Translate this page into:
Grey zones in respiratory cytology: Atypical or suspicious for malignancy and neoplasms of unknown malignant potential

*Corresponding author: Lester J. Layfield, MD, Department of Pathology and Anatomical Sciences, University of Missouri, Columbia, Missouri, United States. layfieldl@health.missouri.edu
-
Received: ,
Accepted: ,
How to cite this article: Layfield LJ. Grey zones in respiratory cytology: Atypical or suspicious for malignancy and neoplasms of unknown malignant potential. CytoJournal 2023;20:42.
Abstract
The purpose of pulmonary cytology is two-fold. First, to establish whether a pulmonary nodule is benign or malignant. Second, pulmonary cytology should classify the type of pathologic process present. When a pulmonary nodule is characterized as malignant, it is of high importance to further classify the malignancy as to type, with non-small cell carcinomas being sub-divided into adenocarcinomas, squamous cell carcinomas, and other types of non-small cell carcinoma. The World Health Organization Reporting System for Lung Cytopathology (WHORSLC) provides an important framework for reporting and classifying material obtained by cytologic techniques, including sputum analysis, bronchial brushings, bronchial washings, and fine-needle aspiration. The system contains five categories for specimen reporting. Clinicians prefer definitive diagnoses separating specimens into definitively benign or definitively malignant categories. The WHORSLC recognizes that it is not invariably possible for cytopathologists to separate specimens into definitively benign or definitively malignant categories. The five categories of the WHORSLC recognize the spectrum of cytologic changes running from clearly benign to clearly malignant, which cytopathologists must place into diagnostically useful and reproduceable categories. The intermediate categories of “atypical” and “suspicious for malignancy” provide structured categories with stringent definitions, estimated malignancy risks, and suggested management and follow-up recommendations. In this way, the categories “atypical” and “suspicious for malignancy” aid in maintaining the high diagnostic accuracy of the “benign” and “malignant” categories.
Keywords
Lung
Pulmonary
Cytology
World Health Organization
Reporting system
INTRODUCTION
Cytologic material obtained from pulmonary nodules demonstrates a spectrum of changes running from those recognizable as definitively benign to those with cytomorphologic features definitively malignant. This spectrum of cytomorphologic change is due to a variety of biological factors including inflammation associated with reparative and reactive change, and radiation and chemotherapy-related abnormalities. Inconclusive diagnoses may reflect limitations of individual cytopathologists to definitively characterize specimens as either benign or malignant. The cytomorphologic features of low-grade carcinomas may substantially overlap those seen in some benign neoplasms as well as reparative or reactive processes. Such cytomorphologic changes and limitations of cytomorphologic analysis may result in the inability of cytopathologists to invariably classify specimens as definitively benign or definitively malignant. A major goal of classification systems is to ensure a high diagnostic accuracy for the benign and malignant categories. Both the Papanicolaou Society of Cytopathology System for Reporting Respiratory Cytology[1] (PSCSRRC) and the more recent World Health Organization Reporting System for Lung Cytopathology,[2] (WHORSLC) contain intermediate categories designed to maximize the diagnostic accuracy of the benign and malignant categories. These intermediate categories, designated “atypical” and “suspicious for malignancy,” are associated with well-characterized definitions, estimated risks for malignancy and recommendations for management and follow-up. The definitions of the WHORSLC closely mirror those originally formulated in the PSCRRC. The “atypical” and “suspicious for malignancy” categories of the PSCSRRC divide the observed cytomorphologic spectrum of changes into categories with increasing risks of malignancy as one moves from the benign or negative category to the “atypical,” to the “suspicious for malignancy,” and finally the malignant category. The risk of malignancy for the “atypical” category is double that of the benign/ negative category and the “suspicious for malignancy” category has a risk of malignancy essentially double that of the “atypical” category. Thus, there appears to be a strong statistical and perhaps biological basis for the creation of the two intermediate categories. The risk of malignancy associated with both the WHORSLC and the PSCSRRC categories depend on the method of specimen acquisition (sputum cytology, bronchial brushing, bronchial washing, or fine-needle aspiration) as well as the reporting category. This represents the systems’ weakness in that the clinician managing the patient must know the method by which the specimen was obtained. In addition, reproducibility of category assignment by cytopathologists is imperfect and often only fair by Kappa scores.[3]
In this publication, we review the utility of the diagnostic categories “atypical” and “suspicious for malignancy,” their associated risks for malignancy, and types of specimens placed in these intermediate categories.
HISTORICAL PERSPECTIVES FOR THE USE OF INTERMEDIATE CATEGORIES
Cytologic examination of specimens obtained from the respiratory tract has a long history, beginning with evaluation of sputum samples. As early as the mid 1840’s, microscopic descriptions of exfoliated cells from the respiratory tract were published.[4,5] Wandall published a series of sputum cytology cases where the technique had been utilized for the diagnosis of pulmonary carcinoma.[6] Further studies by Foot[7,8] and Bamforth[9] confirmed the utility of sputum cytology for the diagnosis of pulmonary carcinomas. These studies focused primarily on bronchogenic carcinoma. The studies by Foot[7,8] report specimens given Papanicolaou diagnostic categories of Class 4 or Class 5 smears.[7] The majority of early studies did not focus on the issue of inconclusive cytology specimens and how best to classify them. Johnston and colleagues, in a series of reports reviewing the cytopathologic diagnosis of lung cancer over a 10-year period, documented the impact of specimen number, specimen type, accuracy of cytopathologic diagnosis, and the significance of inconclusive cytopathologic diagnoses.[10-12] Terminology for these inconclusive specimens has varied, but experts in the field of pulmonary cytopathology have used terms including “suspicious for malignancy” to classify these smears.
Cytopathologists have used a variety of terms to convey their degree of uncertainty regarding the presence of malignancy in inconclusive specimens which cannot be diagnosed as definitively benign or definitively malignant. The terms used for these “grey zone” cases have varied over time, and some terms have been used inconsistently with imprecise definitions. The terms “atypical” and “suspicious for malignancy” have been frequently employed, but their precise definition and usage has varied over time and the terms have been inconsistently used. Biological processes associated with these terms include reactive changes associated with repair or inflammation, metaplastic processes, and reactions to radiation therapy or chemotherapy.
Three terms have been utilized to designate lesions which are neither clearly benign nor clearly malignant. These terms are “atypia,” “atypical,” and “suspicious for malignancy.” They have been used independently by a number of authors[10-15] and have often referred to different biological processes. No consistent or precise relationship existed between the terms and they were not part of a coherent categorization system. Saito et al.[13] used the term “atypical squamous metaplasia” in association with the finding of metaplastic squamous epithelium demonstrating nuclear features of the type seen in squamous cell carcinomas but present in a lesser degree. Rao et al.[14] utilized the term “atypical” to describe features associated with pulmonary viral infections or reparative changes. In the usage described by Saito et al., and Rao et al., the terms “atypical” and “atypia” did not refer to the degree of abnormality present, but were predominantly associated with metaplastic and reactive/reparative changes. The term “suspicious for malignancy” also has had variable usage. Koss et al.[15] used the term “suspicious” (for cancer) in 63 of 1886 specimens from patients undergoing cytologic examination of pulmonary lesions. About 63% of these “suspicious” diagnoses were proven to be carcinomas.[15] Johnston and Bossen utilized the term “atypia suspicious for malignancy” in their practice at Duke University.[10-13] As applied by Johnston,[12] the term “atypia suspicious for malignancy” had a positive predictive value for malignancy of 85%. Despite this strong correlation with malignancy, none of the parameters utilized for establishing the diagnosis “atypia suspicious for malignancy” consistently predicted the presence of malignancy.[16] These studies indicate that the terms “atypia” and “suspicious for malignancy” have some clinical utility but were not used in an organized classification for pulmonary cytology specimens.
In 1999, the Papanicolaou Society of Cytopathology proffered a series of guidelines for the examination and reporting of cytologic specimens obtained from the respiratory tract.[17] These guidelines addressed a wide variety of issues in pulmonary cytology including safety at work, methods for specimen acquisition, and a description of diagnostic categories useful for reporting cytologic specimen results. The guidelines recommended that the terminology should be consistent and the cytopathologist should attempt to render as specific a diagnosis as possible. The task force recommended against using the Papanicolaou class system, but whenever possible the cytologic diagnosis should closely simulate the corresponding surgical pathology diagnosis. They also recommended use of comments to address specimen adequacy, factors limiting diagnostic accuracy, and reasons for the categorization of a specimen as “non-diagnostic,” “atypical,” “probably benign,” or “suspicious.”
These early guidelines did not propose specific definitions or criteria, and did not recommend follow-up or management protocols. Importantly, no data were given associating the diagnostic categories with an estimated risk of malignancy. Two decades later, the Papanicolaou Society of Cytopathology published a more comprehensive system for the reporting of respiratory cytology.[2] This formalized the reporting categories, giving formal definitions, criteria, and estimates for malignancy risk. Several follow-up studies investigated the system’s reproducibility,[3,18] as well as risks for malignancy associated with the diagnostic categories.[19,20] In response to some of the perceived shortcomings of the PSCSRRC system, Japanese experts proposed an alternate system using only four categories (negative for malignancy, atypical cells, suspicious for malignancy, and malignancy).[21,22] Despite the reduction in the number of categories for the Japanese system, and minor changes to the definition for the benign category, diagnostic accuracy, and risk of malignancy for the individual categories remained very similar to the previously published PSCSRRC system. The newly published WHORSLC system closely follows that outlined by the Papanicolaou Society of Cytopathology system with similar risks for malignancy and follow-up recommendations. Importantly, all three recently proposed systems contain intermediate categories, designated “atypical,” and “suspicious for malignancy” to aid in stratification of the spectrum of cytologic changes associated with respiratory cytology samples, give estimated malignancy risks and follow-up recommendations for the intermediate categories.
MORPHOLOGIC FEATURES ASSOCIATED WITH SPECIMENS DESIGNATED “ATYPICAL”
The atypical category is used when the cytopathologists have a low level of concern that a specimen may harbor a malignancy but the morphologic changes present are insufficient for a categorization of “suspicious for malignancy.” Specimens with morphologic changes clearly recognizable as reactive in nature are assigned to the “benign” category and not the “atypical” category. Features helpful in recognizing cellular features associated with reactive change (repair) include a streaming pattern for nuclei present in cell clusters, general maintenance of nuclear polarity, and recognition of cilia on a number of the abnormal appearing cells. [Figure 1a and b] demonstrate the streaming pattern and general maintenance of nuclear polarity in material associated with reactive/ reparative change. The “atypical” category is defined as “specimens demonstrating cytomorphologic features which clearly exceed those seen in benign and reactive conditions but the features fall short of those required for a diagnosis of “suspicious for malignancy.” The morphologic criteria for the WHORSLC and PSCSRRC systems are mainly qualitative rather than quantitative and criteria useful for assignment of a specimen to the “atypical” category include loss of nuclear polarity, absence of features of repair, minor degrees of nuclear crowding, focal nuclear membrane irregularities, mild chromatin distribution abnormalities, and mild increases in the nuclear/cytoplasmic ratio [Table 1]. Figures 2-4 demonstrate criteria characteristic for smears assigned to the “atypical” category. The illustrations demonstrate degrees of loss of nuclear polarity, nuclear crowding, nuclear membrane irregularities, and variability in nuclear size and shape acceptable for assignment to the “atypical” category.

- (a) Sheet of epithelial cells with reactive change characterized by nuclear crowding but preservation of nuclear polarity. (Papanicolaou). (b) Sheet of epithelial cells demonstrating nuclear streaming in a “school of fish” pattern characteristic of reactive change (Papanicolaou).
|
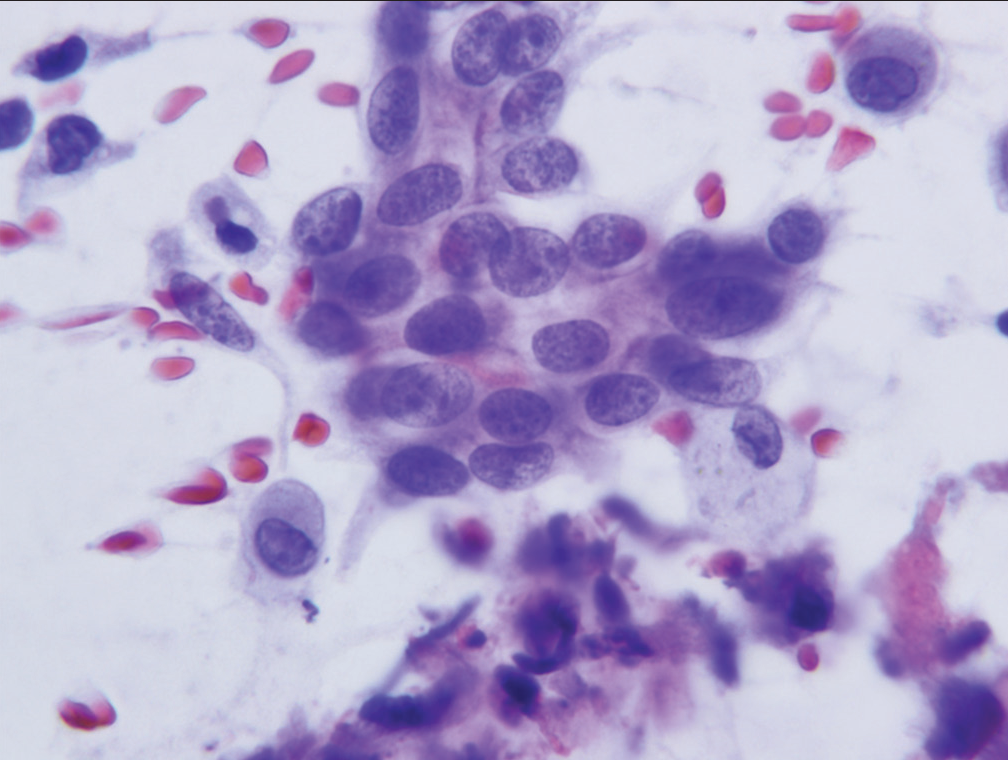
- Group of cells characteristic of the category “atypical.” The cells show mild nuclear crowding but maintain smooth nuclear membranes and little or no anisonucleosis (Papanicolaou).
- Sheet of cells (case designated “atypical”) demonstrating loss of nuclear polarity, slight nuclear membrane irregularities and mild anisonucleosis (Hematoxylin and eosin).
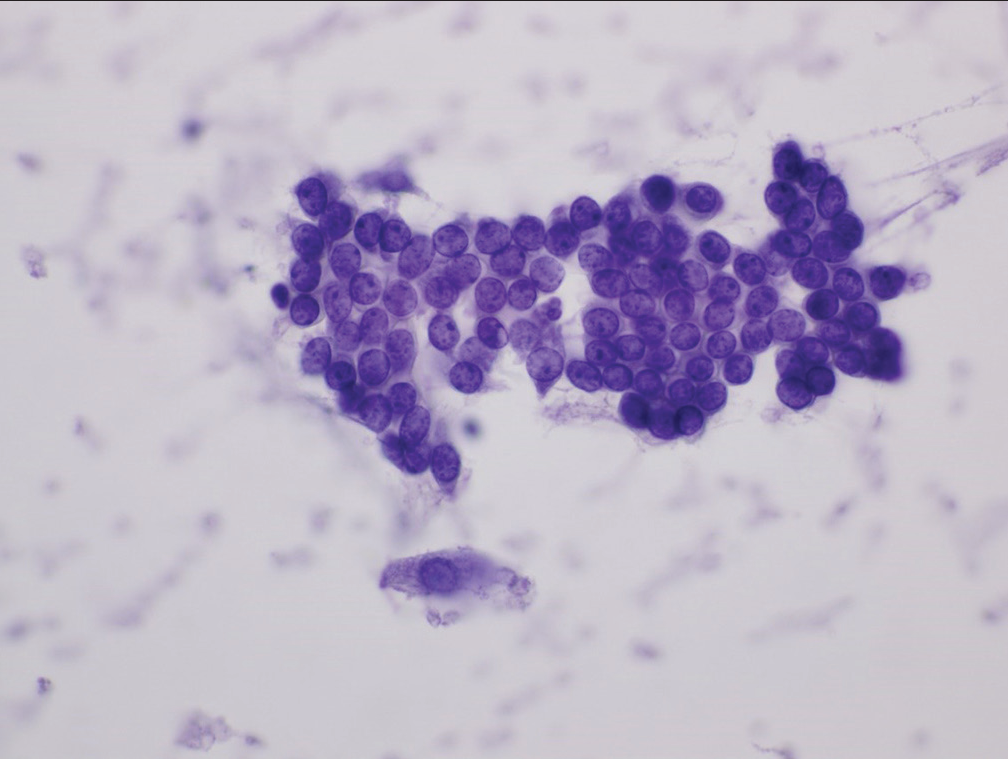
- Sheet of cells characterized as “atypical” showing nuclear crowding, nuclear overlap, loss of polarity, and slight abnormalities of chromatin pattern (Papanicolaou).
Root causes for use of the “atypical” category include morphologic changes which cannot be confidently designated “benign” or “reactive,” insecurity on the part of the cytologist that a specimen is definitively benign, and the presence of a very low-grade neoplasm or carcinoma that morphologically overlaps changes seen in benign epithelium due to inflammation, repair, or minor degrees of dysplasia.
MORPHOLOGIC FEATURES OF SPECIMENS ASSIGNED TO THE “SUSPICIOUS FOR MALIGNANCY” CATEGORY
Since morphologic features for separation of specimens assigned to the “atypical” category, rather than the “suspicious for malignancy” category are predominantly qualitative rather than quantitative, reproducibility of assignment by cytopathologists to the “atypical” or “suspicious for malignancy” categories is only fair. Neither assignment to the “atypical” category or the “suspicious for malignancy” category can form the sole basis for operative intervention. Specimens may fail to meet the stringent criteria for a diagnosis of malignancy in three scenarios. First, a specimen may contain too few cells which meet all criteria. Second, a specimen may contain cells with some, but an insufficient number of well-developed criteria for a malignancy, and finally, the expression of criteria supporting a malignant diagnosis may be present but to a degree insufficient to definitively support a diagnosis of malignancy. The category “suspicious for malignancy” is defined as a specimen demonstrating cytomorphologic features greater than those appropriately placed in the “atypical” category, but having features which fall short of those necessary for a definitive diagnosis of malignancy. [Table 2] lists the criteria necessary for placing a specimen in the “suspicious for malignancy” category. Significant among these are mild-to-moderate anisonucleosis (three-fold variability between the largest and smallest nuclei within a single cluster), as well as significant loss of nuclear polarity, nuclear crowding, nuclear membrane irregularities, and abnormalities of chromatin pattern with hyperchromasia [Figures 5-7]. A precise cut point for increased nuclear/cytoplasmic ratio has not been defined, but an N/C ratio of 0.6–0.7 should be considered significant.
|
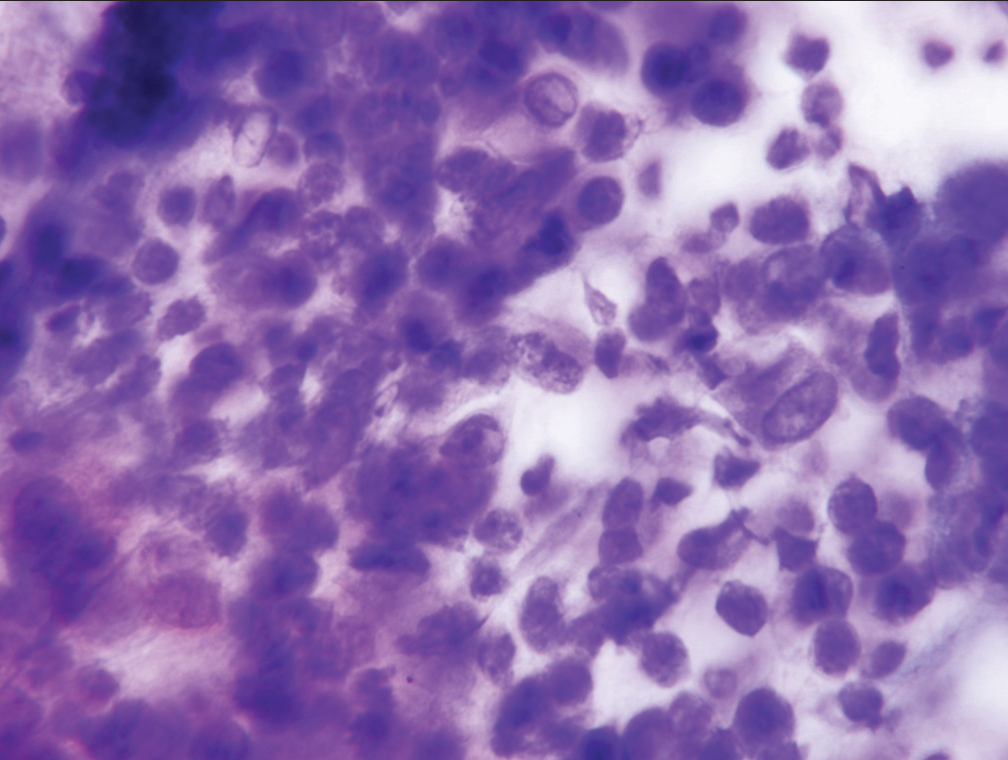
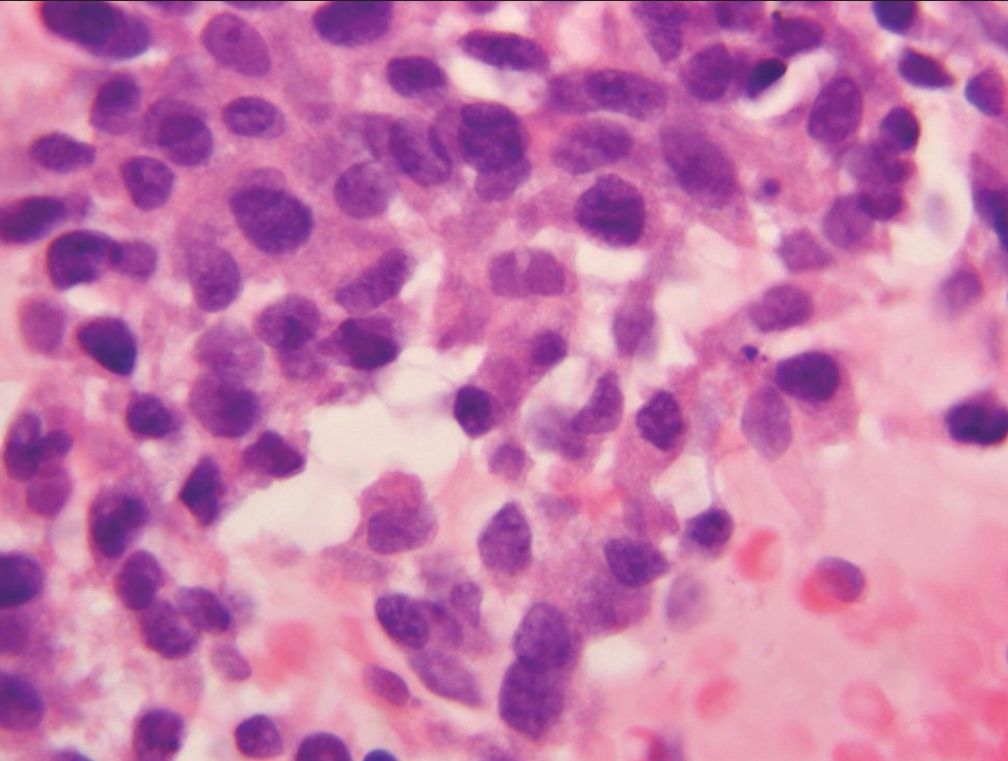
- Sheet of epithelial cells designated “suspicious for malignancy” showing substantial nuclear crowding, loss of nuclear polarity, and moderate anisonucleosis (Papanicolaou).
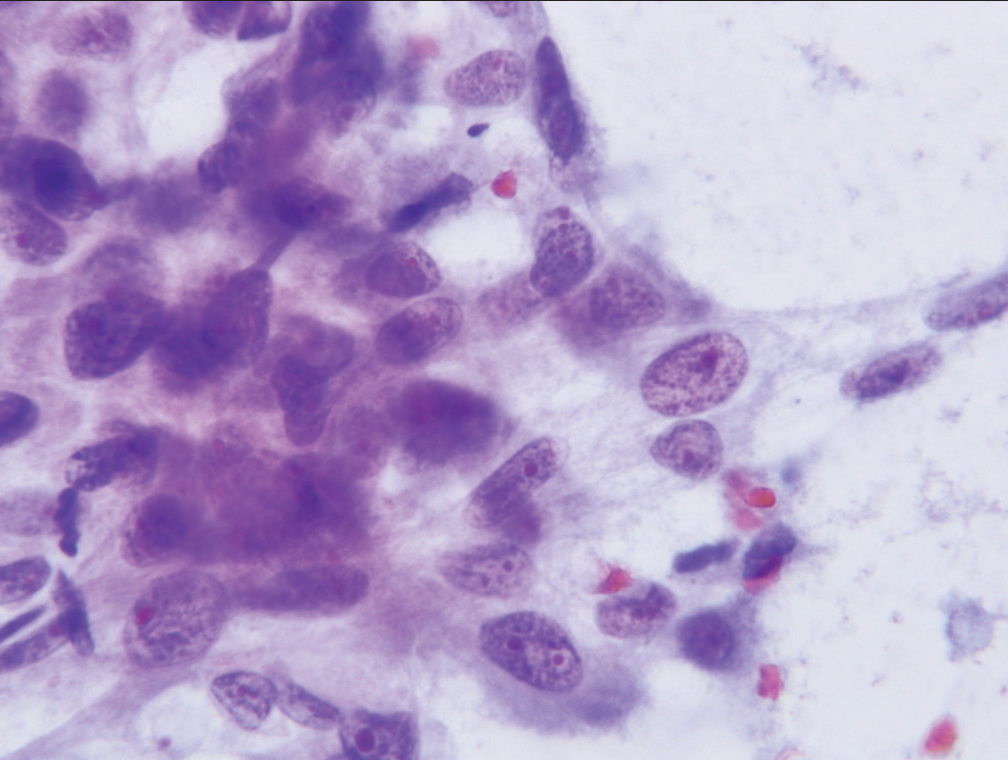
- Cell cluster characterized as “suspicious for malignancy” demonstrate significant loss of cell polarity, abnormalities of chromatin pattern, and at least two-to-three-fold variability in nuclear size (Papanicolaou).
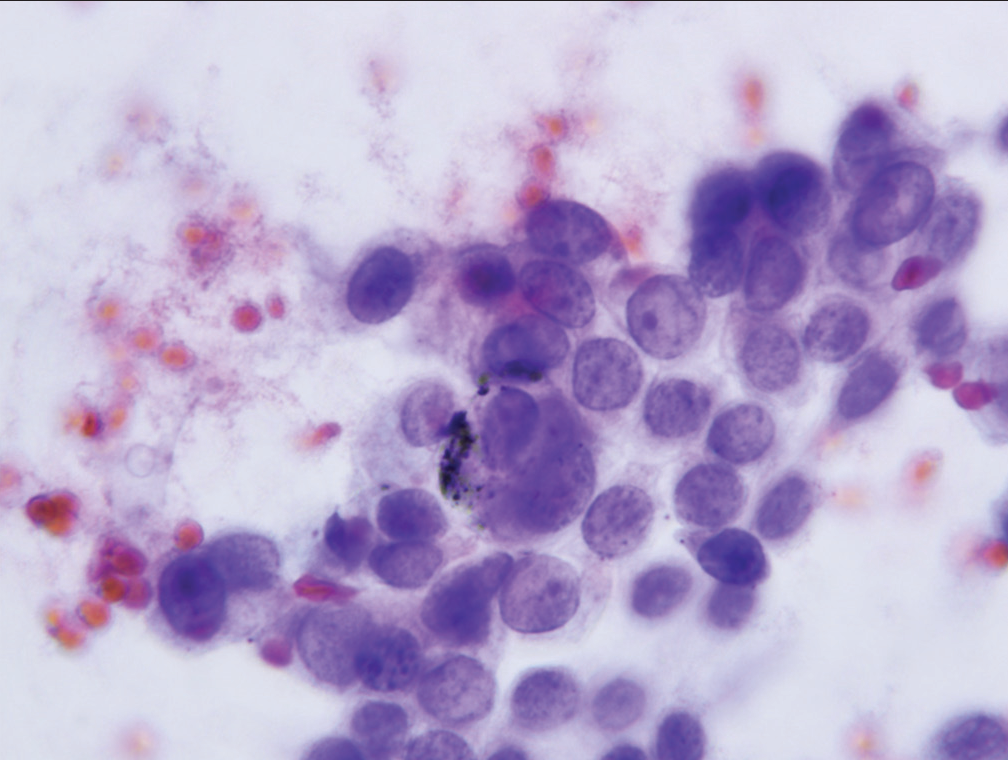
- Cell group characterized as “suspicious for malignancy” showing nucleoli, increased nuclear to cytoplasmic ratio and anisonucleosis (Papanicolaou).
COMPARISON OF MORPHOLOGIC FEATURES BETWEEN CELL POPULATIONS DESIGNATED “ATYPICAL” AND THOSE DESIGNATED “SUSPICIOUS FOR MALIGNANCY”
While the differences in morphologic features between specimens designated “atypical” and those designated “suspicious for malignancy” are qualitative in nature, assessment of the entire cytomorphologic picture is more important that differences in any single criteria. Nonetheless, specimens assigned to the “atypical” category will demonstrate lesser degrees of cytomorphologic abnormality than those assigned to the “suspicious for malignancy” category. Figures 8-12 demonstrate differences in degree of cytomorphologic abnormality between specimens designated “atypical” and those designated “suspicious for malignancy” for each of the major cytomorphologic criteria.
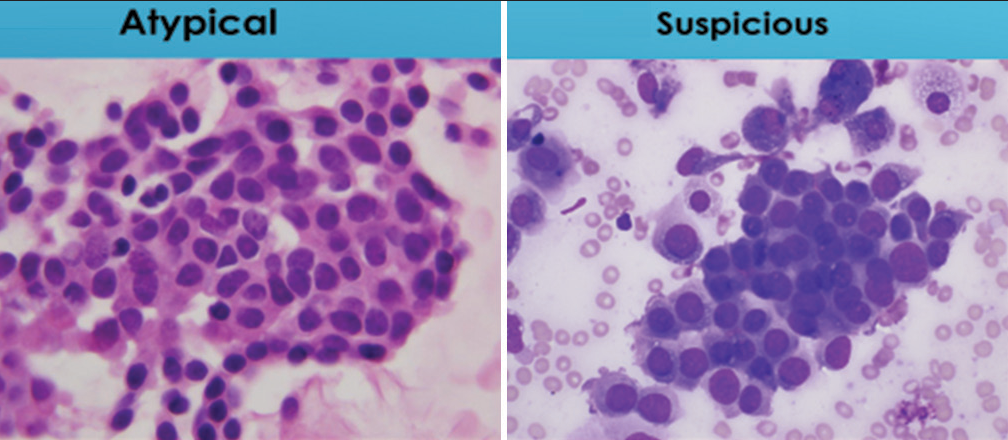
- Comparison of degree of nuclear crowding between cell sheets designated “atypical” and “suspicious for malignancy.” The “atypical” sheet of cells shows a minor degree of cell crowding while the one designated “suspicious for malignancy” shows significant nuclear crowding and nuclear overlap (Papanicolaou and Romansky).
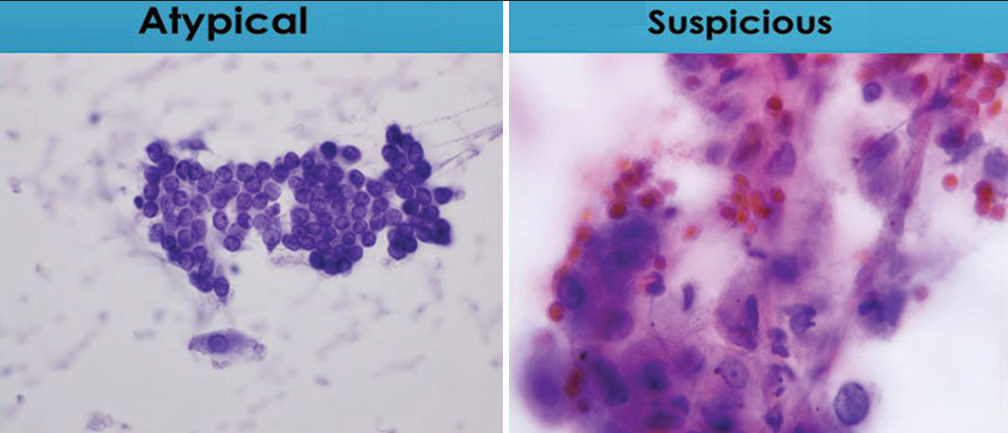
- Comparison of degrees of nuclear membrane irregularities. The sheet of cells characterized as “atypical” lack nuclear membrane irregularities, while the group of cells characterized as “suspicious for malignancy” containing a cell with significant irregularities of nuclear membranes (Papanicolaou).
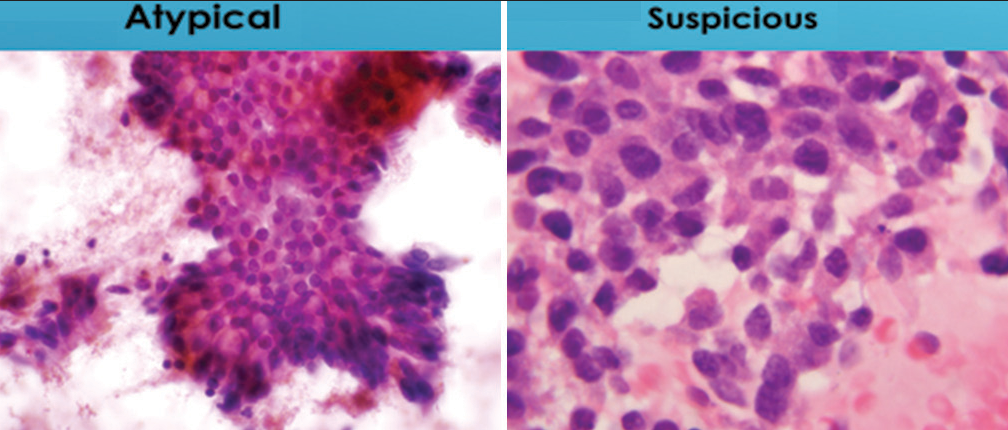
- Illustration shows differences in degree of nuclear polarity for specimens designated “atypical” and “suspicious for malignancy.” The sheet of cells characterize as “atypical” largely maintains nuclear polarity while the cell group designated as “suspicious for malignancy” shows loss of nuclear polarity (Papanicolaou).
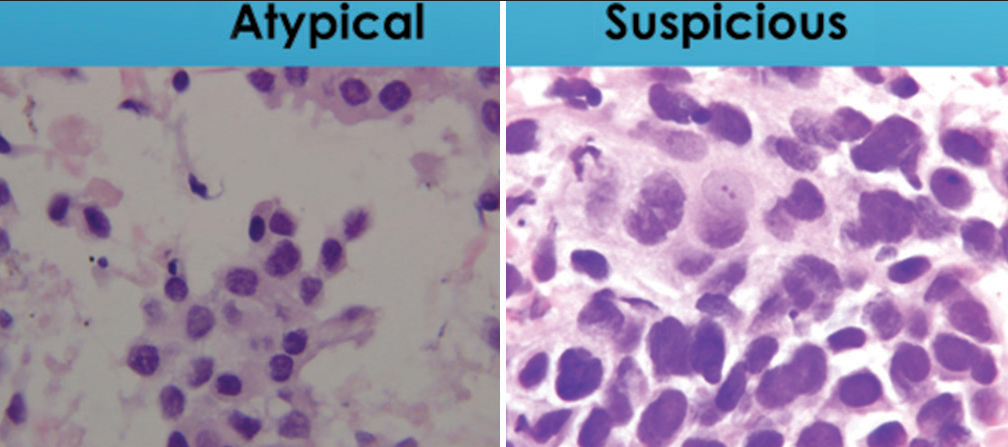
- Comparison of nuclear to cytoplasmic ratio in groups of cells designated as “atypical” or “suspicious for malignancy.” The “atypical” group maintains a low nuclear to cytoplasmic ratio (below 0.6) while the “suspicious for malignancy” group has a nuclear to cytoplasmic ratio (above 0.7) (Papanicolaou).
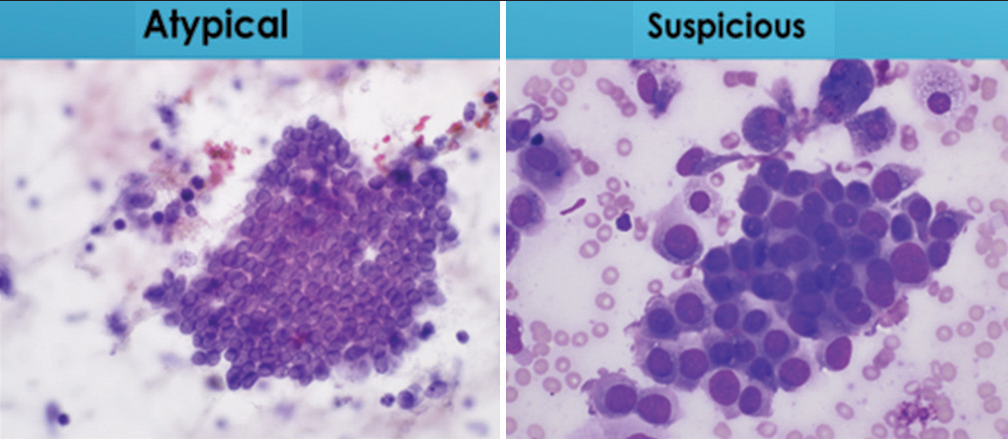
- Comparison of variation in nuclear size in cells groups designated as “atypical” or “suspicious for malignancy.” Cells designated “atypical” show little or no variability in nuclear size or shape while those characterized as “suspicious for malignancy” demonstrate at least a three-fold variation in nuclear size (Papanicolaou).
It is important to remember that the morphologic differences seen in cytology specimens of the lower respiratory tract form a spectrum of change running from clearly benign to definitively malignant. The categories “atypical” and “suspicious for malignancy” are somewhat artificial constructs for dividing these changes into useful clinical categories. The observed differences in malignancy risk between categories are significant and consistent between literature reports.[19-22] Moreover, a recent morphometric study has shown differences in nuclear area and nuclear/cytoplasmic ratio for the benign, malignant, and intermediate categories.[23] These findings support the use of one, if not two, intermediate categories. Distributions for nuclear/cytoplasmic ratio and distribution of nuclear size designated as “benign,” “malignant,” and “atypical” support the use of intermediate categories.
SUMMARY
Cytologic specimens obtained from the respiratory tract by sputum cytology, bronchial brushings, bronchial washings, and fine-needle aspiration specimens are associated with a spectrum of cytomorphologic changes running from clearly benign to clearly malignant. Clinicians treating patients desire definitively benign or definitively malignant diagnoses which are not always achievable by cytologic evaluation. Intermediate categories with definitions, criteria, and estimates for risk of malignancy are helpful in dividing this spectrum into useful clinical categories. While criteria for separation of these categories are more qualitative than quantitative, evaluation of malignancy risks between the categories suggest that they have a biological, as well as a statistical basis. The primary function of the intermediate categories “atypical” and “suspicious for malignancy” is to preserve the high diagnostic accuracy of the “benign” and “malignant” categories. This appears to be largely achieved for fine needle aspiration specimens, in that the malignancy risk of a fine-needle aspiration specimen assigned to the “benign” category is approximately 24%, while the malignancy risk of a fine needle aspiration-derived specimen assigned to the “malignant” category is >90%. Use of the “atypical” categories results in a stepwise increase in malignancy risk. This degree of stratification of malignancy risk between the negative/ benign category and the malignant category supports the value and use of the intermediate categories “atypical” and “suspicious for malignancy.”
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The author has no conflicts or interest relating to this study.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Lester J. Layfield, MD, performed the literature review, wrote the text, and edited the text. He produced all photographs included in the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study meets the requirements of the Helsinki Accord and was approved by the Human Subject Review Committee of the University of Missouri.
LIST OF ABBREVIATIONS (IN ALPHABETIC ORDER)
PSCSRRC - Papanicolaou Society of Cytopathology System for Reporting Respiratory Cytology
WHO RSLC - World Health Organization Reporting System for Lung Cytopathology
EDITORIAL/PEERREVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
References
- The world health organization reporting system for lung cytopathology. Acta Cytol. 2022;67:80-91.
- [Google Scholar]
- The Papanicolaou Society of Cytopathology classification for pulmonary specimens: An overview. Cytopathology. 2016;27:149-52.
- [Google Scholar]
- The Papanicolaou Society of Cytopathology guidelines for respiratory cytology: Reproducibility of categories among observers. Cytojournal. 2018;15:22.
- [Google Scholar]
- A study on neoplastic cells in sputum as a contribution to the diagnosis of primary lung cancer. Acta Chir Scand. 1944;91(Suppl 93):1-143.
- [Google Scholar]
- The identification of types of pulmonary cancer in cytologic smears. Am J Pathol. 1952;28:963-83.
- [Google Scholar]
- Cytologic diagnosis in suspected pulmonary cancer; critical analysis of smears from 1000 persons. Am J Clin Pathol. 1955;25:223-40.
- [Google Scholar]
- The examination of the sputum and pleural fluid in the diagnosis of malignant disease of the lung. Thorax. 1946;1:118-27.
- [Google Scholar]
- Ten years of respiratory cytopathology at Duke University Medical Center. I. The cytopathologic diagnosis of lung cancer during the years 1970 to 1974, noting significance of specimen number and type. Acta Cytol. 1981;25:103-7.
- [Google Scholar]
- Ten years of respiratory cytopathology at Duke University Medical Center. II. The cytopathologic diagnosis of lung cancer during the years 1970 to 1974, with a comparison between cytopathology and histopathology in the typing of lung cancer. Acta Cytol. 1981;25:499-505.
- [Google Scholar]
- Ten years of respiratory cytopathology at Duke University Medical Center. III. The significance of inconclusive cytopathologic diagnoses during the years 1970-1974. Acta Cytol. 1985;26:759-66.
- [Google Scholar]
- Cytologic study of tissue repair in human bronchial epithelium. Acta Cytol. 1988;32:622-8.
- [Google Scholar]
- Bronchial wash cytology: A study on morphology and morphometry. J Cytol. 2014;32:63-7.
- [Google Scholar]
- Pulmonary cytology-a brief summary of diagnostic results from July 1st 1952, until December 31st 1960. Acta Cytol. 1964;8:104-13.
- [Google Scholar]
- Clinical significance of an inconclusive cytopathologic diagnosis I: Positive predictive value. Acta Cytol. 1994;38:193-200.
- [Google Scholar]
- Guidelines of the Papanicolaou Society of Cytopathology for the examination of cytologic specimens obtained from the respiratory tract. Diagn Cytopathol. 1999;21:61-9.
- [Google Scholar]
- “the Papanicolaou Society of Cytopathology system for reporting respiratory cytology”-a retrospective analysis of 101 cases of CT-guided FNAC. Diagn Cytopathol. 2020;48:701-5.
- [Google Scholar]
- The new guidelines of the Papanicolaou Society of Cytopathology for respiratory specimens: Assessment of risk of malignancy and diagnostic yield in different cytological modalities. Diagn Cytopathol. 2018;46:725-9.
- [Google Scholar]
- A modified Papanicolaou Society of Cytopathology system for reporting respiratory cytology specimens: Implications for estimates for malignancy risk and diagnostic accuracy. Diagn Cytopathol. 2021;49:1167-72.
- [Google Scholar]
- Cytology reporting system for lung cancer from the Japan Lung Cancer Society and Japanese Society of Clinical Cytology: An interobserver reproducibility study and risk of malignancy evaluation on cytology specimens. Acta Cytol. 2020;64:452-62.
- [Google Scholar]
- Cytology reporting system for lung cancer from the Japan Lung Cancer Society and the Japanese Society of Clinical Cytology: An extensive study containing more benign lesions. Acta Cytol. 2022;66:124-33.
- [Google Scholar]
- Atypia in pulmonary cytology: A cytomorphometric analysis of the spectrum of changes between benign and malignant. Diagn Cytopathol. 2021;49:909-14.
- [Google Scholar]








