Translate this page into:
Hydatid disease limited to bilateral adrenal glands mimicking tuberculosis
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Hydatid disease is an endemic zoonotic infectious disease caused by Echinococcus species. Adrenal involvement by hydatid disease is rare and often seen unilaterally in disseminated infections. We report a case of hydatid disease limited to bilateral adrenal glands, which is diagnosed with fine-needle aspiration biopsy (FNAB) revealing characteristic microscopic findings. To the best of our knowledge, no cases of bilateral adrenal hydatid cysts have been reported before.
A 57-year-old male being followed for chronic renal failure was admitted to our hospital with fatigue, malaise, weight loss, and symptoms of infection such as fever and elevated C-reactive protein levels. He had a history of pulmonary tuberculosis. Abdominal computed tomography (CT) scan revealed pathologic para-aortic lymph nodes and bilateral adrenal masses with necrotic component. With a preliminary diagnosis of disseminated tuberculosis, FNAB from a para-aortic necrotic lymph node was performed. Biopsy revealed only necrotic material; nevertheless, tuberculosis could not be excluded. Tissue microbiologic culture of the sample obtained from the same lymph node was negative for Mycobacterium tuberculosis. Antituberculosis treatment was started empirically, but after 6 months of therapy, there was no sign of regression. A positron emission tomography-CT scan, performed to rule out lymphoma, showed bilateral adrenal masses having a cystic component with increased uptake of fluorodeoxyglucose (FDG) [Figure 1]. There were also para-aortic lymph nodes with increased FDG uptake, which were compatible with metastasis. The patient underwent an ultrasound-guided FNAB to assess the adrenal masses.
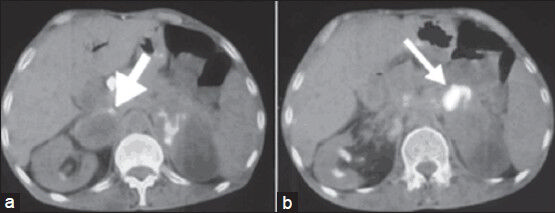
- Axial fused positron emission tomography/computed tomography images show cystic necrotic adrenal masses with the peripheral fluorodeoxyglucose uptake (arrows) on the right (a) and on the left (b)
The aspirate from the right adrenal mass was macroscopically clear. Both air-dried and alcohol-fixed smears were prepared for cytology. The air-dried smears were stained with Diff-Quik for on-site evaluation. The alcohol-fixed smears were stained by the Papanicolaou method. On site evaluation was reported as insufficient for diagnosis. Cell block was prepared by standard protocols and sections were stained with hematoxylin and eosin. On cytologic examination, the smear background was dirty; composed of debris [Figure 2]. Numerous fragments of hooklets and calcareous bodies were seen among a few inflammatory cells [Figures 3 and 4]. In the cell block sections, well-preserved laminated cyst wall fragments were present [Figure 5]. The findings were reported as hydatid cyst of the adrenal gland. Serologic analysis was also consistent with hydatid disease. Even though, FNAB was not performed, contralateral adrenal mass was also clinically regarded as the same lesion, because of the similar radiologic nature.
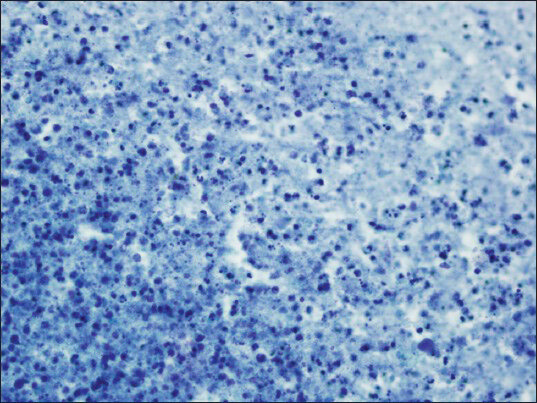
- Cyst fluid is seen as amorphous necrotic background (smear, Papanicolaou, ×400)
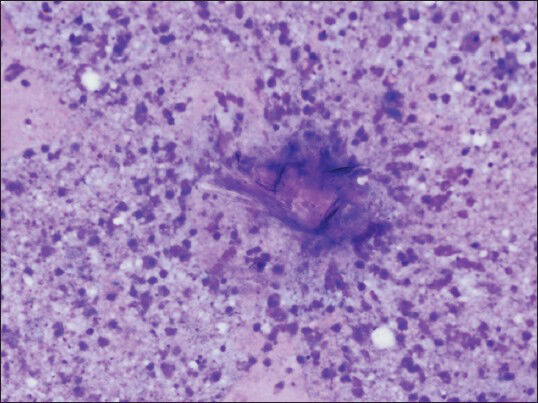
- Fragments of laminated cyst wall with necrotic debris (smear, May-Grünwald-Giemsa, ×400)
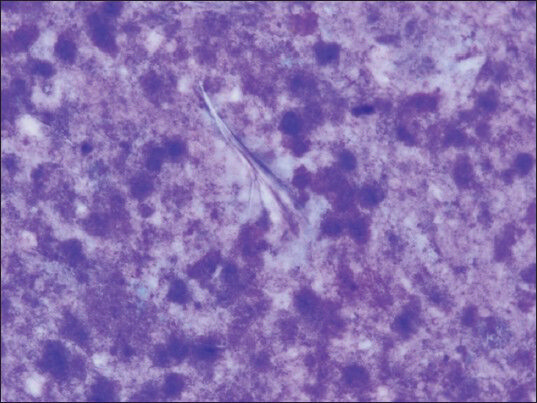
- Pale hooklets can be noticed by attentive search (smear, May-Grünwald-Giemsa, ×1000)
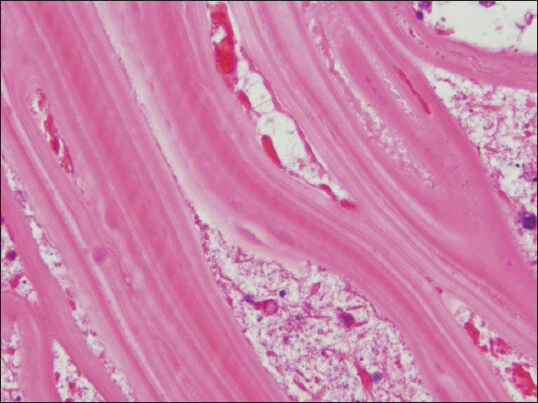
- The laminated cyst wall presents typical appearance in cell block as parallel, acellular striations (cell block, H and E, ×1000)
Cystic lesions of adrenal glands are rare. Cysts of adrenal glands are most commonly seen in the fifth and sixth decades, but they can be seen in any age group. They are usually (92%) unilateral and benign, showing no special predilection for either side. Hydatid disease accounts for 6-7% of all adrenal gland cysts.[1] Other types of the adrenal gland cysts are endothelial cysts (lymphangiomatous and angiomatous cysts) (45%); pseudocysts (39%) and epithelial cysts (true glandular retention cysts, embryonal cysts, and cystic adenomas) (9%).[1]
Clinical presentation of an adrenal cyst is variable. It is most commonly asymptomatic. There is a wide variety of symptoms associated with an adrenal cyst, although often it will cause vague, nonspecific symptoms. The most commonly seen clinical features are dull pain in the renal area, gastrointestinal symptoms including fullness, nausea, vomiting, constipation, anorexia, and a palpable mass. As adrenal cysts are usually asymptomatic, they are discovered as incidental findings on radiological or during surgery, which are done for different abdominal pathologies.[2]
Although FNAB is a relative contraindication for evaluation of hydatid cyst due to potential complication of anaphylactic reaction, hydatid cyst may be sampled unexpectedly with many reports of FNAB of hydatid cyst.[4] Hydatid disease should be taken into consideration in the differential diagnosis of cystic lesions especially in geographic regions where it is endemic. Hydatic disease, a parasitic infection caused by Echinococcus tapeworm, is an endemic condition in many countries. The most common cause is Echinococcus granulosus.[34] Echinococcus has a complex lifecycle requiring two mammalian hosts; dogs are definitive hosts and sheep, cattle, horses are intermediate hosts. Humans are secondarily infected by usage of food and water containing eggs of the parasite which are contaminated by dog feces.[5] Echinococcus cysts are mostly seen in the liver (50-70%) and lungs (20%). Adrenal gland involvement is seen in 0.5% of cases and is usually unilateral. Hydatid disease in organs other than liver or lungs are usually part of generalized echinococcosis and only rarely are primary cysts.[6]
In endemic regions, hydatid cysts are the most common cysts requiring operation. Hydatid cysts involving the adrenals are most of the time part of generalized echinococcosis. It is highly unusual that the echinococcal infection is limited to adrenal glands.[78] In a report dated 1930, Guttman pointed out that approximately 70% of Addison's disease cases were caused by tuberculosis.[9] Tuberculosis remains a common cause of infection of the adrenal gland in the developing countries.[10]
For diagnosis of hydatid cyst in the adrenal gland, ultrasonogram, CT scan, and magnetic resonance imaging can be employed, which demonstrate cystic structures as well as daughter cysts. The key diagnostic tool in cases of hydatid disease is ultrasonography, for which the diagnostic sensitivity for abdominal echinococcosis ranges from 93% to 98%.[311] Laboratory tests such as eosinophilia, Casoni skin test, and indirect hemagglutination are not diagnostic. Moreover, hydatid cysts in lungs, spleen, or kidneys tend to be associated with lower serum antibody levels as compared with the liver. Degenerating cysts are also associated with low serum antibody levels. Many sensitive and specific serological tests such as complement fixation, enzyme linked immunosorbent assay, and specific hydatid IgE tests have come into use.[112]
Microscopically, hydatid cyst has an inner nucleated, germinative layer and an outer nonnucleated, laminated layer. Outside the outer layer, there is an inflammatory reaction that causes an infiltration of fibroblasts, giant cells and mononuclear and eosinophilic cells limiting the affected area.[13] Although FNA may be useful for diagnosing adrenal cystic lesions, it is not recommended in case of hydatid cyst, since there is a risk of disseminated infection and allergic reactions.[5] The fluid aspirated from the hydatid cyst is mostly clear and it consists of debris, few inflammatory cells, and numerous scoleces. The aspirate in old cysts may contain large fragments of stratified layer of the cyst wall with a dirty background of debris including hooklets, which are diagnostic of the disease.[414]
The most effective treatment is surgical removal of the cyst, which often can be curative if the lesion is completely excised.[15] During surgery special attention should be paid to avoid spilling the contents, since dissemination and severe allergic reactions can occur.[5] When complete removal is not accomplished, surgical treatment is not curative. In such instances additional medical treatment with antihelmintic drugs is indicated.[516]
SUMMARY
Hydatid disease can occur in any region of the body.[6] Although, it is rare in adrenal glands, this possibility should be kept in mind, especially in endemic areas or in patients with a history of hydatid disease in other organs.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
All the authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This manuscript is a case description in form of a letter. Therefore, institutional ethical approval was not mandatory.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Hydatid cysts of the adrenal gland: Review of nine patients. World J Surg. 2004;28:97-9.
- [Google Scholar]
- Primary hydatid disease in the adrenal gland: A case report. Braz J Infect Dis. 2006;10:362-3.
- [Google Scholar]
- Hydatid cyst of the adrenal gland: A clinical study of six cases. ScientificWorldJournal. 2006;6:2420-5.
- [Google Scholar]
- Diagnosis of pulmonary hydatid disease presenting with solid nodule and mimicking malignancy by fine needle aspiration cytology. Cytojournal. 2012;9:13.
- [Google Scholar]
- Unusual location of hydatid cyst: Soft tissue mass in the neck. Eur Arch Otorhinolaryngol. 2006;263:1147-50.
- [Google Scholar]
- Uncommon adrenal masses: CT and MRI features with histopathologic correlation. Eur J Radiol. 2007;62:359-70.
- [Google Scholar]
- Addison's disease: A statistical analysis of 566 cases and study of the pathology. Arch Pathol. 1930;10:742-85.
- [Google Scholar]
- Addison's disease due to tuberculosis of the adrenal glands. J Pre-Clin Clin Res. 2012;6:88-92.
- [Google Scholar]
- Urinary retention: Unusual presentation of hydatid cyst. Case report and literature review. 2011. Int J Surg. 27:10. Available from: http://www.yester.ispub.com/journal/the-internet-journal-of-surgery/volume-27-number-2/urinary-retention-unusual-presentation-of-hydatid-cyst- case-report-and-literature-review.html#sthash 5G921 vf4.dpbs
- [Google Scholar]
- Hydatid disease of the urinary tract: An update. Chirurgia (Bucur). 2008;103:621-7.
- [Google Scholar]
- Liver - Non-neoplastic diseases. In: Rosai J, ed. Rosai and Ackerman's Surgical Pathology Vol 2. (10th ed). Amsterdam: Elsevier; 2011. p. :915.
- [Google Scholar]
- Liver and pancreas. In: Bibbo M, Wilbur D, eds. Comprehensive Cytopathology (3rd ed). Amsterdam: Elsevier; 2008. p. :876.
- [Google Scholar]
- Echinococcal cyst of the subcutaneous tissue: A rare case report. Parasitol Int. 2008;57:236-8.
- [Google Scholar]
- Primary hydatid cyst in adrenal gland: A case report. Int Urol Nephrol. 2005;37:21-3.
- [Google Scholar]







