Translate this page into:
Accuracy of diagnosis of solid pseudopapillary tumor of the pancreas on fine needle aspiration: A multi-institution experience of ten cases
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Solid pseudopapillary tumor of the pancreas (SPTP) is a neoplasm of uncertain origin and indolent biologic behavior with distinctive morphological features occurring predominantly in young women. This tumor has an excellent prognosis compared to neuroendocrine and acinar cell carcinoma, which are close differential diagnoses based on morphology, hence making it crucial to diagnose SPTP correctly.
Objectives:
To discuss the cytomorphological features of 10 cases of SPTP reported in two institutions and to evaluate the diagnostic accuracy of endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) cytology in establishing the diagnosis of SPTP.
Methods:
Ten diagnosed cases of SPTP were retrieved from the computerized endoscopy and pathology databases of our two tertiary care institutions. Nine patients had subsequent histological follow-up available. Eight patients underwent EUS-FNA while one patient each had ultrasound and computed tomography-guided FNA. The rapid on-site evaluation was carried out in all 10 cases, and additional material was retained for cell block preparation. Immunohistochemical (IHC) stains ranging from synaptophysin, progesterone receptor, chromogranin, β-catenin, CD10, and NSE were applied on cell blocks. Histological sections of all resected specimens were reviewed, and findings were correlated with those obtained by FNA.
Results:
Adequate material was obtained in all ten cases. IHC stains helped to confirm the cytological impression of SPTP. Histological examination of resection specimens, available in 9/10 cases, confirmed the cytological diagnosis.
Conclusions:
FNA particularly that obtained with EUS guidance is an effective tool in the accurate diagnosis of SPTP.
Keywords
Cytology
endoscopic ultrasound
fine needle aspiration
neoplasm
pancreas
INTRODUCTION
Solid pseudopapillary tumor of the pancreas (SPTP) is a rare neoplasm of uncertain origin, often indolent biologic behavior, and distinctive pathologic features. It constitutes approximately 1% of pancreatic neoplasms and 3% of cystic lesions of the pancreas. SPTP occurs more frequently in the body and tail of the pancreas, and usually in young women.[123] SPTP is usually confined to the pancreas at the time of initial diagnosis; complete surgical excision is often possible and is usually curative. Metastases are rare after excision, and even patients with metastases at the initial diagnosis may survive for many years or even for decades following the treatment. The clinical findings and the radiological features of SPTP can help in making the correct diagnosis and in differentiating these lesions from other pancreatic neoplasms. Abdominal ultrasound and computed tomography (CT) scan usually demonstrate a large, well-encapsulated mass with both solid and cystic components causing displacement of nearby structures.[45]
Endoscopic ultrasound (EUS) with EUS-guided fine needle aspiration (FNA) has an important role in providing an accurate preoperative diagnosis of pancreatic lesions, since it not only provides staging information but also means to establish a cytological diagnosis. EUS-guided FNA can differentiate SPTP from other pancreatic neoplasms of similar radiological and cytological appearance but with different biological behavior, such as pancreatic neuroendocrine neoplasms (PNN), acinar cell carcinoma (ACC), pancreatoblastoma (PB), and pancreatic mucinous cystic neoplasm (PMCN).[6789101112]
This review focuses on the cytomorphological features of 10 cases of SPTP diagnosed by EUS-guided FNA in eight, and by ultrasound and CT-guided FNA in one patient each.
METHODS
Ten diagnosed cases of SPTP were retrieved from the computerized databases of two tertiary care hospitals, one in Pakistan and the other in the United States.
Nine patients had subsequent surgery following the cytological diagnoses so that histological follow-up was available. Eight patients underwent EUS-FNA for cytologic confirmation while one patient each underwent an ultrasound and CT-guided FNA. In all ten cases, rapid on-site evaluation (ROSE) of the material obtained by FNA has carried out the enabling assessment of material adequacy and the formulation of a provisional diagnosis. Smears were made onsite in the endoscopy suite or the radiology department. The aspirated material was smeared onto glass slides; one smear was air dried and immediately stained with Romanowsky stain for ROSE, whereas the remaining smears were fixed immediately in 95% alcohol for subsequent Papanicolaou staining. The additional aspirated material was retained for cell block evaluation using standard techniques. A panel of immunohistochemical stains (IHC), including progesterone receptor, synaptophysin, chromogranin, β-catenin, CD10, and NSE, were applied on the cell block. The details of specific stains used in each case are shown in Table 1.
For the purpose of this study, histological sections of all resected specimens with a full panel of IHC were reviewed, and findings were correlated with those obtained by FNA.
RESULTS
All the patients in our series were female ranging in age from 13 to 50 years (mean 22 years). All patients presented with abdominal pain. Eight patients had EUS-FNA whereas one patient each had an FNA performed under CT- and ultrasound guidance. Two patients had a tumor located in the head of the pancreas, one in the body, five in the tail, and two had a large tumor present in the body extending into the tail. Tumor size ranged from 42 to 80 mm. EUS findings in six patients revealed mixed solid and cystic, circumscribed neoplasms, with heterogenous areas, whereas the tumor was more necrotic on ultrasound in two other patients who underwent EUS. The remaining two patients had features of a solid/cystic neoplasm on imaging (CT and ultrasound). The detailed clinical, EUS, CT, and ultrasound findings are shown in Table 2. Figure 1a and b show EUS and CT scan images of two cases.
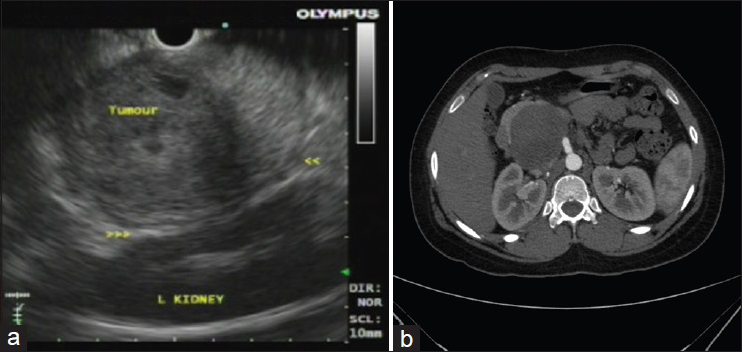
- (a) Endoscopic ultrasound image showing a circumscribed neoplasm in the tail of the pancreas with heterogenous appearance in close relation to the spleen. (b) Computed tomography image showing a circumscribed pancreatic head mass
Cell blocks were available in all ten cases. The initial cytological diagnosis was confirmed in all nine patients in whom resection specimens were available. One patient was lost to follow-up after EUS-FNA, and so histological confirmation was not possible.
Morphological evaluation of smears and cell blocks showed a papillary pattern with central capillaries and myxoid stroma in all 10 cases. Nuclear grooves were present in 4/10 cases; cytoplasm was moderate in 7 cases, abundant in 2, and scant in 1 case. Necrosis was seen in 2/10 cases and all 10 cases mitoses were occasional or not seen. Small nucleoli were present in 3/10 cases. Cytoplasmic vacuolation was seen in 4/10 cases. Cercariform cells were not observed in any of the cases we report.
Histological findings
Resection specimens were available in 9 cases, and they shared similar morphological and IHC findings seen earlier on cell blocks.
Immunohistochemistry
Cytomorphological and IHC findings are shown in Table 1. Figure 2a–e illustrates cytomorphological features, and Figure 3a–c depicts positive IHC staining for β-catenin, NSE, and CD99. β-catenin was applied in six cases, all of which showed positive nuclear and cytoplasmic staining (100%). Chromogranin was negative in all nine cases. Synaptophysin showed variable results with three cases showing focal positivity while two cases were negative. 3/3 cases showed positive Progesterone receptor (PR) nuclear staining. CD10 was performed in one case, and it was strongly positive. An extended panel of IHC was also applied on resection specimens including CD99, which demonstrated characteristic perinuclear dot-like positivity in 8/10 cases [Figure 3c].
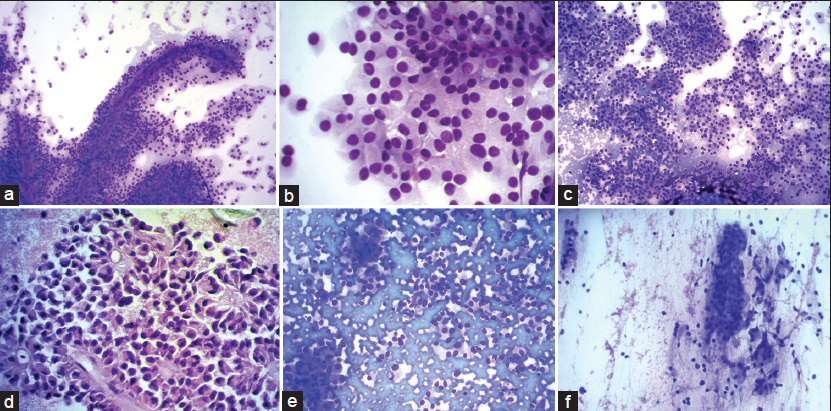
- (a) Romanowsky stain showing tumor cells arranged in pseudopapillae and solid nests. Individual cells have round to oval nuclei with abundant eosinophilic cytopalasm ×200. (b) ×400 view of tumor cells showing evenly dispersed nuclear chromatin, regular nuclear membrane, and inconspicuous nucleoli. Cells are monomorphic with abundant cytoplasm. Cytoplasmic vacuoles present in few cells. (c) Solid sheets of tumor cells, cohesive as well as discohesive pattern ×200. (d) Myxoid fibrillary material forms the central core of these papillae. Nuclear membrane irregular in this case but with fine chromatin ×400. (e) Pancreatic neuroendocrine tumor: Cohesive and discohesive cellular smear. plasmacytoid appearance of tumor cells with eccentrically placed nucleus and nuclei with salt and pepper-like chromatin. (f) Intraductal papillary mucinous neoplasm: Columnar cells, cohesive clusters, bland nuclei with mucinous cytoplasm. Mucin in background
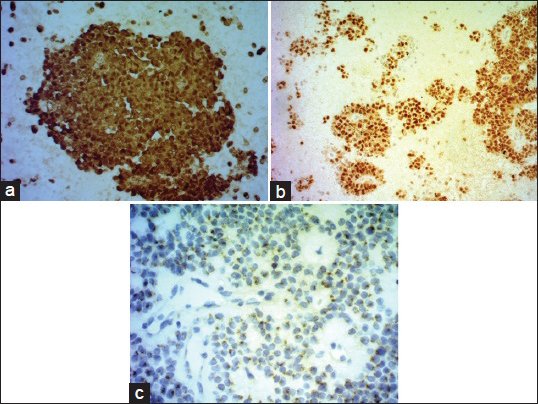
- (a) β-catenin on cell block (nuclear and cytoplasmic positivity). (b) NSE on cell block. (c) CD 99 perinuclear dot like positivity
DISCUSSION
SPTP of the pancreas is a rare neoplasm of unknown pathogenesis with low malignant potential usually affecting young women in the second or third decade of life. All our cases were also seen in females, mean age of 22 years. Despite the low malignant potential of this condition, up to 15% of patients with this tumor develop metastases. The most common sites of metastases are regional lymph nodes, the liver, mesentery/omentum, and peritoneum.[131415] Washington[16] showed that the clinical and pathologic features of SPTP, including diffuse growth, venous invasion, nuclear pleomorphism, mitotic rate, necrosis, and dedifferentiation, were related to its aggressive behavior or metastatic potential.
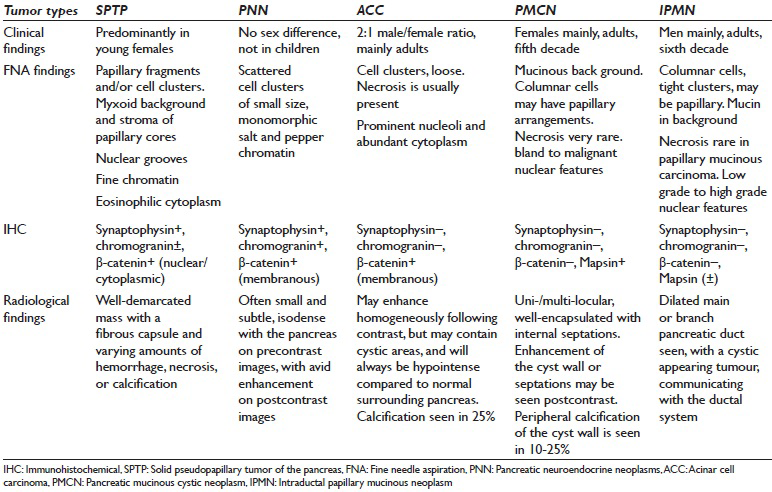
Almost 80% of all cystic lesions of the pancreas are pseudocysts with the remainder being serous cystadenoma, PMCN, intraductal papillary mucinous neoplasm (IPMN), cystic PNN, and SPTP. All these entity were the clinical and radiological differential diagnosis. However once an adequate cytological material is obtained, the differential diagnosis can easily be narrowed down. The major conditions from which SPTP's need to be differentiated pathologically [Table 3] include PNN, ACC, and to some extent PMCN and IPMN.[17]
β-catenin mutations associated with WNT signaling play a major role in tumorigenesis of SPTP. SPTP's may also show alterations in the adenomatous polyposis coli (APC) gene which are not seen in pancreatic ductal neoplasms (PDN).[18] Diffuse immunostaining for β-catenin has been found in the cytoplasm and nuclei of SPTP [Figure 3a], in contrast to the predominantly membranous reactivity in PNN and ACC (25% may show nuclear staining), which may be helpful in distinguishing between SPTP and such neoplasms. Although aberrant nuclear and cytoplasmic expression of β-catenin may also be seen in PBs,[1920] this is easily distinguished on cytomorphological grounds.
In our series, 7/10 cases had cystic solid areas on radiology and EUS imaging [Table 2]. Degenerative cystic change, the mechanism of cyst formation, is seen most commonly in SPTP, less frequently in PNN, and only occasionally in PDN. All these lesions manifest clinically in a similar manner.[172122232425]
Recently, the role of E-cadherin, which is also a member of the WNT signaling pathway, has been established in SPTP. Burford et al.[26] showed 100% membranous staining for E-cadherin in PNN and nuclear and/or cytoplasmic or no expression of E-cadherin in SPTP. Similarly, a recent report from El-Bahrawy et al.[27] showed membranous expression of E-cadherin in 93% cases of PNN. It has also been suggested that a lack of membranous expression by E-cadherin antibody has a role in the pathogenesis of SPTP and explains the discohesive nature of tumor cells. Although we did not perform E-cadherin in our cases, recent studies we quote showed that lack of membranous staining of E-cadherin in SPTP is a helpful diagnostic tool and its role clearly need to be elucidated further.
Deregulated expression of cell cycle associated proteins in SPTP with over expression of cell cycle activating proteins, cyclin D1 and cyclin D3, along with counteracting up regulation of the cyclin-dependent kinase inhibitors.
p21 and p27 has also been studied.[28] These findings explain the low proliferative index in tumor cells and its relationship with a good prognosis.
PNN is a major challenge in the differential diagnosis of SPTP, mainly because of overlapping morphological and IHC features. Many studies have established an accurate diagnostic approach which can differentiate between PNN and SPTP. Important cytomorphological features that can be detected with certainty by a good cytopathologist include the presence of a cohesive and discohesive monotonous population of neoplastic cells. On smears, variable patterns such as solid sheets, rosettes, pseudo-rosettes, or papillary arrangement can be seen. The background may be either clean, hemorrhagic, or contain naked nuclei, or histiocytes. Mitotic figures, amyloid, thin capillaries, calcification, multinucleation, nuclear pleomorphism or necrosis are less frequent features and are rarely seen.[29] Individual cells are round to oval, small to medium-sized, with smooth contours. Nuclei tend to be eccentrically located, giving the cell a plasmacytoid appearance [Figure 2e]. In PNN, the chromatin is characteristically granular or salt and pepper-like. We found that the absence of papillary fronds with myxoid stoma, plasmacytoid appearance and salt and pepper-like chromatin were the three most helpful cytomorphological features favoring PNN over SPTP [Figure 2e].
Since it can be difficult to perform a large battery of IHC stains on the limited cytological material, antibodies to be tested need to be chosen wisely. Burford et al.[26] performed an extended panel of IHC on resection specimens and selected a panel which can be used on FNA material. According to their study, chromogranin A, CD56, and synaptophysin were consistently expressed (100%) in PNN, whereas E-cadherin was noted in 14 of 16 PNN cases in a membranous pattern. E-cadherin expression, if present in SPTP, was noted in a cytoplasmic and/or nuclear pattern but never in a membranous pattern. Chromogranin A was expressed in 3 (38%) of 8 SPTP cases. All PNN cases stained with β-catenin showed cytoplasmic or membranous staining. In comparison, all SPTP cases showed a strong nuclear staining pattern, with three cases having concurrent cytoplasmic staining, although less pronounced than nuclear reactivity. Seventy-five percent of SPTP cases were positive for PR and demonstrated a strong nuclear pattern of staining while approximately 31% of PNN also showed PR staining. Their results show that expression of E-cadherin/β-catenin, CD10, and PR is significantly different between SPTP and PNN. Burford et al.[26] also found E-cadherin and β-Catenin to be100% specific for PNN whereas chromogranin A and PR were 67% and 25% specific. In addition, CD10 is expressed in 100% of SPTP cases and only 30% of PNN cases. Chromogranin A has been long-used to support the diagnosis of PNN expressed in only 38% of SPTP. CD56, NSE, and synaptophysin are positive in both PNN and SPTP. They concluded that characteristic expression of E-cadherin/β-catenin and CD10 could be used to distinguish PNN from SPTP. Another study, by Li et al.[30] emphasized the unique pattern of dot-like staining for CD99 in SPTP in contrast to membranous staining in all PNN's and most ACC, and negative immunostaining in PDC.
In our study, we found β-catenin, chromogranin A, and CD10 to be more sensitive markers for SPTP. We performed CD 99 on our resection specimens [Figure 3c] and found it be a useful marker with a unique perinuclear dot-like staining pattern [Figure 3c]. In our experience, β-catenin, chromogranin A, CD10, and CD99 form the most useful diagnostic panel to diagnose SPTP on cytology.
PMCN and IPMN are true cystic neoplasm and are each in the differential diagnosis of the other, on both clinical and radiologic grounds. PMCN is more common in women and IPMN in men. IPMN often shows a clear connection with the pancreatic ductal system with obvious main duct dilatation.[29] On smears, cellularity is variable with abundant thick viscous mucus often seen in the background and neoplastic mucinous cells demonstrating varying degrees of cytological atypia depending on the grade of the tumor [Table 3]. Distinguishing these two from SPTP is not challenging, and the major distinguishing cytomorphological features include abundant mucinous background in IPMN and PMCN [Figure 2f] in contrast to a myxoid stroma, limited to the fibro-vascular cores in SPTP. Distinguishing between PMCN and IPMN on cytology alone is not possible with the clinical information being essential.[29]
In addition to the cytomorphological findings and IHC described above and shown in Table 3, some studies have described distinctive cytomorphological features which can help in making a definitive diagnosis of SPTP. In one such study, Jhala et al.[31] describe the presence of clear vacuolated cytoplasm in almost 80% of SPTP's. However in our study, cytoplasmic vacuolation was seen in only 40% of cases (4/10). In another study, Samad et al.[32] discussed the presence of cercariform cells as a useful clue in diagnosing SPTP and in differentiating it from other pancreatic neoplasms with overlapping features. Cercariform cells look like cells with cytoplasmic tails, as though they have been detached from the fibrovascular cores during aspiration or smearing. We did not see cercariform cells in any of the cases we report here.
The ACC may have similar cytomorphological features such as monomorphism; however, major differences are seen in nuclear features, where ACC usually have prominent nucleoli without papillary configuration or perivascular myxoid appearance. In ACC, abundant granular cytoplasm with indistinct cell borders is seen in Table 3. Positive IHC for synaptophysin and nuclear, rather than membranous, staining for β-catenin are clues to diagnosing SPTP rather than ACC, although 23% of ACC may show nuclear staining.[33]
Our results suggest that the use of FNA appears to be accurate and reliable for the cytologic diagnosis of SPTP. The fact that we were able to confirm the initial cytologic diagnosis by histological evaluation of the resected specimen in all nine patients who underwent surgery supports this. Branching papillae with associated myxoid stroma limited to the core are characteristic of this tumor, and can easily be seen on initial direct smears performed at the time of ROSE. Clinical correlation, cytomorphological features, and IHC stains applied to the cell-block help to distinguish SPTP's from other pancreatic neoplasms with similar morphologic features such as PNN, ACC, and PMCN.
CONCLUSION
A high index of clinical suspicion is necessary in order to diagnose SPTP. This diagnosis should be borne in mind when a young female patient presents with a pancreatic mass. EUS is well-known and highly effective method for diagnosing and staging pancreatic neoplasms, and EUS-guided FNA appears to be the procedure of choice for obtaining tissue in such patients. More traditional forms of image-guided FNA may be used where EUS-FNA is not available. Clinico-pathological and IHC features help to distinguish SPTP's from pancreatic neoplasms with similar morphological features and aid in planning optimal subsequent treatment. Surgical excision offers the best chance for cure and should always be attempted since patients with SPTP have an excellent prognosis after surgical excision.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
No competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that final version of this study is read and approved by all authors.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of both institutions associated with this study. All authors take responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
ACC - Acinar Cell Carcinoma
APC - Adenomatous Polyposis Coli
CT - Computed Tomography
EUS - Endoscopic Ultrasound
FNA - Fine Needle Aspiration
IHC - Immunohistochemical
IPMN - Intraductal Papillary Mucinous Neoplasm
IRB - Institutional Review Board
PB - Pancreatoblastoma
PDN - Pancreatic Ductal Neoplasms
PMCN - Pancreatic Mucinous Cystic Neoplasm
PNN - Pancreatic Neuroendocrine Neoplasms
PR - Progesterone Receptor
ROSE - Rapid On-Site Evaluation
SPTP - Solid Pseudopapillary Tumor of the Pancreas
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2015/12/1/29/171140
REFERENCES
- Solid-pseudopapillary tumor of the pancreas: A typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000;17:66-80.
- [Google Scholar]
- Cystic forms of typically solid pancreatic tumors. Semin Diagn Pathol. 2000;17:81-8.
- [Google Scholar]
- Tumors of the exocrine pancreas. Tumors of the Pancreas. Atlas of Tumor Pathology. Third Series, Fascicle 20. Washington DC: Armed Forces Institute of Pathology; 1997. p. :31-144.
- [Google Scholar]
- Solid-pseudopapillary tumor of the pancreas: Clinical experience and literature review. World J Gastroenterol. 2005;11:1403-9.
- [Google Scholar]
- Solitary cystic tumor of the pancreas: EUS-pathologic correlation. Gastrointest Endosc. 1997;45:268-76.
- [Google Scholar]
- Role of endoscopic ultrasound in the diagnosis of cystic lesions of the pancreas. Pancreatology. 2001;1:637-40.
- [Google Scholar]
- Ultrasound guided percutaneous fine needle aspiration cytology of pancreas: A review of 61 cases. Trop Gastroenterol. 1995;16:101-9.
- [Google Scholar]
- The use of endoscopic ultrasound in the diagnosis of solid pseudopapillary tumors of the pancreas in children. J Pediatr Surg. 2002;37:1370-3.
- [Google Scholar]
- Fine needle aspiration biopsy of pancreatic endocrine neoplasms by endoscopic ultrasonographic guidance. Acta Cytol. 2001;45:905-7.
- [Google Scholar]
- Serous cystadenoma of the pancreas with papillary features: A diagnostic pitfall on fine-needle aspiration biopsy. Arch Pathol Lab Med. 2001;125:1591-4.
- [Google Scholar]
- World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. p. :246-8.
- [Google Scholar]
- Endoscopic ultrasound guided fine needle aspiration cytology diagnosis of solid pseudopapillary tumor of the pancreas: A case report and literature review. World J Gastroenterol. 2007;13:5158-63.
- [Google Scholar]
- FNAC of papillary and solid epithelial neoplasm of pancreas – A case report. Indian J Pathol Microbiol. 1999;42:369-72.
- [Google Scholar]
- Solid-pseudopapillary tumor of the pancreas: Challenges presented by an unusual pancreatic neoplasm. Ann Surg Oncol. 2002;9:3-4.
- [Google Scholar]
- Endocscopic ultrasound guided fine needle aspiration cytology diagnosis of solid pseudopapillary tumor of the pancreas; a rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol. 2004;121:654-62.
- [Google Scholar]
- Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401-4.
- [Google Scholar]
- Mutation of beta-catenin and its protein accumulation in solid and cystic tumor of the pancreas associated with metastasis. Int J Mol Med. 2003;11:461-4.
- [Google Scholar]
- Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: Frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol. 2001;159:1619-27.
- [Google Scholar]
- Solid pseudopapillary neoplasm of the pancreas: A single institution experience of 14 cases. HPB (Oxford). 2006;8:148-50.
- [Google Scholar]
- Solid-pseudopapillary tumor of the pancreas: Immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361-71.
- [Google Scholar]
- E-cadherin/beta-catenin and CD10: A limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol. 2009;132:831-9.
- [Google Scholar]
- E-cadherin/catenin complex status in solid pseudopapillary tumor of the pancreas. Am J Surg Pathol. 2008;32:1-7.
- [Google Scholar]
- Deregulated expression of cell cycle-associated proteins in solid pseudopapillary tumor of the pancreas. Mod Pathol. 2001;14:47-53.
- [Google Scholar]
- Immunohistochemical evaluation of solid pseudopapillary tumors of the pancreas: The expression pattern of CD99 is highly unique. Cancer Lett. 2011;310:9-14.
- [Google Scholar]
- Large clear cytoplasmic vacuolation: An under recognized cytologic clue to distinguish solid pseudopapillary neoplasms of the pancreas from pancreatic endocrine neoplasms on fine needle aspirates. Cancer. 2008;114:249-54.
- [Google Scholar]
- Cercariform cells: Another cytologic feature distinguishing solid pseudopapillary neoplasms from pancreatic endocrine neoplasms and acinar cell carcinomas in endoscopic ultrasound-guided fine-needle aspirates. Cancer Cytopathol. 2013;121:298-310.
- [Google Scholar]
- Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: Frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953-62.
- [Google Scholar]








