Translate this page into:
Endoscopic ultrasound-guided fine-needle aspiration diagnosis of secondary tumors involving the pancreas: An institution's experience
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Pancreatic masses may seldom represent a metastasis or secondary involvement by lymphoproliferative disorders. Recognition of this uncommon occurrence may help render an accurate diagnosis and avoid diagnostic pitfalls during endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). In this study, we review our experience in diagnosing secondary tumors involving the pancreas.
Materials and Methods:
The electronic database of cytopathology archives was searched for cases of secondary tumors involving the pancreas at our institution and a total of 31 cases were identified. The corresponding clinical presentations, imaging study findings, cytological diagnoses, the results of ancillary studies, and surgical follow-up, if available, were reviewed.
Results:
Nineteen of the patients were male and 12 female, with a mean age of 66 years. Twenty-three patients (74%) had a prior history of malignancy, with the latency ranging from 6 months to 19 years. The secondary tumors involving the pancreas included metastatic carcinoma (24 cases), metastatic sarcoma (3 cases), diffuse large B-cell lymphoma (2 cases), and plasma cell neoplasm (2 cases). The most common metastatic tumors were renal cell carcinoma (8 cases) and lung carcinoma (7 cases). Correct diagnoses were rendered in 29 cases (94%). The remaining two cases were misclassified as primary pancreatic carcinoma. In both cases, the patients had no known history of malignancy, and no ancillary studies were performed.
Conclusions:
Secondary tumors involving the pancreas can be accurately diagnosed by EUS-FNA. Recognizing uncommon cytomorphologic features, knowing prior history of malignancy, and performing ancillary studies are the keys to improve diagnostic performance and avoid diagnostic pitfalls.
Keywords
Ancillary study
endoscopic ultrasound
fine-needle aspiration
pancreas
secondary tumor
INTRODUCTION
Involvement of the pancreas by secondary malignancies is a well-documented, yet uncommon occurrence, accounting for 4–15% of all malignancies in the pancreas found at autopsy.[12] These secondary tumors often present as a single or multiple masses in patients with or without a known history of malignancy. In some cases, secondary neoplasms may share morphologic features with primary pancreatic neoplasms, thus imposing a diagnostic challenge. The distinction of primary pancreatic neoplasms from metastatic disease has a significant impact on patient clinical management. Most metastatic diseases involving the pancreas are treated with non-surgical approaches, although surgical resection is being increasingly performed for isolated metastases to the pancreas.[345] The hematologic malignancies involving the pancreas often require systemic therapies.
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has emerged as a safe and favorable method for evaluating pancreatic lesions.[6] The diagnostic performance of FNA for primary pancreatic tumors, primarily ductal adenocarcinoma, has a sensitivity ranging from 58% to 92% and specificity ranging from 93% to 100%.[7891011121314] However, there are only few reports in the literature that address the use of FNA in secondary tumors involving the pancreas.[151617181920212223] In this retrospective study, we reviewed our institutional experience in diagnosing secondary malignancies involving the pancreas via EUS-FNA and discuss diagnostic clues and pitfalls for these lesions.
MATERIALS AND METHODS
A computer-based data search was conducted to identify all EUS-FNA of pancreatic masses performed at our institution over a 7-year period from July 2005 to June 2012. All pancreatic masses with or without extrapancreatic extension were selected. Exclusive peripancreatic masses or masses that arose from nearby organs and locally invaded to the pancreas were excluded. For the cases selected, we reviewed clinical data, imaging study reports, cytologic diagnosis, ancillary studies, and surgical follow-up if available. The clinical characteristics included age, sex, history of malignancy, and latency period since the initial diagnosis of malignancy. The imaging characteristics of the pancreatic lesion(s) included size, site, solitary versus multiple lesions, and the presence of synchronous extra-pancreatic lesions.
All FNA biopsies were performed under EUS guidance with a 22- or 25-gauge needle. The rapid on-site evaluation was performed by either a cytotechnologist or cytopathologist. Direct smears were prepared and stained with Diff-Quik (Dade Diagnostics, west Monroe, LA, USA) after air-drying or with Papanicolaou stain after alcohol fixation. The remaining aspirate was collected in Cyto-Rich Red Fixative (BD Diagnostics, Franklin Lakes, NJ, USA) and processed for cell block. Hematoxylin and eosin stained sections were used for additional morphologic evaluation. In selective cases, the cell block sections were used for immunocytochemical studies or molecular tests. In cases cytologically suspicious for lymphoproliferative disorders, part of the aspirate was saved in RPMI preservative and submitted for flow cytometric studies.
RESULTS
Out of 1346 pancreatic EUS-FNA cases performed between July 2005 and June 2012, 31 patients (2.3%) were eventually diagnosed with metastatic malignancy to the pancreas or secondary involvement of the pancreas by a hematologic malignancy. Out of these 31 patients, 19 were male (61%) and 12 were female (39%) [Table 1]. The average age at diagnosis was 66 years (ranging from 49 to 86 years). Twenty-three patients (74%) had a prior known history of malignancy. In these patients, the latency period between the primary diagnosis of malignancy and the development of pancreatic lesions ranges from 6 months to 19 years with an average of 5.9 years. The patients with metastatic renal cell carcinoma (RCC) had the longest latency period, averaging 11.8 years.

Secondary tumors presented as a solitary lesion in the pancreas in 17 cases, while the remaining 14 cases had multiple pancreatic lesions [Table 1]. In 6 of 14 cases with multiple pancreatic lesions, the patient also had suspicious metastatic lesions elsewhere in the body. The pancreatic lesions were located in the body/tail region in 18 cases and in the head/uncinate process in 12 cases. The remaining case had multiple lesions involving nearly the entire pancreas. The size of the pancreatic lesions was recorded in 28 cases with an average of 4.3 cm (ranging from 1.1 to 10 cm). If multiple masses were present, the size of the largest mass was used.
The final diagnoses of these secondary tumors were metastatic carcinoma (24 cases, 77%), lymphoproliferative disorder (4 cases, 13%), and metastatic sarcoma (3 cases, 10%) [Table 2]. The metastatic carcinomas included RCC (8 cases), nonsmall cell lung cancer (NSCLC, 4 cases), small cell lung cancer (SCLC, 3 cases), colorectal carcinoma (2 cases), adenocarcinoma of the breast (2 cases), esophageal carcinoma (1 case), ovarian carcinoma (1 cases), cervical carcinoma (1 case), urothelial carcinoma (1 case), and Merkel cell carcinoma (1 case). The lymphoproliferative disorders included diffuse large B-cell lymphoma (DLBCL, 2 cases) and plasma cell neoplasm (2 cases). The metastatic sarcomas included liposarcoma, chondrosarcoma and synovial sarcoma in one case each.
The correct cytological diagnoses were rendered in 29 out of 31 cases (94%) while two cases were misclassified as primary pancreatic carcinomas [Table 2]. In 18 out of the 29 cases with correct cytological diagnoses, ancillary studies were used to reach the final diagnosis. The ancillary studies included immunostaining (16 cases) and flow cytometry (2 cases). The remaining 11 cases with no ancillary testing were solely diagnosed based on morphological features. All of these 11 patients had a known prior history of malignancy in which histologic morphology was available to compare with that seen in the cytological material. These 11 cases included RCC (3 cases), NSCLC (2 cases), ductal carcinoma of the breast (2 cases), small cell carcinoma of the lung (1 case), plasma cell neoplasm (1 case), esophageal adenocarcinoma (1 case), and urothelial carcinoma (1 case).
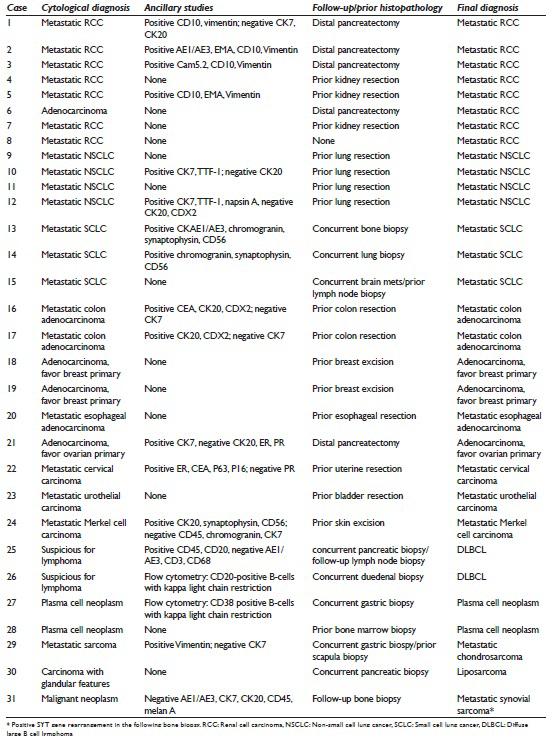
The 2 cases that were misclassified as primary pancreatic malignancies had no known prior history of malignancy. In both cases, ancillary testing was not performed. One patient (patient #6) had a solitary pancreatic lesion in the body/tail region. The imaging finding of a 2 cm lesion in the left kidney was not provided to the cytopathologist at the time of biopsy and cytological evaluation. A distal partial pancreatectomy was performed, and histological examination showed a metastatic RCC [Figure 1]. The second patient (patient #30) had multiple pancreatic lesions in the body/tail region with one dominant mass. The patient also had an associated liver mass and a vague mesenteric lesion. The cytological examination was read as positive for malignant cells with vague glandular features. A core needle biopsy was performed, and the diagnosis of well-differentiated liposarcoma was rendered [Figure 2]. No pancreatectomy was performed on this case. In addition, there was a patient (patient #31) who presented with a solitary mass in the body of pancreas. The aspirate revealed poorly differentiated malignant neoplasm with uncertain cell lineage by both morphologic and immunophenotypic analysis. A follow-up bone biopsy showed synovial sarcoma, which was positive for SYT gene rearrangement [Figure 3].

- Cytomorphologic features and surgical follow-up of metastatic renal cell carcinoma that was initially misclassified pancreatic adenocarcinoma. The aspirates showed clusters of tumor cells with vacuolated cytoplasm (a) Diff-Quik, ×40, and lipid droplets in the cytoplasm (b) Diff-Quick, ×40. The surgical follow-up showed tumor cells with eosinophilic (c) H and E, ×20 and clear cytoplasm (d) H and E, ×20
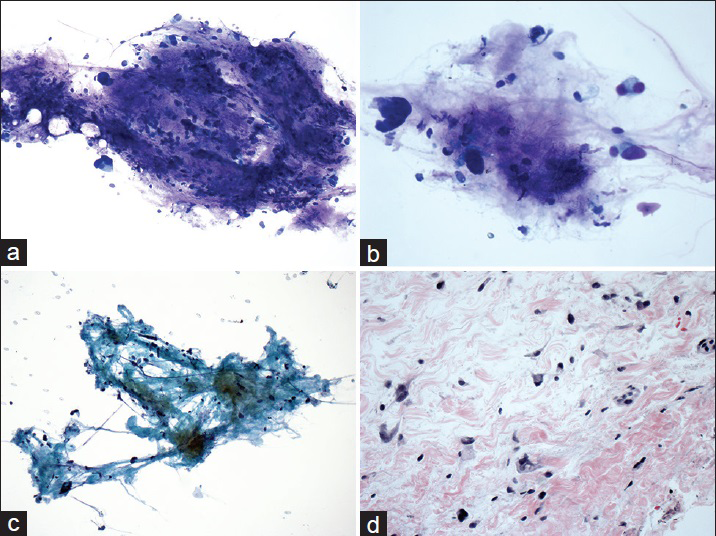
- Cytomorphologic features and surgical follow-up of liposarcoma that was initially misclassified carcinoma with glandular features. The aspirates showed dyscohesive tumor cells admixed with myxoid/collagenous stroma (a) Diff-Quik, ×20 and (c) Papanicolaou, ×20. The tumor cells had eccentrically located nuclei (b) Diff-Quik, ×40. The surgical follow-up showed scattered tumor cells embedded in myxoid/collagenous stroma (d) H and E, ×40
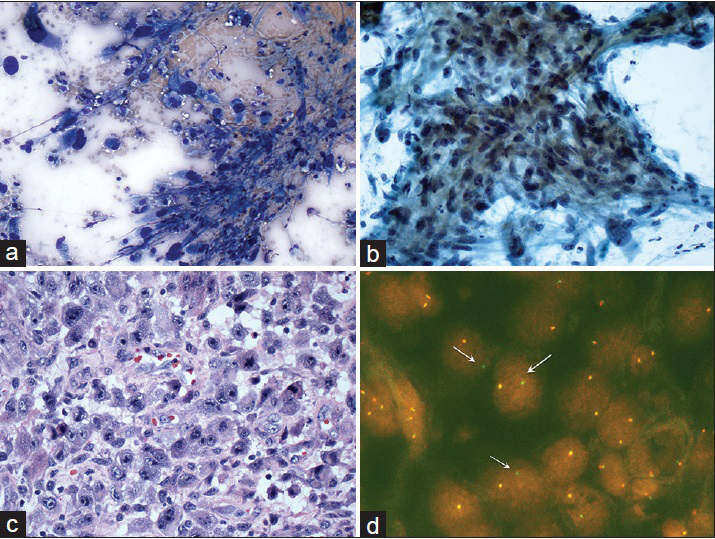
- Cytomorphologic features and surgical follow-up of synovial sarcoma that was initially diagnosed as unclassified malignant neoplasm. The aspirates showed pleomorphic tumor cells with hyperchromatic nuclei (a) Diff-Quik, ×40, and (b) Papanicolaou, ×20. The surgical follow-up showed sheets of tumor cells with pleomorphic nuclei and prominent nucleoli (c) H and E, ×40. Fluorescence in situ hybridization for SYT gene rearrangement demonstrated split green-orange or isolated green signals (arrows) (d) Fluorescence in situ hybridization image, ×80
DISCUSSION
Involvement of the pancreas by secondary tumors including carcinomas, sarcomas, and hematological malignancies is a well-documented, yet uncommon occurrence. Autopsy studies have shown the incidence of pancreatic involvement by secondary tumors to be in the range of 4–15%.[12] Both studies included tumors that involved the pancreas by direct invasion from nearby organs. This involvement can present as a well-defined solitary mass versus a more diffuse involvement at multiple sites of the pancreas as seen on different imaging modalities.[21] Few reports in the literature have tried to establish a characteristic radiologic appearance of primary pancreatic tumors versus metastatic lesions. DeWitt et al. reported that the presence of well-defined borders was significantly associated with metastatic lesions.[21] However, tumor size, echogenicity, location, and lesion number were not found to be significantly different between primary pancreatic tumors versus metastatic tumors, suggesting that by imaging alone, it is not possible to differentiate primary pancreatic neoplasms from metastatic tumors.[1721] The tumors in our series represented solitary or multiple masses with similar frequencies and showed no preferential location. As the treatment and prognosis could be significantly different, it is imperative to render an accurate diagnosis. The cytological examination is considered an extremely useful tool to make this distinction.[1521]
EUS-FNA is a safe and accurate method of evaluating pancreatic lesions.[6] A number of previous studies have repeatedly shown high sensitivity and specificity of cytological examination for primary pancreatic neoplasms.[678913] Few reports have also demonstrated the utility of EUS-FNA in evaluating secondary pancreatic malignancies.[15161820] The reported incidence of secondary malignancies diagnosed by pancreatic FNA ranges from 1% to 11%.[13151617] In our series, the calculated incidence was 2.3% based on 1346 pancreatic EUS-FNA cases, which is within the reported range. Cytological diagnosis of secondary tumors involving the pancreas can be challenging at times. The diagnostic accuracy rate of EUS-FNA could be variable with one report being 89%.[24] In the current study, we rendered a correct diagnosis in 29 of 31 cases (94%), a higher diagnostic accuracy rate than previously reported in the literature. Several factors may contribute to our high diagnostic performance. Most of our patients (74%) had a documented prior history of malignancy and had prior surgical specimens available for morphological comparison. It is important not to discount a remote history of malignancy because the latency for the development of pancreas metastasis could be as long as more than 20 years after initial diagnosis.[2025] Our two cases that were misdiagnosed from the patients who had no documented history of malignancy. Second, at our institution, all EUS-FNA procedures for solid pancreatic masses are performed with rapid on-site evaluation either by a cytopathologist or a cytotechnologist. This practice enables adequate sampling, which has been shown to increase diagnostic yield.[2627] Third, ancillary studies may be required for rendering a diagnosis or classification of secondary tumors, especially in cases without a prior history of malignancy. The rapid on-site evaluation also allows the cytopathologist or cytotechnologist to triage specimens for performing ancillary studies. In the current series, ancillary studies were performed in 18 of 31 cases (58%), including immunostaining (16 cases) and flow cytometry (2 cases).
The present study showed that metastatic carcinomas were the most common secondary tumors, seen in 24 of 31 cases (77%), which is similar to that previously reported.[171920212223] Correct diagnosis and accurate classification of metastatic carcinomas rely on knowledge of the patient's malignancy history, careful evaluation of morphologic features, and performance of immunocytochemical studies if indicated. Since the metastatic carcinomas could be morphologically diverse and originate from a number of organs, the patient's prior history along with the morphologic features of the current specimen may provide clues for selection of immunostaining markers. The most common tumor metastatic to the pancreas has been reported to be RCC.[16202829] Eight metastatic carcinomas (33%) in our series were RCC, in which six patients had prior history and were treated with nephrectomy. These cases except one were correctly diagnosed based on the characteristic morphology and in some cases, the confirming immunostains. The working immunopanel may include cytokeratin, EMA, vimentin, and CD10. More recently, PAX8, a pan-renal marker and CAIX, a clear cell RCC maker, have been recommended for the work-up of metastatic RCC.[30] The importance of rendering the accurate diagnosis in these patients has been highlighted by the reports showing that a solitary RCC metastasis had a better prognosis than primary adenocarcinoma of the pancreas and metastatic RCC was associated with prolonged survival as compared to other metastatic tumors to the pancreas.[41831] The outcome of partial pancreatectomy in these patients has also been shown to be excellent.[32] Metastatic carcinoma of the lung has been reported to be as high as 42% in an autopsied case series.[2] Seven of our twenty-four cases were metastatic lung cancers, including adenocarcinoma (4 cases) and SCLC (3 cases). Metastatic lung adenocarcinoma has overlapping morphology with primary pancreatic adenocarcinoma, the most common malignancy in the pancreas.[1520] Primary small cell carcinoma, although rare, could be seen in the pancreas and should be included in the differential diagnosis metastatic small cell carcinoma.[153334] Prior history of lung cancers including SCLC would be of great help for suggesting a metastasis. All of our patients with metastatic lung cancer had an either prior history or current biopsy to suggest a lung primary. thyroid transcription factor-1 (TTF-1) is a relatively specific marker for adenocarcinoma of the lung with a reasonable sensitivity and should be included in the workup if suspecting a metastatic lung adenocarcinoma. The panel for small cell carcinoma should include neuroendocrine markers such as chromogranin, synaptophysin, CD56 as well as TTF-1. In cases with negative TTF-1, Napsin A may add additional diagnostic value.[3536] Although the vast majority of SCLCs stain positive for TTF-1, the positivity of TTF-1 has been reported in small cell carcinoma of extra-pulmonary origins.[3738] Therefore, the clinical history and presentation may provide additional help in determining the possible primary sites. Other less commonly seen metastatic carcinomas in our series included colon, breast, esophagus, ovary, cervix, bladder, and Merkel cell carcinoma. All of these patients had a documented history of malignancy and immunostains were performed in 3 cases to substantiate the diagnosis.
Involvement of the pancreas by lymphoproliferative disorders has been previously reported.[815163940] In our series, secondary lymphoproliferative disorders were seen in 4 cases, including DLBCL and plasma cell neoplasm, 2 cases each. The diagnosis of DLBCL was rendered based on the cytomorphologic features, immunophenotypic features by immunostaining or flow cytometry, as well as the results of concurrent tissue biopsies. Previous studies have repeatedly demonstrated that ancillary studies are required to render a primary diagnosis and classification of lymphomas.[414243] The ancillary studies may include flow cytometry, immunostains, and molecular tests. Rapid on-site evaluation is cruial in this circumstance, which helps procure specimen and recommend tissue biopsy if necessary. The two patients with plasma cell neoplasm had prior history, and the tumors showed characteristic cytomorphologic features, which may be sufficient for a diagnosis. Nevertheless, flow cytometry was performed in one of the cases.
Although rare, sarcomas can present as a metastasis to the pancreas. A variety of metastatic sarcomas have been reported in the literature, including leimyosarcoma, angiosarcoma, rhabdomyosarcoma, chondromosarcoma, liposarcoma, and malignant fibrous histocytoma.[831444546474849] In our series, three patients were diagnosed with metastatic sarcomas. One patient had a history of dedifferentiated chondrosarcoma of the scapula and presented with multiple pancreatic masses and extrapancreatic masses. Based on the clinical history, morphologic comparison, and immunostaining results, the diagnosis of metastatic chondrosarcoma was rendered. The second patient presented with a dominant pancreatic mass and a liver mass. Morphological examination showed malignant cells with vague glandular features. However, the concurrent core biopsy showed liposarcoma and the patient was treated accordingly. The third patient presented with a solitary pancreatic mass, the biopsy of which showed poorly differentiated neoplasm with negative immunostaining for cytokeratin, CD45, and melan A. The follow-up bone biopsy revealed synovial sarcoma, confirmed by SYT gene rearrangement.
In our series, there were two cases that were cytologically misclassified. Thefirst case (patient #6) was initially diagnosed as pancreatic adenocarcinoma. The distal pancreatectomy showed metastatic clear cell RCC with focal eosinophilic features, which appeared to be overlapping, at least focally, with the features of adenocarcinoma [Figure 1]. The second case (patient #30) showed dyscohesive or single tumor cells had eccentrically located hyperchromatic nuclei, which was interpreted as glandular differentiation. The current core biopsy resulted in a diagnosis of liposarcoma involving the pancreas. The myxoid/collagenous stroma present on the original aspirate specimen was underappreciated during initial cytological evaluation [Figure 2]. In both of these two cases, the patients have not known the previous history of malignancy and no ancillary studies were performed on cytological specimens.
CONCLUSION
In summary, secondary tumors involving the pancreas are uncommon, which can be accurately diagnosed by EUS-FNA. Provision of detailed clinical history and close communication with clinicians is imperative. In the setting of absent or unknown clinical history of previous neoplasia, recognition of cytomorphologic features, and unusual for primary pancreatic neoplasms should prompt further workup. In addition to the recognition of a prior history of malignancy, rapid on-site evaluation, and ancillary studies may help improve the diagnostic performance of secondary malignancies involving the pancreas.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by the ICMJE http://www.icmje.org/#author.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board.
LIST OF ABBREVIATIONS (In alphabetic order)
EUS-FNA - Endoscopic Ultrasound-Guided Fine Needle Aspiration
TTF-1 - Thyroid Transcription Factor-1.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Secondary tumors of the pancreas: Clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001;51:686-90.
- [Google Scholar]
- Secondary tumors of the pancreas: An analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527-35.
- [Google Scholar]
- Pancreaticoduodenectomy for metastatic tumors to the periampullary region. J Gastrointest Surg. 1999;3:119-22.
- [Google Scholar]
- Pancreatic resection for metastatic renal cell carcinoma: Presentation, treatment, and outcome. Ann Surg Oncol. 2003;10:922-6.
- [Google Scholar]
- Surgical treatment of metastatic tumors to the pancreas: A single center experience and review of the literature. World J Surg. 2006;30:1536-42.
- [Google Scholar]
- EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am. 2012;22:155-67.
- [Google Scholar]
- Percutaneous fine needle aspiration biopsy of the pancreas. Cytodiagnosis of pancreatic carcinoma. Acta Cytol. 1978;22:215-20.
- [Google Scholar]
- Fine needle aspiration cytology of the pancreas. An analysis of its use in 52 patients. Acta Cytol. 1985;29:873-8.
- [Google Scholar]
- Cytologic criteria for the diagnosis of pancreatic carcinoma. Am J Clin Pathol. 1985;83:171-6.
- [Google Scholar]
- CT-guided needle biopsy of the pancreas: A retrospective analysis of diagnostic accuracy. Am J Gastroenterol. 1992;87:1610-3.
- [Google Scholar]
- Detection and tumor staging of malignancy in cystic, intraductal, and solid tumors of the pancreas by EUS. Gastrointest Endosc. 2001;53:722-7.
- [Google Scholar]
- Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459-64.
- [Google Scholar]
- Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma: Emphasis on atypical, suspicious, and false-negative aspirates. Cancer Cytopathol. 2003;99:285-92.
- [Google Scholar]
- Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118-26.
- [Google Scholar]
- Fine needle aspiration of metastatic and hematologic malignancies clinically mimicking pancreatic carcinoma. Acta Cytol. 1992;36:471-6.
- [Google Scholar]
- Utilization of fine-needle aspiration biopsy in the diagnosis of metastatic tumors to the pancreas. Diagn Cytopathol. 1995;12:8-13.
- [Google Scholar]
- Detection of pancreatic metastases by EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:65-70.
- [Google Scholar]
- Multiple late asymptomatic pancreatic metastases from renal cell carcinoma: Diagnosis by endoscopic ultrasound-guided fine needle aspiration biopsy with immunocytochemical correlation. Dig Dis Sci. 2002;47:1839-42.
- [Google Scholar]
- Diagnosis of nonprimary pancreatic neoplasms by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2004;31:313-8.
- [Google Scholar]
- Metastases in the pancreas from nonhematologic neoplasms: Report of 20 cases evaluated by fine-needle aspiration. Diagn Cytopathol. 2004;31:216-20.
- [Google Scholar]
- EUS-guided FNA of pancreatic metastases: A multicenter experience. Gastrointest Endosc. 2005;61:689-96.
- [Google Scholar]
- Metastatic disease to the pancreas documented by endoscopic ultrasound guided fine-needle aspiration: A seven-year experience. Diagn Cytopathol. 2012;40:228-33.
- [Google Scholar]
- Endoscopic ultrasound-guided biopsy of pancreatic metastases: A large single-center experience. Pancreas. 2013;42:524-30.
- [Google Scholar]
- Accuracy of endoscopic ultrasound-guided fine-needle aspiration in the suspicion of pancreatic metastases. BMC Gastroenterol. 2013;13:63.
- [Google Scholar]
- Clinicopathological features and surgical outcome of isolated metastasis of renal cell carcinoma. Hepatogastroenterology. 2007;54:1836-40.
- [Google Scholar]
- Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-94.
- [Google Scholar]
- The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159-71.
- [Google Scholar]
- Pancreatic metastasis 25 years after nephrectomy for renal cancer. Tumori. 1989;75:503-4.
- [Google Scholar]
- The role of fine-needle aspiration cytology in the evaluation of metastatic clear cell tumors. Cancer. 1999;87:380-9.
- [Google Scholar]
- Best practices recommendations in the application of immunohistochemistry in urologic pathology: Report from the International Society of Urological Pathology consensus conference. Am J Surg Pathol. 2014;38:1017-22.
- [Google Scholar]
- Clinical features of metastatic tumors of the pancreas in Korea: A single-center study. Gut Liver. 2011;5:61-4.
- [Google Scholar]
- Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002;9:675-9.
- [Google Scholar]
- Management of primary hepatopancreatobiliary small cell carcinoma. J Surg Oncol. 2013;107:692-5.
- [Google Scholar]
- Napsin A (TA02) is a useful alternative to thyroid transcription factor-1 (TTF-1) for the identification of pulmonary adenocarcinoma cells in pleural effusions. Diagn Cytopathol. 2007;35:493-7.
- [Google Scholar]
- Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010;41:20-5.
- [Google Scholar]
- An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am J Surg Pathol. 2010;34:525-32.
- [Google Scholar]
- Extrapulmonary small cell carcinoma: A clinicopathological study with identification of potential diagnostic mimics. Histopathology. 2012;61:454-64.
- [Google Scholar]
- Relapse of IgA lambda multiple myeloma presenting as obstructive jaundice and abdominal pain. Onkologie. 2009;32:119-21.
- [Google Scholar]
- Use of endoscopic ultrasound in diagnosing plasmacytoma of the pancreas. JOP. 2012;13:26-9.
- [Google Scholar]
- EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-91.
- [Google Scholar]
- Fluorescence in situ hybridization studies on direct smears: An approach to enhance the fine-needle aspiration biopsy diagnosis of B-cell non-Hodgkin lymphomas. Cancer. 2009;117:338-48.
- [Google Scholar]
- Targeted use of fluorescence in situ hybridization (FISH) in cytospin preparations: Results of 298 fine needle aspirates of B-cell non-Hodgkin lymphoma. Cancer Cytopathol. 2010;118:250-8.
- [Google Scholar]
- Dedifferentiated chondrosarcoma metastasizing to the pancreas in pregnancy. Acta Obstet Gynecol Scand. 1989;68:467-8.
- [Google Scholar]
- Computed tomography and histologic appearance of pancreatic metastases from distant sources. Acta Radiol. 1989;30:615-9.
- [Google Scholar]
- Isolated pancreatic metastasis of extremity myxoid liposarcoma: Report of a case. Jpn J Clin Oncol. 2006;36:662-4.
- [Google Scholar]
- Pleomorphic liposarcoma of the axilla metastatic to the pancreas. Dig Surg. 2009;26:262-3.
- [Google Scholar]
- Diagnosis of metastatic pancreatic mesenchymal tumors by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2009;37:792-802.
- [Google Scholar]
- Role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in diagnosing metastasis to the pancreas: A tertiary center experience. Pancreatology. 2011;11:390-8.
- [Google Scholar]








