Translate this page into:
Enhancing the cytological features and diagnostic significance of cerebrospinal fluid in bacterial meningitis

*Corresponding author: Chenyi Wan, Department of Neurology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China. wanchenyi2022@163.com
-
Received: ,
Accepted: ,
How to cite this article: Long Y, Peng Y, Huang Z, Zhu M, Wan C. Enhanc ing the cytological features and diagnostic significance of cerebrospinal fluid in bacterial meningitis. CytoJournal. 2024;21:24. doi: 10.25259/Cytojournal_111_2023
Abstract
Objective:
The objective of this study was to investigate the cytological features and diagnostic significance of cerebrospinal fluid (CSF) in bacterial meningitis (BM).
Material and Methods:
Patients diagnosed with BM at the First Affiliated Hospital of Nanchang University Hospital between August 2021 and April 2022 were enrolled. Clinical, cranial imaging, CSF-next-generation sequencing, CSF examination, and CSF cytology data were retrospectively analyzed. CSF cytology samples were prepared using a CSF cell pelletizer (precipitation method) and stained using the May–Grunwald–Glemsa (MGG) method. The χ2 test was employed to compare the positive rate of routine CSF count and CSF cytology.
Results:
Eight patients (four males and four females), aged 41–67 years, were included. Among them, two patients had undergone brain surgery within the past 4 months, one patient had an 8-year history of otitis media, and two patients had a history of sudden toothache. Clinical manifestations included fever, headache, sudden disturbance of consciousness, and neck stiffness. CSF cytology revealed abnormal inflammatory changes dominated by neutrophils in seven patients. Routine CSF cell counts exceeded 100/uL in only four cases, indicating a higher positive rate of CSF cytology for detecting CSF inflammatory reactions compared to routine cell count.
Conclusion:
Comparative detection of bacteria through the observation of CSF cytology inflammatory status in BM patients are more useful for diagnosing BM than routine CSF counts.
Keywords
Bacterial meningitis
Cerebrospinal fluid
Cytology
Inflammatory
INTRODUCTION
Central nervous system infections are severe neurological diseases associated with high morbidity and mortality rates. Despite the identification of over 100 pathogens responsible for these infections, more than half of the cases remain attributed to unknown pathogens.[1-3] Among central nervous system infections, bacterial meningitis (BM) represents a significant proportion, with an estimated 1.2 million new cases reported annually.[4] Clinical manifestations of BM include non-specific symptoms such as fever, headache, vomiting, and neck stiffness, with severe cases potentially presenting altered mental status and consciousness disturbances. Lumbar puncture typically reveals cerebrospinal fluid (CSF) abnormalities, including elevated white blood cell counts (predominantly neutrophils), increased protein levels, decreased glucose levels, reduced CSF-to-blood glucose ratio, and elevated intracranial pressure (generally >200 mmH2O).[5,6] However, the diagnostic specificity of routine CSF analysis and neuroimaging for identifying pathogens is limited, leading to the difficulty of definite diagnosis.[7] Final diagnosis often relies on etiological investigations such as CSF culture, smear microscopy, polymerase chain reaction, and next-generation sequencing.
In recent years, advancements in molecular biology technologies have revolutionized clinical testing, elevating laboratory diagnosis to the molecular level, notably with the introduction of metagenomic next-generation sequencing (mNGS) of CSF. Compared to traditional methods, mNGS offers distinct advantages, significantly enhancing the detection rate of pathogen.[8] Theoretically, mNGS can detect nearly all pathogens. However, its high cost impedes repeated testing, necessitating the exploration of more cost-effective and convenient alternatives in clinical practice.
BM is characterized by a predominant neutrophilic inflammatory response in CSF. CSF cytology emerges as a more sensitive method for detecting inflammatory cell responses compared to conventional CSF cell counting techniques. Moreover, CSF cytology is practical and cost-effective compared to expensive CSF high-throughput genetic testing. Despite the predominance of neutrophilic inflammation in BM-associated CSF, further summarization of its clinical changes is warranted, as its diagnostic value has been relatively overlooked.
This study retrospectively analyzed clinical and CSF cytology data from 8 BM patients admitted to the First Affiliated Hospital of Nanchang University Hospital over a 10-month period. The aim was to delve deeper into the characteristics and diagnostic value of CSF cytology in BM.
MATERIAL AND METHODS
Research subjects
Patients diagnosed with BM and hospitalized at the First Affiliated Hospital of Nanchang University Hospital between August 2021 and April 2022 were included in the study. Initial diagnosis of BM was based on clinical manifestations, routine CSF count, and biochemical examinations. In addition, CSF-mNGS was performed, meeting the diagnostic criteria for BM. The study adopted a rigorous inclusion-exclusion process. Inclusion criteria were as follows: (1) Initially diagnosed with BM based on clinical presentation, routine CSF and biochemical tests, and (2) positive bacterial cultures from CSF; and (3) quantification of lactate concentration in the CSF. Exclusion criteria were as follows: (1) Patients with negative bacterial cultures from CSF; (2) identification of viruses in CSF; and (3) incomplete clinical data from patients.
BM diagnostic criteria are as follows:[9] (1) Symptoms of vomiting, convulsions, fever, skin petechiae, and brain dysfunction; (2) positive meningeal irritation signs and neurological examination; and (3) CSF leukocyte level over 5 × 108/L; (4) cloudy appearance of CSF. The clinical data, cranial imaging, CSF routine examination, CSF-mNGS, CSF cytology, treatment, and prognosis were retrospectively analyzed. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University and patients or their families gave informed consent.
CSF inspection methods
CSF examination comprises CSF routine cell count, CSF biochemistry, CSF-mNGS, and CSF cytology. CSF cytology was conducted using the CSF cell precipitation method. Specifically, 0.5 mL of CSF was added to the CSF fluid cell pelletizer and refrigerated at 4°C. After 24 h of natural precipitation and drying, the cells were stained using the May-Grunwald-Giemsa (MGG).
Statistical analysis
Statistical analysis was conducted using SPSS software (Version R27.0.1.0, IBM Corp., Chicago, IL, USA). The χ2 test was utilized to compare the positive rate of CSF routine count and CSF cytology. A significance level of P < 0.05 was considered statistically significant.
Protocol for preparation of cytological reading slides
The slides for cytological reading were prepared using the CSF cell pelletizer (precipitation method) and stained using the MGG method. Specifically, 0.5 mL of CSF was added to the CSF cell pelletizer and placed in a 4°C refrigerator for 24 h to allow natural precipitation and drying of the cells. The dried cells were, then, stained using the MGG method, following standard laboratory procedures. Based on the prepared slides, cytological examination was conducted by skilled laboratory personnel under a microscope. The cytological features, including cell morphology, presence of inflammatory cells, and any observed bacteria, were carefully evaluated. The presence of abnormal inflammatory changes, such as neutrophilic inflammation dominated by neutrophils, was indicative of BM, and this information contributed to the diagnostic process.
RESULTS
General clinical information
A total of eight patients, aged 41–67 years with a mean age of 57 years, were included in this study, comprising four male and four female patients. All patients exhibited fever, with five patients experiencing headaches, and seven patients presenting with neck stiffness. Among them, three patients were conscious, while the remainder displayed varying degrees of impaired consciousness or coma.
Regarding head imaging findings, four patients showed no apparent abnormalities, one patient exhibited cerebral edema, another had a widening of the brain pool, one displayed thickening of the meninges, and one showed enhancement of the meninges [Table 1].
| Patients | Gender | Age | Time (days)* |
Headache | Fever | Consciousness | Others | Neck resistance | Imaging results | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| I | Male | 41 | 9 | + | + | Clear | 4 months after intracranial hematoma drainage | + | Abnormal signal and edema in the right frontotemporal lobe with enhancement |
Better prognosis |
| II | Male | 55 | 2 | – | + | Confused | 2 months after intracranial acoustic neuroma | - | The cistern of the right cerebellopontine angle was widened without abnormal enhancement |
Better prognosis |
| III | Female | 67 | 2 | + | + | Clear | Rheumatoid arthritis, diabetes, high blood pressure | + | Dural enhancement and thickening | Better prognosis |
| IV | Male | 62 | 2 | + | + | Clear | Toothache 11 days before onset | + | Cerebellar meningeal enhancement | Better prognosis |
| V | Male | 57 | 5 | + | + | Light coma | Toothache, preexisting diabetes mellitus 5 days before onset | + | Normal | Better prognosis |
| VI | Female | 47 | 1 | – | + | Confused | Suffered from autoimmune encephalitis 1 month ago |
+ | Normal | Better prognosis |
| VII | Female | 64 | 2 | – | + | Light coma | Mitral valve replacement 7 years ago, hypertension | + | Normal | Give up |
| VIII | Female | 64 | 16 | + | + | Coma | Past history of otitis media, pus from external auditory canal 2 days ago |
+ | Normal | Better prognosis |
Imaging
The right frontotemporal-parietal lobe exhibited large edema signal shadows, and the right frontal lobe displayed beaded annular enhancement shadows on the enhanced scan (I); the right cerebellopontine angle cistern was widened, and postoperative changes were not found, and no obvious abnormal enhancement signal shadows were found (II); dural thickening and enhancement, obstructive hydrocephalus (III); obvious enhancement of cerebellar meninges (IV); and no abnormality in imaging data (V~VI) [Figure 1a-d].

- (a) The right frontotemporal and parietal lobe show large edema signal shadows, and the right frontal lobe shows beaded ring enhancement shadows on the enhanced scan (White arrow indicates beaded ring enhancement shadows) (I); (b) thickening and enhancement of dura mater (White arrow indicates dural enhancement) (III); (c) the dural thickening and enhancement disappeared after treatment (White arrow indicates dural enhancement is gone) (III); and (d) significant enhancement of cerebellar meninges (White arrow indicates meningeal enhancement) (IV).
CSF TESTING RESULTS
Table 2 presents the results of CSF examination in eight patients. The CSF routine leukocyte count was (0–10)/μL in four cases, (100–1000)/ μL in two cases, and (>1000)/ μL in two cases. CSF sugar ranged from 0.01–4.92 mmol/L, and CSF protein ranged from 58–1099 mg/dL. Intracranial pressure ranged from 160 to 400 mmH2O, with five patients exhibiting pressures >200 mmH2O. CSF-mNGS examination was completed for all eight patients, revealing the presence of various pathogens: Three Gram-positive cocci, including Staphylococcus aureus (I), Streptococcus gordonii (II), Streptococcus pneumonia (VI); four Gram-positive cocci were detected: Negative bacilli: Klebsiella (III/V), Haemophilus haemophilus (IV), and Proteus panethi (VI); and one case of Listeria monocytogenes (VII) were detected. CSF cytology demonstrated abnormal inflammatory changes, with one patient dominated by lymphocytes (comprising approximately 60% of cells) and seven patients dominated by neutrophils (comprising approximately 60–90% of cells), along with activated lymphocytes and mononuclear phagocytes. Notably, streptococcus bacteria were observed in one case under the light microscope (at ×100 magnification), appearing in pairs and arranged in clusters [Figure 2a-d]. A subsequent review of routine and biochemical tests of CSF were conducted for all 8 patients. However, only one patient (III) underwent CSF cytology re-examination after a 7-day interval treatment, which revealed a transition from neutrophil-dominated mixed inflammation to mild lymphocyte reaction.
| Patients | Intracranial pressure (mmH2O) | CSF (/uL) |
Protein (mmol/L) |
Glucose (mmol/L) |
Chloride (mmol/L) |
CSF-mNGS | Cerebrospinal fluid cytology | Result |
|---|---|---|---|---|---|---|---|---|
| I | 400 | 120 | 229.7 | 1.62 | 112.5 | Staphylococcus aureus | Number of cells: 2500, L10%, M10%, N80%, AL+, N-based |
Inflammatory response |
| II | 250 | 10 | 195.3 | 4.92 | 126.1 | Streptococcus gordonii | Number of cells: >3000, L60%, M10%, N30%, AL+, L-based |
Inflammatory response |
| III | 220 | 10 | 419.9 | 2.00 | 119.0 | Klebsitin | Number of cells: 2500, L20%, M10%, N70%, AL+, N-dominant mixed |
Inflammatory response |
| IV | 200 | 5600 | 169.4 | 2.87 | 125.7 | Haemophilus haemophilus | Number of cells: 3000, L5%, M5%, N90%, AL+, N-based |
Inflammatory response |
| V | 350 | 15000 | 1099.8 | <0.2 | 110.5 | Klebsiella pneumoniae | Number of cells: >3000, L5%, M5%, N90%, N-based | Inflammatory response |
| VI | 160 | 1 | 180.7 | 3.45 | 124.3 | Proteus panethi | Number of cells: >3000, L10%, M10%, N80%, AL+, N-based |
Inflammatory response |
| VII | 175 | 5 | 58.06 | 3.24 | 124.9 | Listeria monocytogenes | Number of cells: >3000, L20%, M10%, N70%, N-based |
Inflammatory response |
| VIII | 180 | 630 | 1072 | 0.01 | 114.0 | Streptococcus pneumonia | Number of cells: 2500, L30%, M10%, N60%, AL+, N-based | Inflammatory response |
L% is the ratio of lymphocytes, M% is the ratio of monocytes, N% is the ratio of neutrophils, AL+: Activated lymphocytes. CSF: Cerebrospinal fluid, mNGS: Metagenomic next-generation sequencing
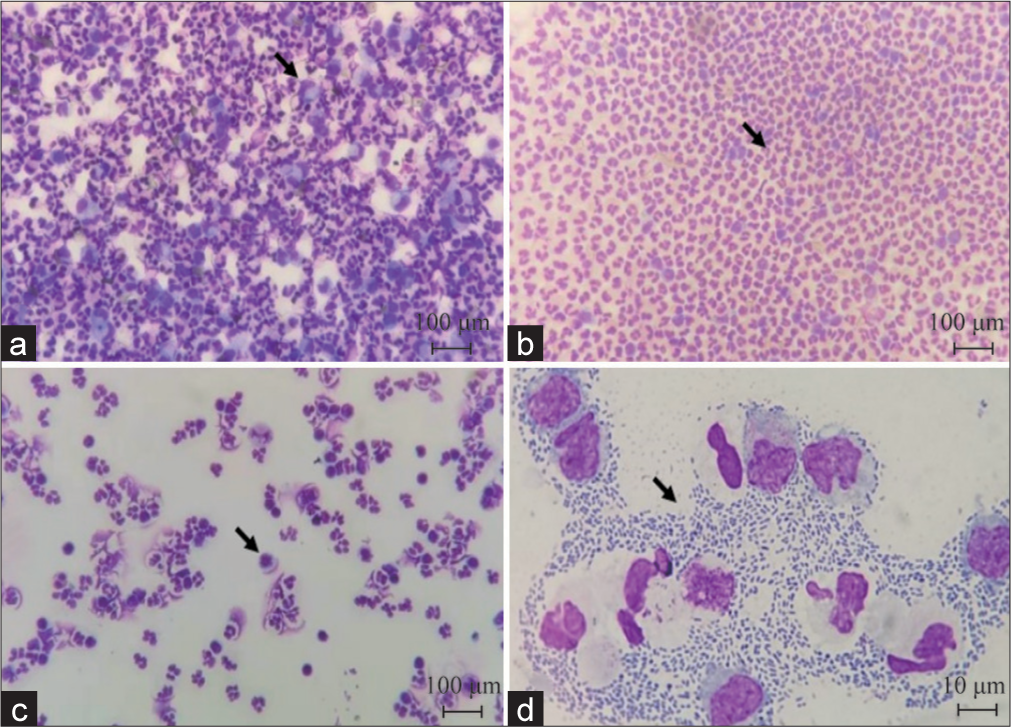
- Cerebrospinal fluid cytology (May-Grunwald-Glemsa staining). (a) Neutrophil inflammatory response (black arrow indicates monocytes-macrophages)(×100); (b) neutrophil inflammatory response (black arrow indicates neutrophils)(×100); (c) inflammatory response of neutrophils and lymphocytes (black arrow indicates activated lymphocytes)(×100); and (d) a large number of bacterial cells in the intercellular space (indicated by arrow), the cells are in pairs and arranged in clusters, under the light microscope (×10).
We compared the positive rate of CSF cytology with CSF routine count. Among the cases, only four had a CSF cell count exceeding 100/uL, resulting in a positive rate of 50% for routing count. However, CSF cytology revealed neutrophil inflammatory changes in seven cases, with a higher positive rate of 87.5%. The positive rate of detecting CSF inflammatory reaction using CSF cytology was found to be higher than that of routine cell count but the difference is not statistically significant.
Outcome
After standard treatment, the shortest hospitalization duration was 16 days; the longest was 35 days, with an average stay of 26 days. Following treatment, patients experienced resolution of symptoms such as headache and fever. They regained consciousness and exhibited a supple neck. Lumbar puncture, CSF routine, and biochemical tests normalized, and imaging indicated reduced lesions or meningeal enhancement. Regrettably, one patient succumbed to multi-organ failure after discontinuing treatment.
DISCUSSION
BM is a globally prevalent disease with a high mortality rate, particularly affecting neonates and the elderly. Besides age, factors such as trauma, immunocompromised states, genetic predispositions, and unhealthy lifestyles contribute significantly to the risk of BM. The pathogenesis of BM often involves bacteria entering the subarachnoid space either through the bloodstream or from adjacent infection sites such as paranasal sinuses and inner ear mastoids, eventually breaching the blood–brain barrier. Common pathogens responsible for BM include S. pneumonia, Neisseria meningitides, and L. monocytogenes.[9,10] Patients enrolled in this study had diverse predisposing factors such as post-operative intracranial surgery, sudden toothache, external ear pus, autoimmune encephalitis, and diabetes, placing them at high risk for BM. Initial symptoms typically included headache, fever, altered consciousness, and neck stiffness, highlighting the importance of considering bacterial infection as a primary diagnostic consideration. The imaging manifestations of BM are diverse. Due to its acute onset, enhancement of the meninges at the base of the brain is not very common on enhanced scans, and only a small number of patients may present with communicating hydrocephalus. These typical features are not obvious in the early stages of the disease course, and there are no specific changes.[11] The increase in inflammatory exudates following disease development manifests as blurred boundaries between gyri. Localized and diffuse long T1 and long T2 signals may appear when complicated with brain parenchymal infection, accompanied by a pronounced mass effect. Enhanced scanning may reveal abnormal enhancement resembling gyri, meningeal thickening, or aqueduct compression, leading to cerebral cistern blockage and subsequent hydrocephalus. Abnormal meningeal enhancement, cerebral cortex signals on diffusion-weighted imaging, and long T2 signals in the cerebral cortex are key magnetic resonance imaging (MRI) diagnostic features of BM. In this study, three patients exhibited imaging changes characterized by extensive brain parenchymal edema. Contrast-enhanced scans revealed bead-like annular enhancement, dura mater thickening, meningeal enhancement in the cerebellum, and obstructive hydrocephalus, consistent with MRI findings in BM. A retrospective analysis conducted abroad, along with the study by Bentlin et al.[12,13] suggested that meningitis caused by Gram-negative bacilli infection is more likely to exhibit imaging changes such as ependymatitis, subdural effusion, hydrocephalus, ventriculomegaly, and brain abscess, indicating a worse prognosis for patients. In this study, three patients presented with imaging changes, including one gram-positive coccus and two Gram-negative bacilli cases, consistent with the aforementioned literature. In general, the clinical manifestations and imaging changes of BM lack distinct characteristics. Early diagnosis of BM through imaging alone is challenging and may be easily confused with other central nervous system infections such as tuberculous meningitis.
BM is a condition requiring emergency treatment, where rapid and accurate diagnosis, followed by timely treatment, is crucial.[14] The gold standard for diagnosis still relies on CSF analysis. In clinical practice, CSF bacterial culture or next-generation sequencing is primarily utilized, while routine CSF cell counting appears to be less accurate. Among the eight patients in this group, only four had leukocytes counts exceeding 100/uL. CSF bacterial culture demands a high bacteria concentration, harsh culture conditions, and a lengthy incubation period of at least a week. In patients not receiving antibiotics, the diagnosis yield is approximately 85%.[15] However, false negatives may occur in patients previously treated with antibiotics. Although CSF-mNGS significantly improves pathogen detection rates,[16] its high cost limits its repeatable use. Notably, CSF cytology provides preliminary results within 24 h and, due to its convenience and low cost, allows for repeated testing. It represents the most rapid diagnostic measure if bacteria are detectable under a microscope. CSF cytology involves the morphological examination of CSF cells. The diagnostic technique is originated in the early 20th century. In 1954, Sayk pioneered the use of centrifugation for CSF cell slides. In China, the application and advancement of CSF cytology technology have been introduced and led by esteemed neurology seniors such as Hou Xide. Clinical CSF cytology has evolved into a vital subspecialty of neurology, playing a crucial role in diagnosing central nervous system diseases.[17-19] In this study, all patients presented with fever symptoms, and five exhibited disturbances of consciousness and had higher cell counts. Neutrophils dominate the CSF cytological classification during the acute phase of BM, comprising up to about 90% of cells. Cell morphology primarily reflects neutrophilic inflammation, although activated lymphocytes, mononuclear phagocytes, and even individual streptococcus cells may be observed. Effective antibiotic therapy leads to a significant decrease in nucleated cells in CSF, particularly neutrophils, while lymphocytes gradually increase. Dynamic monitoring of CSF cytology was conducted in only one of the eight patients. Post-treatment CSF cytology in this patient shifted from neutrophil-dominated mixed inflammation to a mild lymphocytic reaction, with cell counts decreasing from 2500 to 300. This indicates the effectiveness of anti-inflammatory treatment and its value in guiding antibiotic adjustments. The previous studies have also shown the accumulation of different immune cells in the central nervous system, which may contribute to local immune responses. Researchers such as Pashenkov et al.[20] suggest that regulating the transport of dendritic cells into and out of the CSF may represent a mechanism for controlling inflammation in the central nervous system. Nau et al.[21] believe that during the resolution of inflammation in BM, a significant portion of granulocytes undergo apoptosis. These granulocytes are cleared through phagocytosis by macrophages, indicating changes in macrophages during bone marrow turnover.
CSF cytology in patients with BM typically reveals a neutrophilic response during the acute phase. As antibiotics therapy is initiated and the disease progresses, the proportion of neutrophils gradually decreases while lymphocytes and monocytes increase, indicating a mixed cellular response. Given the similarity between BM and tuberculous meningitis, early recognition of disease progression and effective intervention is crucial for prognosis. However, diagnosing BM is often delayed due to atypical clinical symptoms, delayed CSF examination through lumbar puncture, and low detection rates of pathogenic bacteria. Although CSFNGS has significantly improved diagnosis rates, its limited availability, high cost, and logistical challenges hinder widespread adoption in medical facilities nationwide.
On the other hand, CSF cytology, as a simple and rapid diagnostic tool for central nervous system diseases, holds practical significance. Promoting the use of this low-cost CSF diagnostic technology is essential for widespread accessibility and improved patient outcomes. The limitations of this study primarily include the small sample size of dynamically observed cases, which lacks statistical persuasiveness. Second, there is no comparison of the analysis efficiency with other testing methods and economic practicality. In addition, the cytological results of CSF in the article are not the sole determinants of infection types, hence requiring additional indicators for supplementary conclusions in the future.
Although our study focused on the diagnostic significance of CSF cytological features in patients with BM, we acknowledge that the immune cell changes in meningitis may be influenced by various factors. Meningitis can be caused by a wide range of pathogens, including bacteria, viruses, fungi, and parasites. Each type of meningitis may elicit different immune cell responses, necessitating consideration of the possibility of different pathogens in the diagnosis and treatment of meningitis. Our study results demonstrate that observing CSF cytological features, particularly the inflammatory response of neutrophils, can improve the accuracy of diagnosing BM, which is crucial for guiding clinical treatment. However, we also recognize that cytological features may vary among different types of meningitis, highlighting the need for further research to deepen our understanding of the pathophysiological processes of meningitis. Future studies can further investigate the immune cell responses in different types of meningitis and compare them with the cytological features observed in BM to enhance the accuracy of meningitis diagnosis and individualized treatment.[22,23]
SUMMARY
CSF cytology in patients with BM often reveals inflammatory changes, such as neutrophilic inflammation. Compared to routine cell counts in CSF, CSF cytology provides a more sensitive assessment of the inflammatory state of CSF and can even detect bacteria, making it a valuable tool for the diagnosis of BM.
AVAILABILITY OF DATA AND MATERIALS
Data and materials are available upon reasonable request. All data are available from the corresponding author.
ABBREVIATIONS
BM - Bacterial meningitis
CSF - Cerebrospinal fluid
MGG - May–Grunwald–Glemsa
mNGS - Metagenomic next-generation sequencing
MRI - Magnetic resonance imaging
AUTHOR CONTRIBUTIONS
CW and YL: Designed the research study; ZH and MZ: Performed the research; CW and YP: Provided help and advice on the ELISA experiments; CW: Analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research/study approved by the Institutional Review Board at the First Affiliated Hospital of Nanchang University, number (2022)CDYFYYLK(06-013), dated 20 22. Written informed consent was obtained from all the patients or their families prior to the enrollment of this study. The study was conducted in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
This study was supported by the Science and Technology Project of Jiangxi Health Commission (No. 202310021), the Traditional Chinese Medicine Science and Technology Project of Jiangxi(2022A309).
References
- Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877-97.
- [Google Scholar]
- Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology. 2010;75:924-32.
- [CrossRef] [PubMed] [Google Scholar]
- The Vietnam initiative on zoonotic infections (VIZIONS): A strategic approach to studying emerging zoonotic infectious diseases. Ecohealth. 2015;12:726-35.
- [CrossRef] [PubMed] [Google Scholar]
- Acute bacterial meningitis during and after pregnancy. BJOG. 2012;119:1555-7.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of patients with acute bacterial meningitis in a teaching hospital in Ethiopia: A prospective study. PLoS One. 2018;13:e0200067.
- [CrossRef] [PubMed] [Google Scholar]
- Adjunctive dexamethasone therapy in unconfirmed bacterial meningitis in resource limited settings: Is it a risk worth taking? BMC Neurol. 2016;16:153.
- [CrossRef] [PubMed] [Google Scholar]
- Adults with suspected central nervous system infection: A prospective study of diagnostic accuracy. J Infect. 2017;74:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol. 2017;243:16-24.
- [CrossRef] [PubMed] [Google Scholar]
- ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22:S37-62.
- [CrossRef] [PubMed] [Google Scholar]
- Listeria monocytogenes sequence type 6 and increased rate of unfavorable outcome in meningitis: Epidemiologic cohort study. Clin Infect Dis. 2013;57:247-53.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical application of viral cerebrospinal fluid PCR testing for diagnosis of central nervous system disorders: A retrospective 11-year experience. Diagn Microbiol Infect Dis. 2014;80:207-15.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of complications of neonatal and infant meningitis on MRI by organism: A 10 year review. Eur J Radiol. 2011;80:821-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal meningitis according to the microbiological diagnosis: A decade of experience in a tertiary center. Arq Neuropsiquiatr. 2010;68:882-7.
- [CrossRef] [PubMed] [Google Scholar]
- Community-acquired bacterial meningitis. Nat Rev Dis Primers. 2016;2:16074.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical use of multiplex-PCR for the diagnosis of acute bacterial meningitis. J Family Med Prim Care. 2022;11:593-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of the metagenomic next-generation sequencing (mNGS) for detection of bacterial meningoencephalitis: A systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2022;41:881-91.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical cerebrospinal fluid cytology Nanjing: Jiangsu Science and Technology Press; 1985. p. :1-120.
- [Google Scholar]
- Clincal cerebrospinal fluid cytology diagnosis Shijiazhuang: Hebei Science and Technology Press; 2007. p. :1-150.
- [Google Scholar]
- Clinical application of cerebrospinal fluid cytology in neurological diseases. Chin J Contemp Neurol Neurosurg. 2004;14:566-9.
- [Google Scholar]
- Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J Neuroimmunol. 2002;122:106-16.
- [CrossRef] [PubMed] [Google Scholar]
- Granulocytes in the subarachnoid space of humans and rabbits with bacterial meningitis undergo apoptosis and are eliminated by macrophages. Acta Neuropathol. 1998;96:472-80.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J Neuroinflammation. 2019;16:219.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura. Cephalalgia. 2017;37:36-48.
- [CrossRef] [PubMed] [Google Scholar]








