Translate this page into:
Diagnostic accuracy of bronchial brush cytology and the added value of immunohistochemistry and fluorescence in situ hybridization of pulmonary neuroendocrine tumors
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Bronchial brush (BB) cytology carries low sensitivity for detecting neuroendocrine carcinomas (NECs), including typical carcinoid (TC) tumors of the lung. We aimed to investigate the detection of neuroendocrine tumors including TC through BB routine cytology cell block (CB), immunohistochemistry (IHC), and fluorescence in situ hybridization (FISH).
Materials and Methods:
A SNOMED search showed 187 lung biopsy or resection specimens from 2008 through 2011 containing neuroendocrine or carcinoid in the diagnosis. Residual BB specimens retained in PreservCyt were used to prepare a ThinPrep slide for FISH analysis. CBs were stained with H and E and IHC for chromogranin and synaptophysin.
Results:
Of the 187 cases, 16 had residual BB material available within 1 year of diagnosis and were used in CB preparation for IHC and FISH slides. Cytologic evaluation determined 1 case positive for malignancy (small cell lung carcinoma [SCLC]), 1 suspicious for adenocarcinoma, and 14 negative for malignancy. On the basis of histologic diagnosis, FISH was performed. SCLC showed polysomy (86% abnormal cells); 2 TC tumors showed a gain of 7p12 (15% abnormal cells) and a gain of 5q15 (72% abnormal cells), respectively. Two cases had CBs with positive immunoreactivity for chromogranin and synaptophysin. The sensitivity for detection of NEC was 18.8%, 15.4%, and 25% for cytologic evaluation, CB, and FISH, respectively.
Conclusion:
Neuroendocrine tumors, including TC are difficult to detect with BB cytologic evaluation, most likely because tumor cells lack in the specimen. Assessment of further studies is needed to explore the role of cytology and ancillary methods for detection of these tumors.
Keywords
Ancillary studies
carcinoid tumor
cytology
endobronchial sampling
pulmonary
INTRODUCTION
Incidental pulmonary nodules are increasingly detected, largely because of screening programs for high-risk patients.[1] Detection of a nodule may prompt endobronchial evaluation to distinguish between benign and malignant processes. Although fine-needle aspiration (FNA) is more specific in diagnosing cancer, bronchial brushing (BB) can serve as a sensitive, less invasive method to sample lesions, specifically endobronchial specimens. Fluorescence in situ hybridization (FISH) with the LAVysion probe set (Abbott Molecular Inc.) has shown an increase in sensitivity for detecting malignancy for non-small cell lung carcinoma (SCLC).[2]
Most pulmonary carcinomas are successfully detected cytologically; however, neuroendocrine tumors can be more difficult to diagnose. The bland nuclei and striking resemblance to bronchial epithelial cells are pitfalls in missing these lesions, especially in BB specimens. In addition, interpretation of crushed samples may result in over diagnosis of small cell carcinoma.[3]
A BB cytologic evaluation carries a low sensitivity for detecting neuroendocrine carcinoma (NEC) and typical carcinoid (TC) tumors of the lung. The aim of the present study was to investigate the use of BB in the detection of neuroendocrine tumors including TC, as well as investigates ancillary testing including cell block (CB) preparation and FISH with the LAVysion probe set.
MATERIALS AND METHODS
The Mayo Clinic Institutional Review Board approved this study. Electronic medical records were searched for lung biopsy or resection specimens and the terms neuroendocrine and carcinoid from January 2008 through December 2011. Cases with a concurrent cytologic specimen were searched to determine whether a BB specimen was obtained before surgery. A specimen was considered concurrent if the BB specimen was provided within 1 year before biopsy and/or resection. A ThinPrep (Hologic, Inc.) slide was prepared for the diagnosis. Specimen vials were retrieved from the archive, and residual BB specimens retained in PreservCyt (Hologic, Inc.) media were retrieved for further analysis. At our institution, all BB case vials were preserved since January 2008. Patient demographic and clinical follow-up information was collected from the electronic medical record. All final diagnoses were made on follow-up resection specimens, and pathologic measurements were derived from the surgical pathology report.
Fluorescence in situ hybridization
An additional ThinPrep slide was prepared for FISH analysis with probes to pericentromeric regions of 6p11 and the locus-specific regions of 7p12 (EGFR), 8q24 (MYC), and 5p15 (Abbott Molecular Inc.).[4] Slides were screened for signal gains and losses by a certified technologist in cytogenetics (S.M.B), and results were reviewed and confirmed by a cytopathologist. A specimen was considered polysomy when ≥5 atypical cells exhibited hypertetrasomy, defined as gains in ≥2 of the 4 probes, with 1 of the probes showing ≥5 copies. Any other molecular abnormality was documented.
Immunohistochemistry
The remaining BB material was used to prepare a CB with H and E stain and immunohistochemistry (IHC) for chromogranin (Chemicon International Inc.; 1:500, LK2H10) and synaptophysin (ICN; 1:40, SY38). The CBs were screened for tumor cells, and the immunostains were interpreted as negative, weak, or strongly positive staining. Membranous and cytoplasmic staining chromogranin or synaptophysin, or both, was considered positive. Comparison with control specimens was performed to exclude aberrant immunostaining. An absence of staining was scored as negative.
RESULTS
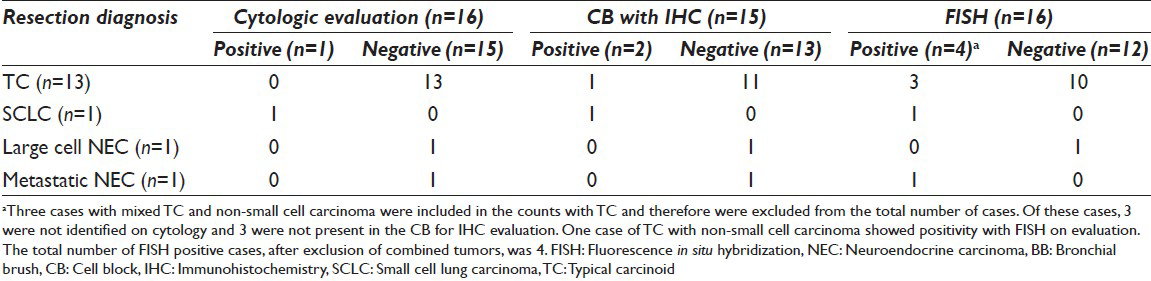
From 2008 through 2011, a total of 187 patients (mean age, 75 years) had the diagnosis of NEC, TC tumor, or SCLC on the biopsy or resection specimen. Sixteen patients had a BB evaluation within 1 year of the diagnosis. The surgical pathologic diagnoses were 13 TC tumors, 3 of which occurred with concurrent non-SCLC (adenocarcinoma) [Table 1]. In addition to these TC tumors were 1 SCLC, 1 large-cell NEC, and 1 metastatic NEC from the gastrointestinal tract. Nine tumors were located centrally; 7 were peripheral. Most TC tumors were < 2 cm in size (<1 cm, n = 6; 1–2 cm, n = 6; >4 cm, n = 4). Sixteen FISH slides and 15 CBs were prepared for additional analyses. One case of TC tumor did not contain enough material to create a CB.
Diagnostic value of cytologic analysis
Of the 16 BB cytology cases, the initial cytologic diagnosis was 1 case positive for malignancy (SCLC), 1 suspicious for adenocarcinoma, and 14 negative for malignancy. After rescreening all cases, only 1 additional BB contained cytologic features of TC tumor. This additional case showed vague rosetting structures [Figure 1a and b], as well as rare, atypical scattered single cells architecturally. The nuclear features included bland-appearing nuclei with evenly distributed chromatin and small, inconspicuous nucleoli. These cells were difficult to distinguish from bronchial epithelial cells.[5] This case was reclassified as atypical cells present. The tumor also was present on the CB and stained positive for chromogranin and synaptophysin [Figure 1c]. This case showed positivity in FISH and 72% of cells showed a gain in the 5q probe [Figure 1d]; 5% of cells showed polysomy.
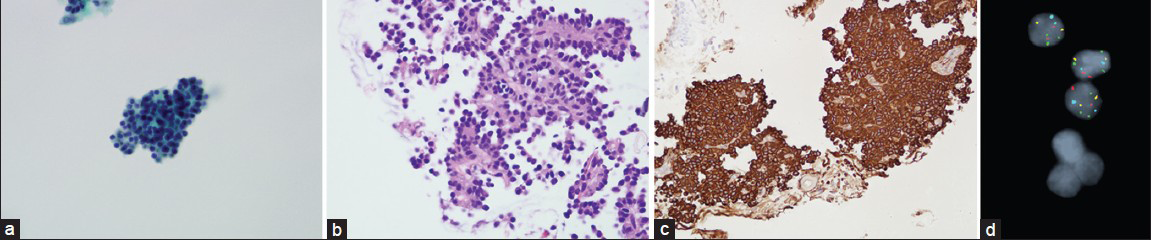
- Results of cytologic evaluations. (a) clusters of monotonous cells with a vague rosetting appearance show bland nuclei (Papanicolaou, original magnification × 40). No evidence of ciliated epithelium is present. These cells are difficult to distinguish from bronchial cells because of morphologic similarity. (b) The rosette pattern is more evident on cell block staining (H and E, original magnification × 20). The tumor cells contain more amphophilic cytoplasm than the normal bronchial cells. (c) Strong membranous synaptophysin staining confirms the neuroendocrine origin of the tumor cells (synaptophysin immunohistochemistry, original magnification × 20). (d) Fluorescence in situ hybridization with LAVysion probe set shows 3 copies of the green probe (5p15)
Diagnostic value of immunohistochemistry and cell block preparation
The CB preparations with IHC for chromogranin and synaptophysin showed that 2 of 15 cases were positive. The other 13 were negative and contained only macrophages and bronchial cells, indicating a lack of tumor in the specimen. The 2 positive cases (1 TC tumor and 1 SCLC) showed both chromogranin and synaptophysin immunopositivity in tumor cells.
Diagnostic value of fluorescence in situ hybridization testing
Fluorescence in situ hybridization results with the LAVysion probe set are summarized in [Table 2]. Four cases showed detectable cytogenetic abnormalities. One TC tumor concurrent with non-SCLC showed polysomy (86% abnormal cells) and was present in the CB. One TC tumor showed a gain of 5q15 (72% abnormal cells) and also was present in the CB. A separate TC tumor showed a gain of 7p12 (15% abnormal cells); however, tumor cells were not present in the CB. One case of metastatic NEC from the gastrointestinal tract showed polysomy (1% abnormal cells) and contained no tumor cells in the CB.
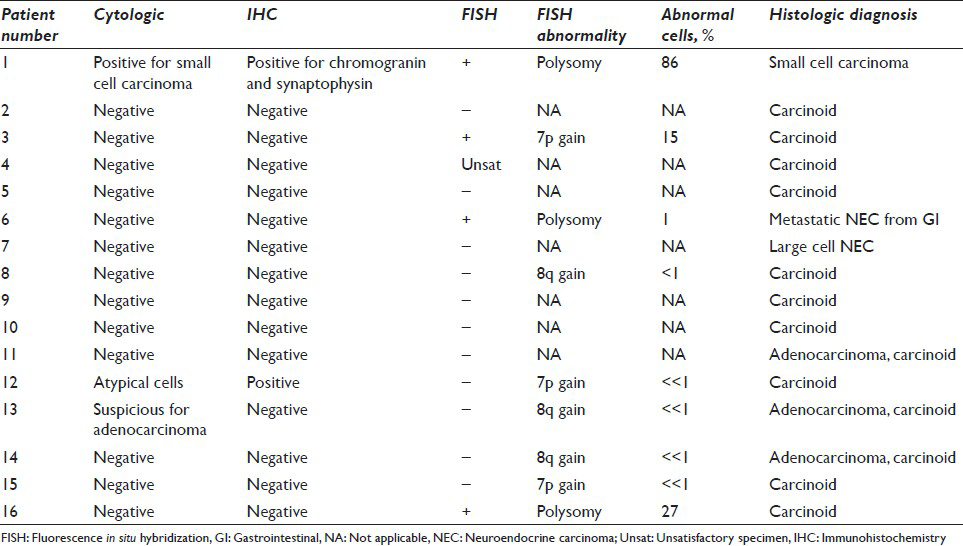
For the other 12 cases, we provide the percentage of abnormal cells in FISH and the proposed clinical interpretation. Cases with rare chromosomal anomalies (<1% abnormal cells) likely would be reported as negative or non-diagnostic molecular changes. Although 9 of the 16 cases may have rare or few atypical cells on FISH, 5 of these cases had <1% atypical cells, and the clinical interpretation of the test would be negative because of scant tumor cellularity.
In the detection of all NECs with BB sampling, the sensitivities were 18.8% for cytology and CB preparation without IHC, 15.4% for IHC on CB, and 25% for FISH. Three primary NECs (1 SCLC and 2 TC tumors) that were positive with FISH were centrally located, with sizes of 3 cm (the SCLC) and 1.8 cm and 2 cm (the 2 TC tumors).
DISCUSSION
In our study, we retrospectively retrieved BB specimens from lung resection cases diagnosed as a neuroendocrine tumor. Although all modalities carried a low sensitivity, FISH with LAVysion found abnormal cells in 25% of cases; cytology was second at 18.8% and CB with IHC for chromogranin and synaptophysin was the lowest at 15.4%. The most likely explanation for the low detection rate is that the tumor is not present in the examined sample because of sampling error. However, when it is present, all modalities have low rates of detecting carcinoid tumors of the lung.
Cytologically, the diagnosis of neuroendocrine cells in BB specimens was difficult because of wide morphologic overlap with surrounding native respiratory epithelial cells.[5] Morphologically, neuroendocrine and bronchial epithelial cells share similar architectural and cytologic characteristics. In liquid-based preparations, single scattered bronchial epithelial cells show polarization and resemble the plasmacytoid formations noted in neuroendocrine cells.[6] In Papanicolaou-stained preparations, finding cilia–a hallmark of benign respiratory epithelial cells–may be difficult. Identification of terminal bars helps distinguish these benign bronchial epithelial cells from neuroendocrine cells.
The terminal bars and cilia are more easily seen on Romanowsky stained slides, which offer more cytoplasmic detail and contrast. With this stain, cilia appear eosinophilic against the predominantly blue background. They may be obscured when the bronchial epithelial cells are arranged in monolayered sheets, further hindering their identification. Furthermore, the architectural arrangement of benign respiratory epithelial cells may then resemble acini and further mimic neuroendocrine tumors. Another characteristic suggestive of a neuroendocrine population or neoplasm is the rosetting of the cells. Subtle and inconspicuous, the neuroendocrine cells could easily be confused with flat, monolayer sheets of native, benign bronchial epithelial cells.
The nuclei of bronchial cells and carcinoid tumor cells are similar in size. In addition, both exhibit evenly distributed chromatin; however, bronchial cells contain more condensed chromatin, thereby creating the appearance of a solid nucleus. Neuroendocrine tumor cells show a more finely stippled chromatin, giving the classic salt-and-pepper appearance.[7] On Romanowsky stain, these features are barely detectable, whereas on Papanicolaou stain, these subtle nuclear differences are more noticeable.
On the other end of the spectrum, carcinoid tumors of the lung may be misdiagnosed as SCLC. Renshaw et al.[8] used responses from the College of American Pathologists non-gynecologic cytology program and demonstrated that poorly preserved specimens with smudgy chromatin may mimic SCLC. In addition, spindle cell areas may contribute to the misclassification of small cell carcinoma.
Cell block preparation has been shown to improve the rate of tumor detection in pulmonary cytologic specimens.[9] Most of the CB specimens in this study lacked diagnostic tumor cells, indicating that the carcinoid tumor cell population was sampled. This finding highlights the limitations of BB specimens, especially when attempting to sample peripherally based lesions. In addition, these lesions are, usually, located in the submucosa and are not amenable to sampling superficially. This characteristic is comparable to other studies detecting neuroendocrine tumors in cytologic specimens, which also show a high rate of non-sampling with a low detection rate (19%; 12/63 cases).[10] With the advent of superTrax (superDimension, Inc.) needle sampling, better CB preparations can be expected for further analysis, including IHC.
The LAVysion probe was originally developed for increasing the sensitivity of diagnosing non-SCLC on BB specimens.[2] However, recent molecular studies have shown that some probes can be used to detect molecular abnormalities in carcinoid neoplasms. Comparative genomic hybridization array has shown gains in chromosome 15, as well as deletions in 11q22.3[1112] in carcinoid tumors of the lung. Molecular probes targeting these loci may be more helpful than the currently available probes in detecting neuroendocrine tumors. FISH was used on bronchial washings in previous studies.[13] While bronchial washings may be available in conjunction with BB cases, FISH testing is not performed on bronchial washings at our institution.
Limitations of the present study that should be considered are its small sample size and that some of our neuroendocrine tumors occurred concomitantly with a non-small cell carcinoma, which may have accounted for FISH abnormalities. Another limitation is that some samples had no tumor cells on the CB, which may have attributed to exhaustion, depletion of the sample, or to non-sampling of the target lesion in the first place.
CONCLUSIONS
Typical carcinoid tumors and low-grade NECs are difficult to diagnose on BB cytologic analysis alone, most likely because of a lack of or scant tumor cells in the specimen. This difficulty is expected because most carcinoid tumors are submucosal by nature. A paraffin-embedded CB performed on 15 cases detected only 1 additional TC tumor, whereas use of FISH detected abnormal cells in 3 additional cases. Performing the LAVysion FISH test on FNA samples or smears with adequate tumor sampling methods (ie, FNA or core biopsy) in future studies could help to determine the efficacy of FISH. Further studies also are needed to fully elucidate the role of combined FISH and cytologic evaluations for the detection of clinically apparent, centrally located tumors in BB specimens.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
Dr. Kevin Halling and Mayo Clinic receive royalties from the sale of the FISH probe set discussed in this paper and also receive grant funding from Abbott Molecular Inc.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this artile declare that we qualify for authorship as defined by ICMJE http://www/icmje.org/#author. Each author has participated sufficiently in the work and take public responsibility for the appropriate portions of the content of this article. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
The Mayo Clinic institutional review board and biospecimens granted approval for this study.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENTS
We would like to thank Ms. Colleen Sauber and Margery Sherman for their editorial services.
REFERENCES
- Benefits and harms of CT screening for lung cancer: A systematic review. JAMA. 2012;307:2418-29.
- [Google Scholar]
- A comparison of cytology and fluorescence in situ hybridization for the detection of lung cancer in bronchoscopic specimens. Chest. 2006;130:694-701.
- [Google Scholar]
- Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: A major pitfall in the management of lung cancer patients. Am J Surg Pathol. 2005;29:179-87.
- [Google Scholar]
- Fluorescence in situ hybridization testing algorithm improves lung cancer detection in bronchial brushing specimens. Am J Respir Crit Care Med. 2010;181:478-85.
- [Google Scholar]
- Pulmonary neuroendocrine neoplasms: A review of clinicopathologic and cytologic features. Diagn Cytopathol. 2010;38:607-17.
- [Google Scholar]
- A review of cytologic findings in neuroendocrine carcinomas including carcinoid tumors with histologic correlation. Cancer. 2000;90:148-61.
- [Google Scholar]
- Distinguishing carcinoid tumor from small cell carcinoma of the lung: Correlating cytologic features and performance in the college of American pathologists non-gynecologic cytology program. Arch Pathol Lab Med. 2005;129:614-8.
- [Google Scholar]
- The diagnostic value of cell block as an adjunct to liquid-based cytology of bronchial washing specimens in the diagnosis and subclassification of pulmonary neoplasms. Cancer Cytopathol. 2012;120:134-41.
- [Google Scholar]
- Cytologic diagnosis and differential diagnosis of lung carcinoid tumors a retrospective study of 63 cases with histologic correlation. Cancer Cytopathol. 2010;118:457-67.
- [Google Scholar]
- Deletions of 11q22.3-q25 are associated with atypical lung carcinoids and poor clinical outcome. Am J Pathol. 2011;179:1129-37.
- [Google Scholar]
- Typical and atypical carcinoid tumors of the lung are characterized by 11q deletions as detected by comparative genomic hybridization. Am J Pathol. 1998;153:1089-98.
- [Google Scholar]
- A fluorescence in situ hybridization-based assay for improved detection of lung cancer cells in bronchial washing specimens. Cancer. 2002;96:306-15.
- [Google Scholar]








