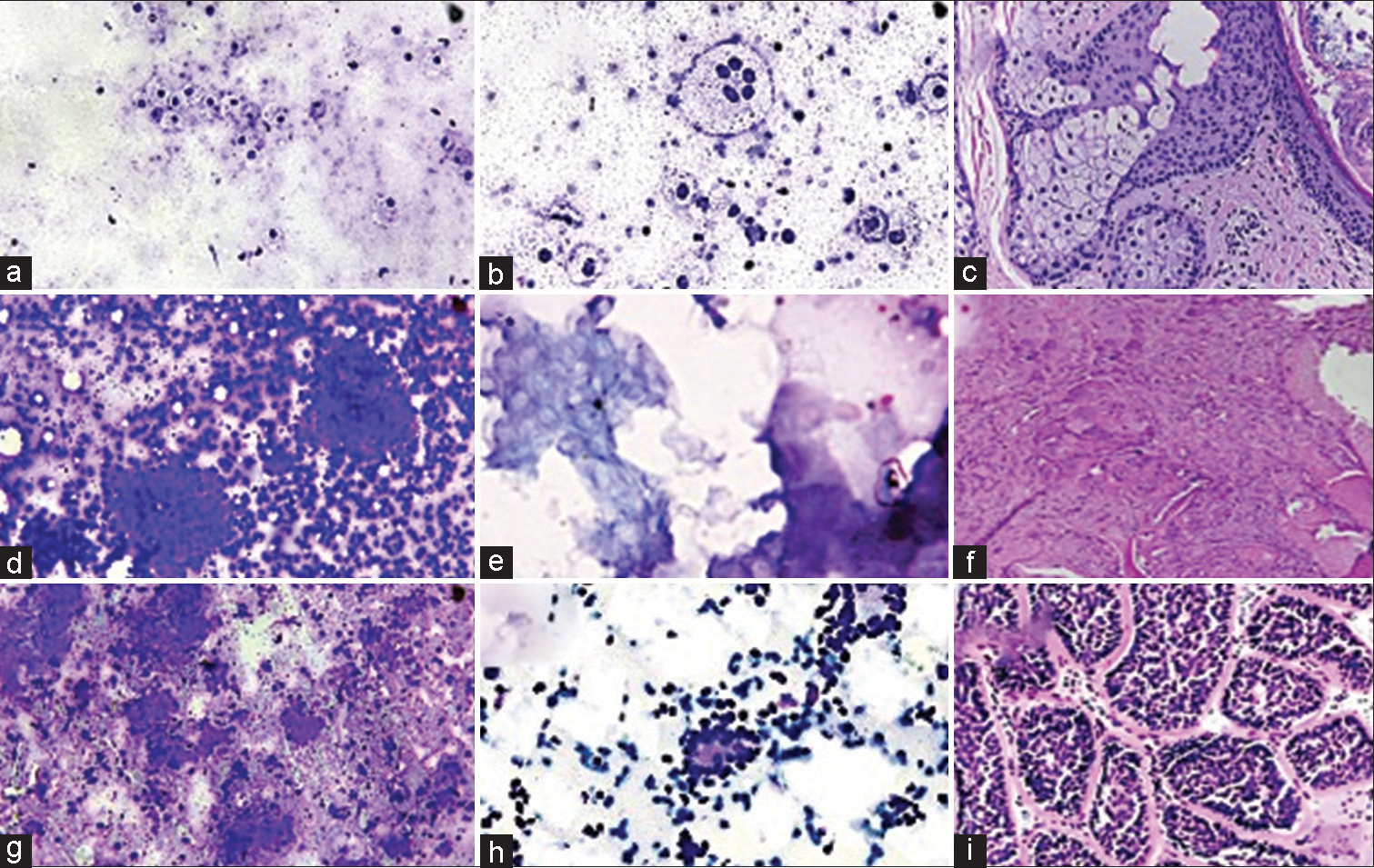
- Benign skin adnexal tumors (a-c) Sebaceous adenoma – Giemsa-stained smears show small clusters of cells with bubbly cytoplasm uniform eccentric round to oval nuclei and a touton giant cell (a and b). Hematoxylin and eosin stained section shows lobules consisting of admixture of basaloid cells and mature sebocytes (c) (×100, ×200). (d-f) Pilomatrixoma – Giemsa-stained smears show clusters of basaloid cells and sheets of ghost cells (d and e). Hematoxylin and eosin stained section shows exhibiting transition of basaloid cells to ghost cells along with foreign body giant cell reaction (f) (×100, ×200). (g-i) Cylindroma – Giemsa and Papanicolaou stained smears show basement material like matrix enclosing the basaloid cells. Cells are arranged around cylinders of dense, acellular substance (g and h). Hematoxylin and eosin stained section shows irregularly shaped islands of epithelial cells in jigsaw puzzle appearance (i) (×100).

- Benign skin adnexal tumors (a and b) Nodular hidradenoma – Giemsa stained smears show clusters of variably cohesive uniform basaloid cells with moderate amount of cytoplasm and small dark ovoid nuclei. Hematoxylin and eosin stained section shows tumor cells arranged in sheets with few cystic areas filled with homogenous eosinophilic material. Cells are squamoid and some show clear cell change (×100, ×200). (c and d) Chondroid syringoma – Giemsa stained smears show epithelial cells embedded in chondromyxoid stroma. Hematoxylin and eosin stained section shows a biphasic tumor containing cords and islands of bland epithelial cells embedded in a fibrous and chondromyxoid stroma (×100).

- Malignant skin adnexal tumors (a-d) Porocarcinoma – Giemsa stained slides show cells in poorly cohesive clusters in an inflammatory background. They exhibit marked pleomorphism, high nucleocytoplasmic ratio, hyperchromatic nuclei, and scanty cytoplasm (a and b). Hematoxylin and eosin stained sections show tumor in diffuse infiltrative pattern comprising atypical poroma cells with hyalinized stroma and blood vessels. Poorly formed ductal structures are also seen (c and d) (100, ×200). (e-h) Sebaceous carcinoma – Giemsa stained slides clusters of atypical sebocytes exhibiting anisonucleosis (e and f). Hematoxylin and eosin stained sections show sheets of multivacuolated sebocytes and undifferentiated basaloid cells (g and h) (×100, ×200, ×400).
Figure 4 depicts the algorithmic approach to cytological diagnosis of skin adnexal tumors.
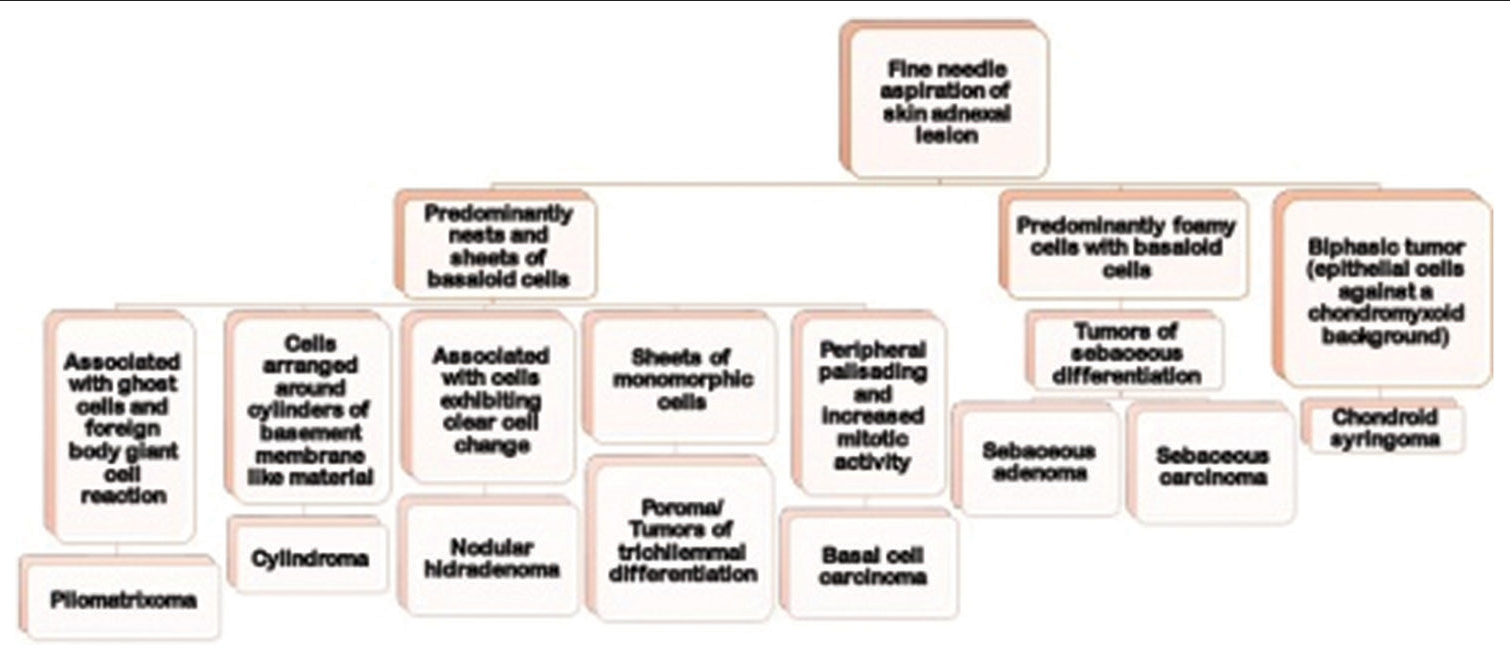
- Algorithmic approach for cytological diagnosis of skin adnexal tumors.
DISCUSSION
Skin adnexal tumors are a large group constituting a wide variety of tumors that often clinically present as asymptomatic nodule. In our study, 60 cases of skin adnexal tumors, with their cytologic diagnosis along with detailed cytomorphology, were correlated with their subsequent histology. FNAC successfully established the benign or malignant nature of the skin tumors in most of the cases. The presence of the pinkish basement membrane material in the background gave an important clue toward the diagnosis of an adnexal neoplasm.
The age group most affected in the present study was 20–40 years, which is in concordance with the previous studies.[1,2] Our study showed male predominance with male-to-female ratio being 1.8:1 in contrast to Arora et al. and Chand et al. who showed a female preponderance.[1,2] In a study done by Rajalakshmi et al., the male-female ratio was 1.1:1.[3] Incidence of benign tumors in our study – 91.7% (Benign: Malignant – 11:1) which is in tandem with the study done by Chand et al., Rajalakshmi et al., and Kumar et al., while it was 77% in a study done by Radhika et al.[2-5] In our study, tumors of sebaceous and follicular differentiation were the most common which was concordant to Kumar et al. who observed a predominance of tumors with follicular differentiation (48.93%).[4] This is contrary to the findings of Chand et al. and Nair who observed tumors of sweat gland origin to be most common.[2,6] Tumors of sweat gland can occur anywhere in the body with a predilection in the head and neck region. Dubb and Michelow observed that aspirate from nodular hidradenomas exhibits a distinct epithelial cell component arranged as clusters, sheets, and tubules with the cells resembling ductal cells with bland round-to-oval nuclei, vesicular chromatin, small inconspicuous nucleoli, and moderate eosinophilic to clear cytoplasm.[7]
These eccrine tumors have clinicopathological resemblance to basal cell carcinoma, cutaneous leiomyoma, neurofibroma, and subcutaneous metastasis from an internal malignancy.[8] Therefore, knowledge of cytological features of these tumors can allow correct management of the patient and prevent misdiagnosis as a malignancy. Chondroid syringoma is a rare benign skin adnexal tumor which is the soft-tissue counterpart of pleomorphic adenoma and is seen most commonly in head and neck region.[9] Cytologically, it shows a biphasic tumor composed of bland epithelial cells embedded in chondromyxoid stroma.[2,10,11]
The diagnostic triad of pilomatricoma include ghost cells, basaloid cells, and foreign body giant cell reaction in addition to nucleated squames, calcification, and inflammatory cells.[12] However, the age of the lesion determines the proportion of the various components. When FNAC is done in an early lesion or from the periphery of the lesion, there is preponderance of basaloid cells with high nucleocytoplasmic ratio and scant cytoplasm which may lead to the false diagnosis of malignancy. In contrast, FNAC from a mature lesion or from the center of the lesion may lead to preponderance of ghost cells, leading to a misdiagnosis of epidermal inclusion cyst.[13,14]
Adnexal tumors are usually benign, and a complete local surgical excision is curative. The malignant counterpart however is rare, locally aggressive with poor clinical outcome. Therefore, establishing a diagnosis of malignancy in skin adnexal tumors is important. Most common malignant tumor in our study was sebaceous carcinoma which was in concordance with all three of the studies mentioned above.[3-5]
CONCLUSION
Skin adnexal tumours are a large and heterogenous group. Most skin adnexal tumours are benign. Even though histopathology remains the gold standard, FNAC stands out as a valuable modality and a promising tool to diagnose skin adnexal tumours and to classify them as benign or malignant. It aids in early diagnosis and aids the surgeon whether excision is needed.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare no potential conflicts of interest and no source of financial support.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors state that they contributed to this publication according to the guidelines of the journal and no part of this manuscript was plagiarized.
ETHICS STATEMENT BY ALL AUTHORS
This material is the author’s own original work which has not been published elsewhere. The paper is not currently being considered for publication elsewhere. The paper reflects the author’s own analysis in a truthful and complete manner. The paper properly credits the meaningful contributions of co-authors and co-researchers. The results are appropriately placed in the context of prior and existing research. All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such using quotation marks and giving proper references.
LIST OF ABBREVIATIONS (In alphabetic order)
FNAC- Fine needle aspiration cytology
EDITORIAL/PEERREVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
References
- Cyto-histopathological correlation of skin adnexal tumors: A short series. J Cytol. 2018;35:204-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic evaluation of skin adnexal tumors by fine-needle aspiration cytology. Acta Cytol. 2016;60:246-53.
- [CrossRef] [PubMed] [Google Scholar]
- Case series of skin adnexal tumours. J Clin Diagn Res. 2014;8:FC07-10.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological study of skin adnexal tumors-a ten years study. Int Arch Integrated Med. 2018;5:95-100.
- [Google Scholar]
- A study of biopsy confirmed skin adnexal tumors: Experience at a tertiary care teaching hospital. J Clin Sci Res. 2013;2:132-8.
- [CrossRef] [Google Scholar]
- A clinicopathologic study of skin appendageal tumors. Indian J Dermatol Venereol Leprol. 2008;74:550.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic features of hidradenoma in fine needle aspiration biopsies. Acta Cytol. 2009;53:179-82.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelial nevi, neoplasms, and cysts In: Odom RB, James WD, Berger JG, eds. Andrew's Diseases of the Skin (Clinical Dermatology) (9th ed). Philadelphia, PA: W.B. Sauder's Company; 2000. p. :845-55.
- [Google Scholar]
- Chondroid syringoma of the axilla: An unusual tumor diagnosed by fine needle aspiration. Diagn Cytopathol. 2016;44:342-6.
- [CrossRef] [PubMed] [Google Scholar]
- Aspiration cytology in the diagnosis of primary tumors of skin adenexa. Acta Cytol. 2000;45:715-721.
- [CrossRef] [PubMed] [Google Scholar]
- Chondroid syringoma: Fine-needle aspiration cytology of a rare entity at an unusual site. J Clin Diagn Res. 2017;11:ED06-7.
- [CrossRef] [PubMed] [Google Scholar]
- An interesting cytology and histopathological correlation of adnexal tumor. J Dent Med Sci. 2014;13:13-6.
- [CrossRef] [Google Scholar]
- Pilomatricoma mimicking small round cell tumor on fine needle aspiration cytology: A case report. Acta Cytol. 2008;52:627-30.
- [CrossRef] [PubMed] [Google Scholar]
- Pilomatricoma of the face: A benign skin appendage mimicking squamous cell carcinoma. Otolaryngol Head Neck Surg. 2004;130:483-5.
- [CrossRef] [PubMed] [Google Scholar]








