Translate this page into:
Immunocytochemistry of effusion fluids: Introduction to SCIP approach

*Corresponding author: Vinod B. Shidham, MD, FIAC, FRCPath, Department of Pathology, Wayne State University School of Medicine, Karmanos Cancer Center and Detroit Medical Center, Detroit, Michigan, United States. vshidham@med.wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Shidham VB, Atkinson BF. Immunocytochemistry of effusion fluids: Introduction to SCIP approach. CytoJournal 2022;19:3.
Abstract
Due to the remarkably wide morphologic spectrum of reactive mesothelial cells, some of the effusion fluids may be difficult to interpret with objective certainty by cytomorphology alone. Cytomorphology of well to moderately differentiated adenocarcinomas (responsible for the bulk of malignant effusions) may overlap with floridly reactive mesothelial cells. Even mesotheliomas including diffuse malignant epithelioid mesothelioma, are usually cytomorphologically bland without unequivocal features of malignancy.
The intensity of challenge depends on the interpreter’s training or experience level, institutional demographics of patients (such as type of prevalent diseases, predominant sex and age group), technical support, and quality of cytopreparatory processing. In general immunocytochemistry is valuable adjunct to facilitate objective interpretation with or without other ancillary techniques as indicated. An increasing number of immunomarkers is further refining the contribution of immunohistochemistry to this field.
However, application of immunohistochemistry to effusion fluids is relatively challenging because of many variables. Multiple factors such as delay after specimen collection, specimen processing related factors including fixation and storage; ambient conditions under which paraffin blocks are archived (for retrospective testing); antigen retrieval method; duration of antigen retrieval step; antibody clone and dilution; and antibody application time are identical to application of immunohistochemistry in other areas. The significant challenge related to the potential compromization of the immunoreactivity pattern due to exposure to non-formalin fixatives / reagents is also applicable to effusion fluid specimens.
The immunoreactivity results would be compared and corelated with cumulative metadata based on the reported studies performed and validated on formalin-fixed paraffin-embedded tissue sections. Deviating from such protocols may lead to suboptimal results, which is not uncommon in clinical practice with potential compromization of patient care and related liability. Because of this, it is critical to perform immunocytochemistry on formalin-fixed cell-block sections only.
In addition, unless the interpretation criteria for immunohistochemical evaluation of effusion fluids are not modified specifically, it may not be productive in resolving some challenging cases. However, this aspect is not well elaborated in the literature. A basic and critical challenge is finding and locating the cells of interest in cell-block sections of effusion fluids. A unique approach is to choose a fundamental immunopanel which highlight the mesothelial and inflammatory cells in reactive effusion fluids to create the basic map. This allows detection of a ‘second-foreign’ population which can be immunocharacterized further with the help of subtractive coordinate immunoreactivity pattern (SCIP) approach elaborated here.
Keywords
Immunohistochemistry
Immunocytochemistry
SCIP
cell-block
formalin fixation
effusions
cytology

Effusion cytology is one of the most challenging areas in diagnostic cytopathology. Reactive mesothelial cells exhibit a remarkably wide morphologic spectrum, which overlaps with various reactive and malignant processes.[1–4] • Similarly, most of the examples of diffuse malignant epithelioid mesothelioma (DMEM) may not exhibit unequivocal malignant features and may instead resemble reactive mesothelial cells at one end of the spectrum and well to moderately differentiated adenocarcinomas (responsible for the bulk of malignant effusions) at the other end.[1–4] Due to these limitations, some of the effusion fluids are difficult to interpret with objective certainty by cytomorphology alone. The proportion of cases in this category may vary from institution to institution depending on the patient demographics (such as types of prevalent diseases, predominant sex and age group etc.), quality of technical support for cytopreparatory processing, and level of training or experience of the interpreter. Immunocytochemistry is an extremely valuable adjunct for objective interpretation. It may be used along with other ancillary techniques as indicated. Ongoing refinement in immunostaining technology with an ever-increasing number of immunomarkers is pushing it further to the forefront.
The most important issue to be considered when applying immunocytochemistry to effusion fluids is the significant variation in results due to the the number of variables from the time of collection of the specimen to its final immunostaining. Variables which may affect the final results include specimen processing, fixation, and storage; circumstances in which paraffin blocks are archived, such as duration and ambient conditions (for elective testing at later date); antigen retrieval method; duration of antigen retrieval step; antibody clone and dilution; antibody application time; and, above all, the interpretation criteria. Although interpretation criteria are taken for granted, they are difficult to reproduce in relation to many immunomarkers. These challenges related to the interpretation of effusion fluid immunocytochemistry is usually not discussed clearly in the literature.
UNIQUENESS OF EFFUSION IMMUNOCYTOCHEMISTRY
Intricacies in finding and locating the cells of interest in cell-block sections of effusion fluids is a very important limiting factor, with the potential to adversely affect the final results. If it is not approached with special consideration, it may lead to improper immunocytochemical interpretation and, eventually, a suboptimal final result. Although it is not unique for effusion fluid cytology, it is quite common in this scenario to face the challenge associated with evaluation of coordinate immunoreactivity because of the higher frequency of small cell groups and solitary cells in effusion specimens. While processing and interpreting effusion fluid immunocytochemistry with objectivity, the following attributes should be considered:
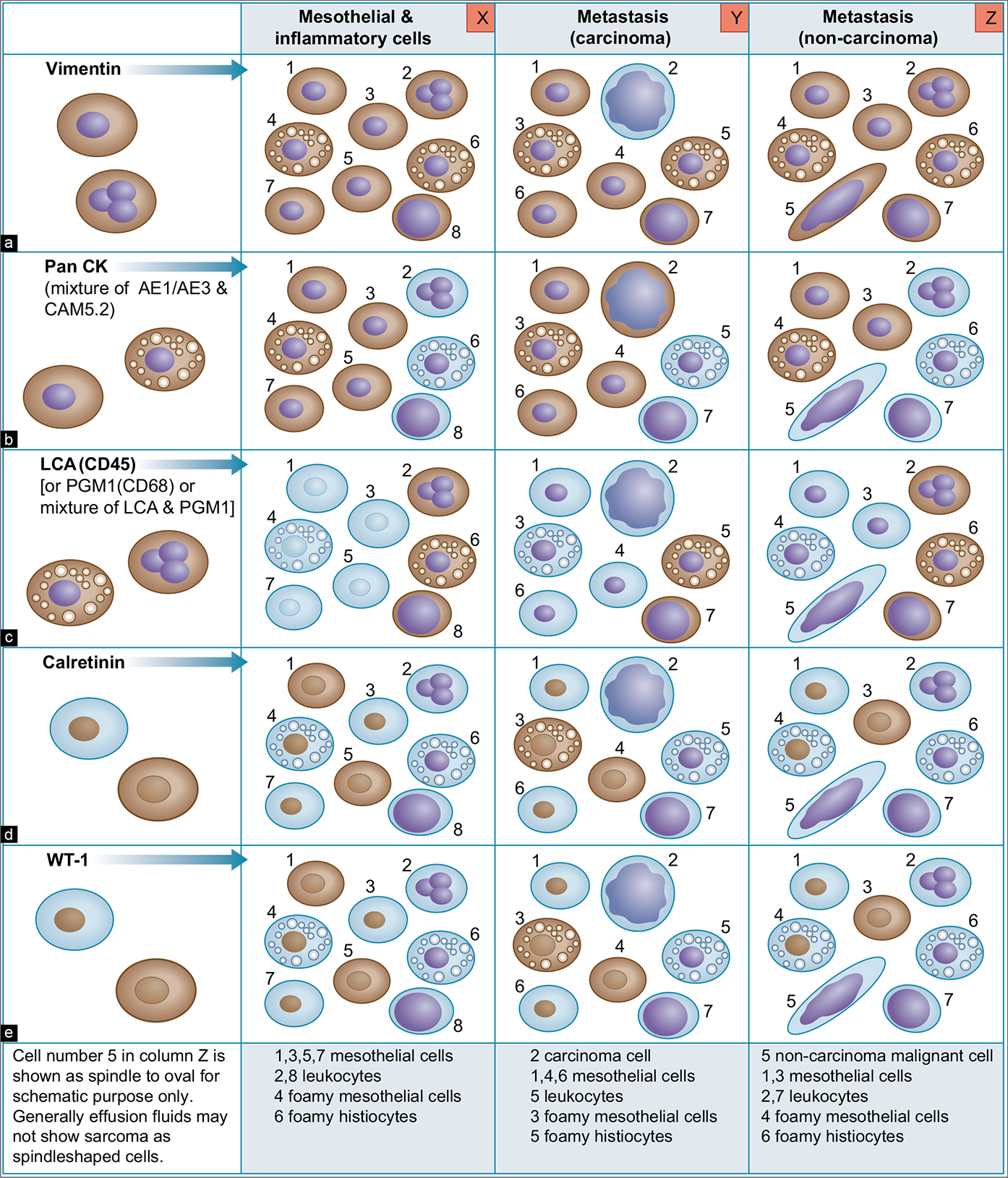
Application of SCIP (Subtractive co-ordinate Immunoreactivity Pattern) approach: The pattern of cancer cells in effusions varies from solitary scattered cells to small, cohesive groups. It may be more difficult to find the same cells or groups of cells in adjacent serial sections on different slides. Most of the individually scattered, abnormal cells, however, will be present in at least a few 4 μm thick serial sections [see Figures 1, 2, 3,4, 6,9 ]. It is important to know the sequence of these serial sections and to orient them identically on the slides to identify the same cells (or small group of cells) more precisely on the adjacent serial sections for proper evaluation of coordinate immunoreactivity pattern [Figure 1]. To achieve this, we routinely orient all serial sections identically on slides and label them sequentially [Figures 2, 3, 4]. This simple approach can help in some unexpected situations where the immunostaining pattern is not straightforward. If this SCIP approach is practiced routinely, it expedites and simplifies the immunocytochemical evaluation of effusion fluids in all cases.
The most effective approach for diagnosis of metastatic effusions is the confirmation of a ‘second-foreign’ noninflammatory population of cells other than mesothelial cells. But this does not help to distinguish between reactive and neoplastic mesothelial cells. The interpretation of epithelioid mesothelioma should be based on other features after demonstrating the abnormal cells to be mesothelial in nature. • For reproducible results, it is important to select an immunopanel which will fundamentally highlight all of the mesothelial and inflammatory cells to create the basic map for confirmation of a ‘second-foreign’ population by the subtractive coordinate immunoreactivity pattern (SCIP) approach [see Figure 1].
The challenge of distinguishing cells of epithelioid mesothelioma from reactive mesothelial cells has to be approached differently and must be based on the quantity (numerous vs a few) and the quality (numerous large groups vs a few small groups) of abnormal cells with proper clinical and radiologic correlation. Once it is determined that the cytologic features favor mesothelioma rather than reactive mesothelial cells, it is relatively simple, with the aid of immunocytochemistry to exclude adenocarcinoma and more recently with molecular pathology testing [Figures 5, 19].[5]
-
The final interpretation of any immunoprofile is the result of comparative evaluation of the database accumulated from the information in reported studies that were performed predominantly on formalin-fixed paraffin-embedded tissue sections. The immunoreactivity pattern for a variety of immunomarkers may not remain the same when other fixation and processing protocols are used.[6] • Consequently, it is prudent to perform immunocytochemistry on formalin-fixed cell-block sections only and to avoid other protocols such as the evaluation of inappropriately processed cell-block with exposure to alcohol and alcoholic fixatives (e.g. CytoLyt) prior to formalin-fixation[7,8] or various cytology smears (direct smears: wet fixed in alcohol or acetone; air-dried fixed with alcohol; air-dried smears rehydrated and postfixed in formol alcohol; liquid-based cytology smears: SurePath or ThinPrep, Cytospin smears, etc.).[9,10]
Thus, for reproducible results, a standardized protocol with steps comparable to the processing of formalin-fixed paraffin-embedded tissue sections is essential.[11-14] Deviating from such a practice may lead to suboptimal results with loss of reproducibility, which is frequently observed in clinical practice and related publications.
For the objective confirmation of an immunoprofile highlighting different types of cells in the effusion, it is important to evaluate the coordinate immunoreactivity pattern of in the same cells [see Figure 1]. • This is not possible with cytology smears since the same cells cannot be followed on different smears. In contrast, serial sections of cell-block allow evaluation of immunoreactivity by different immunomarkers in same cells in adjacent serial sections (coordinate immunoexpression).[15,16]
The proteinaceous effusion fluid around suspended cells may contribute to unexpected non-specific immunoreactivity. The non-specific staining of adjacent inflammatory cells, especially when it is substantial, may hinder the evaluation of membranous immunostaining patterns.
Discrepant results between formalin-fixed paraffin-embedded tissue sections of surgical pathology material and effusion fluid cell-block sections are not uncommon. The variables responsible for such discrepancies include sample size (tiny cell groups or single cells), selection of fixatives, antigen retrieval methods (i.e. heat-induced epitope retrieval, enzyme digestion, etc.), antibody clones used, antibody titer, and other variations in immunostaining protocols. Additional causes for variable results reported by different studies include variation in laboratory sensitivities and study size.[17,18] Furthermore, both the qualitative (pattern of immunostaining: membranous, cytoplasmic, nuclear, etc.) [Table 1] and quantitative criteria for interpretation of immunoreactivity may vary between pathologists and different institutions.[19]
- Basic immunopanel for evaluation by subtractive coordinate immunoreactivity pattern (SCIP) approach.
-
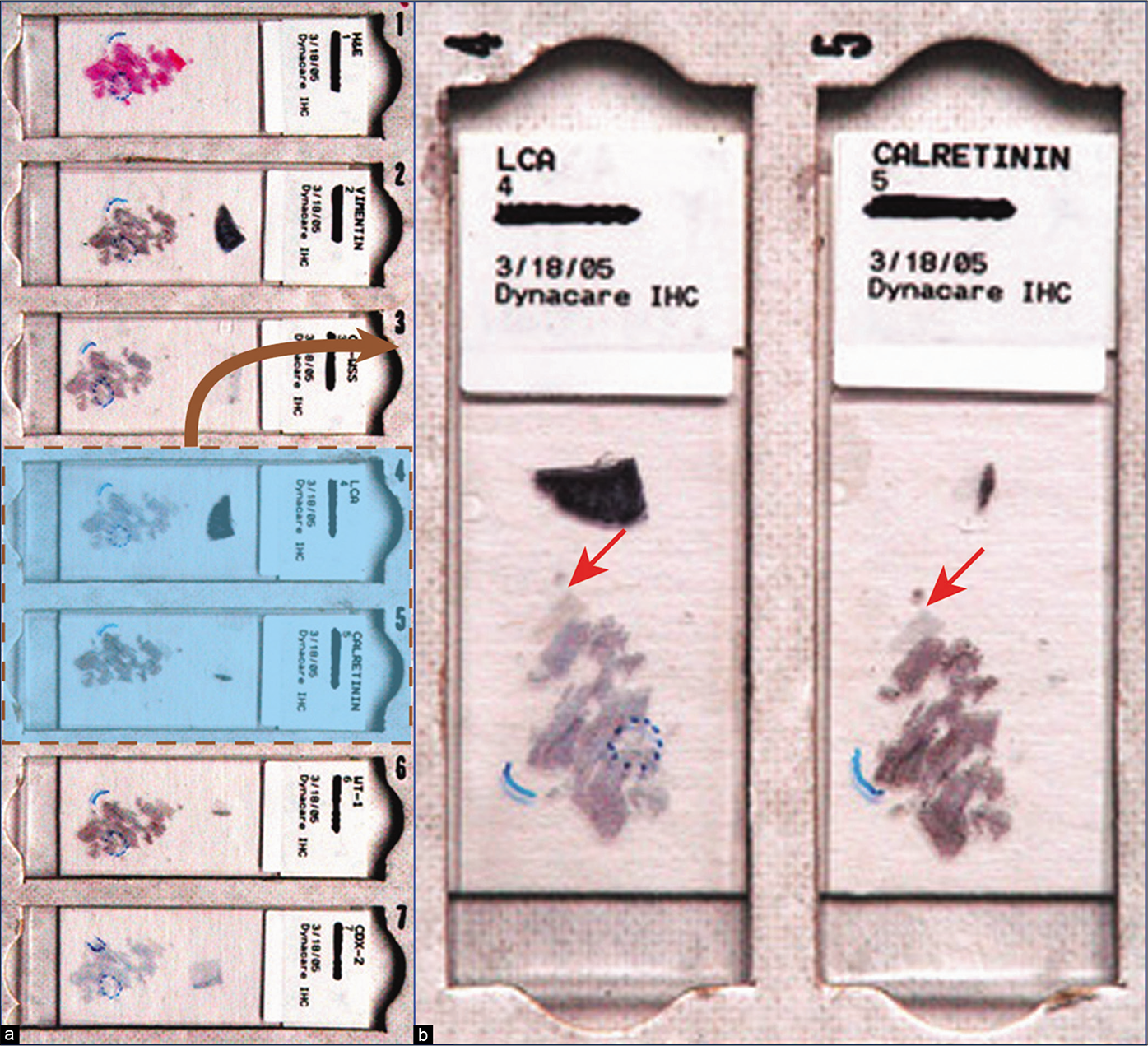
Metastatic colonic adenocarcinoma, peritoneal fluid. a. Serial sections of cell-blocks oriented identically on all slides in a sequential order. The respective numbers identify their relative position with each other: 1, HE; 2, vimentin; 3, cytokeratin; 4, LCA (CD45); 5, calretinin; 6, WT-1; 7, CD-X2. b. Enlarged view showing slide numbers 4 and 5, emphasizing identical orientation (arrows) of cell-block sections on all glass slides. Identical orientations of all serial sections facilitate immunocytochemical evaluation with the SCIP approach. If not properly organized in this fashion, it may be impossible in effusion specimens with a predominance of small cell groups and solitary cells to evaluate the coordinate immunoreactivity pattern in relation to various immunomarkers.
-
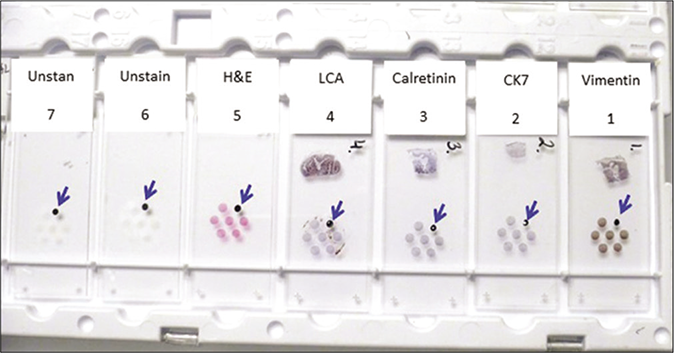
Cell-blocking (with NGCBTM kit) and AV marker: subtractive coordinate immunoreactivity pattern (SCIP) approach. Identical orientation of all sections on glass slides with proper labeling of their exact sequence in relation to each other.
- From Open Access publication: CytoJournal 2021;18:2. https://dx.doi.org/10.25259/Cytojournal_83_2020
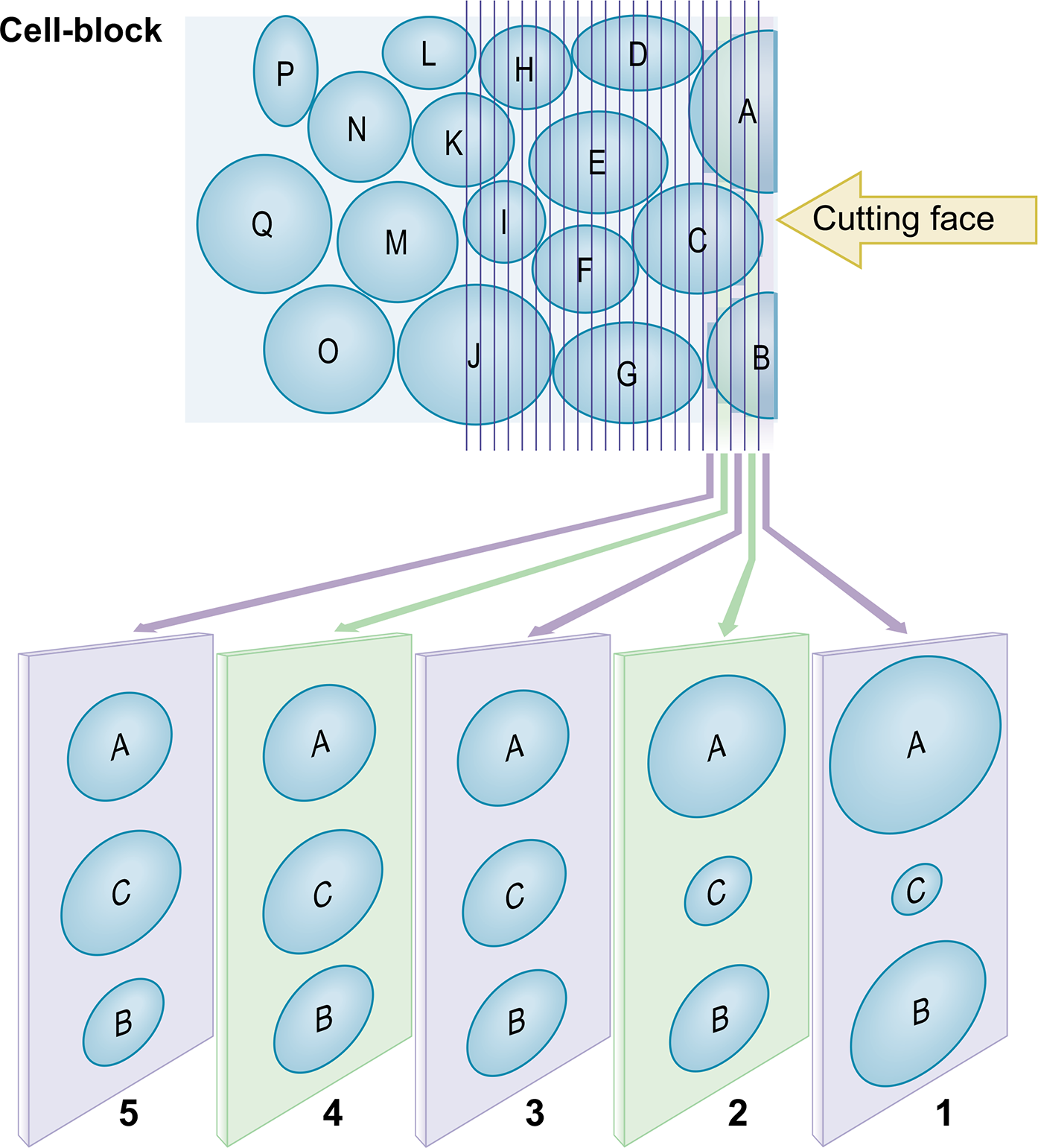
- Serial numbering and orientation of cell-block sections.

- Algorithm for immunocytochemical evaluation of effusions (in conjunction with Figure 1).
![Metastatic colonic adenocarcinoma, peritoneal fluid. The neoplastic cells (red arrow NC) are immunoreactive for pan-cytokeratin (B) and non-immunoreactive for vimentin (A), CD45 (C), calretinin (D), and WT-1 (E). They have nuclear immunoreactivity for CDX2 (F), which is consistent with a colonic primary. The reactive mesothelial cells [with immunoreactivity for calretinin (nuclear-cytoplasmic) (D), WT-1 (nuclear-cytoplasmic) (E), vimentin (A)] and inflammatory cells [with immunoreactivity for vimentin (A) and LCA (C) ] in the background can be subtracted from neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. LCA, leukocyte common antigen; NC, neoplastic cell; RM, reactive mesothelial cell; WT-1, Wilms’ tumor1. [A–F, Immunostained cell-block sections.]](/content/105/2022/19/1/img/Cytojournal-19-3-g007.png)
-
Metastatic colonic adenocarcinoma, peritoneal fluid. The neoplastic cells (red arrow NC) are immunoreactive for pan-cytokeratin (B) and non-immunoreactive for vimentin (A), CD45 (C), calretinin (D), and WT-1 (E). They have nuclear immunoreactivity for CDX2 (F), which is consistent with a colonic primary. The reactive mesothelial cells [with immunoreactivity for calretinin (nuclear-cytoplasmic) (D), WT-1 (nuclear-cytoplasmic) (E), vimentin (A)] and inflammatory cells [with immunoreactivity for vimentin (A) and LCA (C) ] in the background can be subtracted from neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. LCA, leukocyte common antigen; NC, neoplastic cell; RM, reactive mesothelial cell; WT-1, Wilms’ tumor1. [A–F, Immunostained cell-block sections.]
![Metastatic ovarian carcinoma, peritoneal fluid. The neoplastic cells (red arrow NC) are immunoreactive for pan-cytokeratin (B), BerEP4 (D), and non-immunoreactive for vimentin (A) and calretinin (C). The reactive mesothelial cells [with immunoreactivity for calretinin (nuclearcytoplasmic) (C), vimentin (A), and cytokeratin 7 (E)] and inflammatory cells [with immunoreactivity for vimentin (A)] in the background can be subtracted from neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. With a history of ovarian carcinoma, coordinate immunoreactivity for cytokeratin 7 (E) and non-immunoreactivity for cytokeratin 20 (F), it is consistent with metastatic ovarian carcinoma. In smears, the morphology of the carcinoma cells overlapped that of reactive mesothelial cells. NC, neoplastic cell; RM, reactive mesothelial cell. [A–F, Immunostained cell-block sections.]](/content/105/2022/19/1/img/Cytojournal-19-3-g008.png)
-
Metastatic ovarian carcinoma, peritoneal fluid. The neoplastic cells (red arrow NC) are immunoreactive for pan-cytokeratin (B), BerEP4 (D), and non-immunoreactive for vimentin (A) and calretinin (C). The reactive mesothelial cells [with immunoreactivity for calretinin (nuclearcytoplasmic) (C), vimentin (A), and cytokeratin 7 (E)] and inflammatory cells [with immunoreactivity for vimentin (A)] in the background can be subtracted from neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. With a history of ovarian carcinoma, coordinate immunoreactivity for cytokeratin 7 (E) and non-immunoreactivity for cytokeratin 20 (F), it is consistent with metastatic ovarian carcinoma. In smears, the morphology of the carcinoma cells overlapped that of reactive mesothelial cells. NC, neoplastic cell; RM, reactive mesothelial cell. [A–F, Immunostained cell-block sections.]
![Metastatic lung carcinoma, pleural fluid. The specimen shows scattered solitary neoplastic cells (red arrows NC) with rare mesothelial cells (blue arrow RM). The neoplastic cells are immunoreactive for pan-cytokeratin (B) and non-immunoreactive for vimentin (A), LCA (C), and calretinin (D). Rare reactive mesothelial cells (blue arrow RM) [with immunoreactivity for calretinin (nuclear-cytoplasmic) (D)] and inflammatory cells (arrowhead) [with immunoreactivity for LCA (C)] in the background can be subtracted from the neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. This is consistent with poorly cohesive singly scattered carcinoma cells, which were also non-immunoreactive for the melanoma marker. Nuclear immunoreactivity for TTF-1 (E) with a clinical history of lung mass is consistent with a lung primary. NC, neoplastic cell; RM, reactive mesothelial cell; TTF-1, thyroid transcription factor-1. [A–E, Immunostained cell-block sections.]](/content/105/2022/19/1/img/Cytojournal-19-3-g009.png)
-
Metastatic lung carcinoma, pleural fluid. The specimen shows scattered solitary neoplastic cells (red arrows NC) with rare mesothelial cells (blue arrow RM). The neoplastic cells are immunoreactive for pan-cytokeratin (B) and non-immunoreactive for vimentin (A), LCA (C), and calretinin (D). Rare reactive mesothelial cells (blue arrow RM) [with immunoreactivity for calretinin (nuclear-cytoplasmic) (D)] and inflammatory cells (arrowhead) [with immunoreactivity for LCA (C)] in the background can be subtracted from the neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. This is consistent with poorly cohesive singly scattered carcinoma cells, which were also non-immunoreactive for the melanoma marker. Nuclear immunoreactivity for TTF-1 (E) with a clinical history of lung mass is consistent with a lung primary. NC, neoplastic cell; RM, reactive mesothelial cell; TTF-1, thyroid transcription factor-1. [A–E, Immunostained cell-block sections.]
![Metastatic mammary adenocarcinoma, pleural effusion. Specimen with proliferation spheres. The neoplastic cells (red arrows NC) are immunoreactive for cytokeratin 7 (D) and BerEP4 (E). They are non-immunoreactive for vimentin (A), CD68 (B), and calretinin (C). Rare mesothelial cells (blue arrow RM) [with immunoreactivity for vimentin (A), calretinin (nuclear-cytoplasmic) (C), and cytokeratin 7 (D)] and inflammatory cells (arrowheads) [with immunoreactivity for vimentin (A) and CD68 (C)] in the background can be subtracted from the neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. Cytokeratin 7 immunoreactivity with a clinical history of mammary carcinoma is consistent with a breast primary. NC, neoplastic cell; RM, reactive mesothelial cell. [A–E, Immunostained cell-block sections.]](/content/105/2022/19/1/img/Cytojournal-19-3-g010.png)
- Metastatic mammary adenocarcinoma, pleural effusion. Specimen with proliferation spheres. The neoplastic cells (red arrows NC) are immunoreactive for cytokeratin 7 (D) and BerEP4 (E). They are non-immunoreactive for vimentin (A), CD68 (B), and calretinin (C). Rare mesothelial cells (blue arrow RM) [with immunoreactivity for vimentin (A), calretinin (nuclear-cytoplasmic) (C), and cytokeratin 7 (D)] and inflammatory cells (arrowheads) [with immunoreactivity for vimentin (A) and CD68 (C)] in the background can be subtracted from the neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. Cytokeratin 7 immunoreactivity with a clinical history of mammary carcinoma is consistent with a breast primary. NC, neoplastic cell; RM, reactive mesothelial cell. [A–E, Immunostained cell-block sections.]
![Metastatic mammary adenocarcinoma, pleural effusion. Specimen with solitary neoplastic cells as the predominant population. The neoplastic cells (red arrows NC) are immunoreactive for BerEP4 (D) and estrogen receptors (E). They are non-immunoreactive for vimentin (A), CD68 (B), and calretinin (C). Rare mesothelial cells (blue arrow RM) [with immunoreactivity for vimentin (A) and calretinin (nuclear-cytoplasmic) (C)] and inflammatory cells (arrowheads) [with immunoreactivity for vimentin (A) and CD68 (B)] in the background can be subtracted from the neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. Immunoreactivity for ER with a clinical history of mammary carcinoma is consistent with a breast primary. ER, estrogen receptors; NC, neoplastic cell; RM, reactive mesothelial cell. [A–F, Immunostained cell-block sections.]](/content/105/2022/19/1/img/Cytojournal-19-3-g011.png)
- Metastatic mammary adenocarcinoma, pleural effusion. Specimen with solitary neoplastic cells as the predominant population. The neoplastic cells (red arrows NC) are immunoreactive for BerEP4 (D) and estrogen receptors (E). They are non-immunoreactive for vimentin (A), CD68 (B), and calretinin (C). Rare mesothelial cells (blue arrow RM) [with immunoreactivity for vimentin (A) and calretinin (nuclear-cytoplasmic) (C)] and inflammatory cells (arrowheads) [with immunoreactivity for vimentin (A) and CD68 (B)] in the background can be subtracted from the neoplastic cells to deduce a diagnostic coordinate immunoreactivity pattern. Immunoreactivity for ER with a clinical history of mammary carcinoma is consistent with a breast primary. ER, estrogen receptors; NC, neoplastic cell; RM, reactive mesothelial cell. [A–F, Immunostained cell-block sections.]
![Metastatic small cell carcinoma, pleural fluid. Specimen with proliferation spheres. The cytology preparations demonstrated morphologic features of small cell carcinoma (see Figure 5). The neoplastic cells (red arrows NC) are immunoreactive for cytokeratin 7 (A), TTF-1 (nuclear) (C), and various neuroendocrine markers (D,E,F). Rare mesothelial cells and inflammatory cells were present in the background. They could be subtracted from these cells by constructing a coordinate immunoreactivity pattern based on findings in different serial sections. The immunoreactivity for cytokeratin 7 (A) and TTF-1 (C) with nonimmunoreactivity for cytokeratin 20 (B) is consistent with a lung primary. NC, neoplastic cell; TTF1, thyroid transcription factor-1. [A–F, Immunostained cell-block sections.]](/content/105/2022/19/1/img/Cytojournal-19-3-g012.png)
- Metastatic small cell carcinoma, pleural fluid. Specimen with proliferation spheres. The cytology preparations demonstrated morphologic features of small cell carcinoma (see Figure 5). The neoplastic cells (red arrows NC) are immunoreactive for cytokeratin 7 (A), TTF-1 (nuclear) (C), and various neuroendocrine markers (D,E,F). Rare mesothelial cells and inflammatory cells were present in the background. They could be subtracted from these cells by constructing a coordinate immunoreactivity pattern based on findings in different serial sections. The immunoreactivity for cytokeratin 7 (A) and TTF-1 (C) with nonimmunoreactivity for cytokeratin 20 (B) is consistent with a lung primary. NC, neoplastic cell; TTF1, thyroid transcription factor-1. [A–F, Immunostained cell-block sections.]
![Large B-cell lymphoma, peritoneal fluid. PAP-stained Cytospin smears (a–c) showed non-cohesive solitary cells (red arrow NC in c) with many apoptotic bodies (arrowheads in c). These cells in PAP-stained smears resemble non-cohesive poorly differentiated non-small cell carcinoma with a solitary cell pattern (compare with Figure 8). They are present in the HE-stained cell-block section as small groups (d). By the SCIP approach, neoplastic cells could be separated out from cytokeratin 7 (A) and calretinin (B) immunoreactive mesothelial cells (blue arrow RM). After following them in serial sections, the neoplastic cells (red arrow NC) were immunoreactive for CD20 (C) and nonimmunoreactive for CD3 (E). They expressed Bcl2 (D). Flow cytometry demonstrated the monoclonal nature of the CD20 immunoreactive cells. The patient had lymphoma of the small bowel. This was a consult case and a DQ-stained smear, which is usually helpful to evaluate lymphoma cells, was not available. NC, neoplastic cell; RM, reactive mesothelial cell. [a–c, PAP-stained Cytospin smear; d, HE-stained cell-block section, A–E, immunostained cell-block sections.]](/content/105/2022/19/1/img/Cytojournal-19-3-g013.png)
- Large B-cell lymphoma, peritoneal fluid. PAP-stained Cytospin smears (a–c) showed non-cohesive solitary cells (red arrow NC in c) with many apoptotic bodies (arrowheads in c). These cells in PAP-stained smears resemble non-cohesive poorly differentiated non-small cell carcinoma with a solitary cell pattern (compare with Figure 8). They are present in the HE-stained cell-block section as small groups (d). By the SCIP approach, neoplastic cells could be separated out from cytokeratin 7 (A) and calretinin (B) immunoreactive mesothelial cells (blue arrow RM). After following them in serial sections, the neoplastic cells (red arrow NC) were immunoreactive for CD20 (C) and nonimmunoreactive for CD3 (E). They expressed Bcl2 (D). Flow cytometry demonstrated the monoclonal nature of the CD20 immunoreactive cells. The patient had lymphoma of the small bowel. This was a consult case and a DQ-stained smear, which is usually helpful to evaluate lymphoma cells, was not available. NC, neoplastic cell; RM, reactive mesothelial cell. [a–c, PAP-stained Cytospin smear; d, HE-stained cell-block section, A–E, immunostained cell-block sections.]
![Adenocarcinoma, peritoneal fluid. Neoplastic cells are immunoreactive for EMA (a) and HBME-1 (b) with a cytoplasmic immunostaining pattern (arrows) with focal blotchy immunostaining along the cell membrane. Compare Figure 16 with membranous immunostaining highlighting the microvilli in mesothelial cells. [a,b, Immunostained cell-block sections (a,b, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-3-g014.png)
- Adenocarcinoma, peritoneal fluid. Neoplastic cells are immunoreactive for EMA (a) and HBME-1 (b) with a cytoplasmic immunostaining pattern (arrows) with focal blotchy immunostaining along the cell membrane. Compare Figure 16 with membranous immunostaining highlighting the microvilli in mesothelial cells. [a,b, Immunostained cell-block sections (a,b, 100X zoomed).]
![SCIP with dual color immunostaining (metastatic mammary carcinoma, effusion fluid). Reactive mesothelial cells (blue arrowheads in a,b) are immunoreactive for both vimentin (brown) and cytokeratin 7 (red) (inset of a). The metastatic carcinoma cells (arrows), both isolated (c) and small cohesive groups (d), do not show immunostaining for vimentin (no brown color) but only immunostaining for cytokeratin 7 (red) (inset of c). The corresponding cells in adjacent serial section with dual color immunostaining were also negative for calretinin (brown) but immunoreactive for BerEP4 (red) (not shown here). As expected, the background inflammatory cells are highlighted with vimentin (brown) immunostaining only; they are negative for cytokeratin 7 (red) (a,b,c,d). [a–d, Dual color immunostained cell-block sections—vimentin (brown) followed by cytokeratin 7 (red), (a–d 100X).]](/content/105/2022/19/1/img/Cytojournal-19-3-g015.png)
- SCIP with dual color immunostaining (metastatic mammary carcinoma, effusion fluid). Reactive mesothelial cells (blue arrowheads in a,b) are immunoreactive for both vimentin (brown) and cytokeratin 7 (red) (inset of a). The metastatic carcinoma cells (arrows), both isolated (c) and small cohesive groups (d), do not show immunostaining for vimentin (no brown color) but only immunostaining for cytokeratin 7 (red) (inset of c). The corresponding cells in adjacent serial section with dual color immunostaining were also negative for calretinin (brown) but immunoreactive for BerEP4 (red) (not shown here). As expected, the background inflammatory cells are highlighted with vimentin (brown) immunostaining only; they are negative for cytokeratin 7 (red) (a,b,c,d). [a–d, Dual color immunostained cell-block sections—vimentin (brown) followed by cytokeratin 7 (red), (a–d 100X).]
![Metastatic mammary adenocarcinoma, pleural fluid. Metastatic carcinoma cells (arrows in a–c) are immunoreactive only for cytokeratin 7 (red) without vimentin (brown) immunostaining (b). For additional confirmation, these cells are weakly immunoreactive for BerEP4 (red) and negative for calretinin (brown) (c). As expected, the background inflammatory cells (blue arrowhead in b) are highlighted with vimentin (brown) only; they are negative for cytokeratin 7 (red) (b). As noted in c, the reactive mesothelial cells were rare in this specimen. [a, HE-stained section; b, vimentin (brown) followed by cytokeratin 7 (red) (dual color immunostained cell-block section); c, calretinin (brown) followed by BerEP4 (red) (dual color immunostained cell-block section); (a–c, 40X).]](/content/105/2022/19/1/img/Cytojournal-19-3-g016.png)
- Metastatic mammary adenocarcinoma, pleural fluid. Metastatic carcinoma cells (arrows in a–c) are immunoreactive only for cytokeratin 7 (red) without vimentin (brown) immunostaining (b). For additional confirmation, these cells are weakly immunoreactive for BerEP4 (red) and negative for calretinin (brown) (c). As expected, the background inflammatory cells (blue arrowhead in b) are highlighted with vimentin (brown) only; they are negative for cytokeratin 7 (red) (b). As noted in c, the reactive mesothelial cells were rare in this specimen. [a, HE-stained section; b, vimentin (brown) followed by cytokeratin 7 (red) (dual color immunostained cell-block section); c, calretinin (brown) followed by BerEP4 (red) (dual color immunostained cell-block section); (a–c, 40X).]
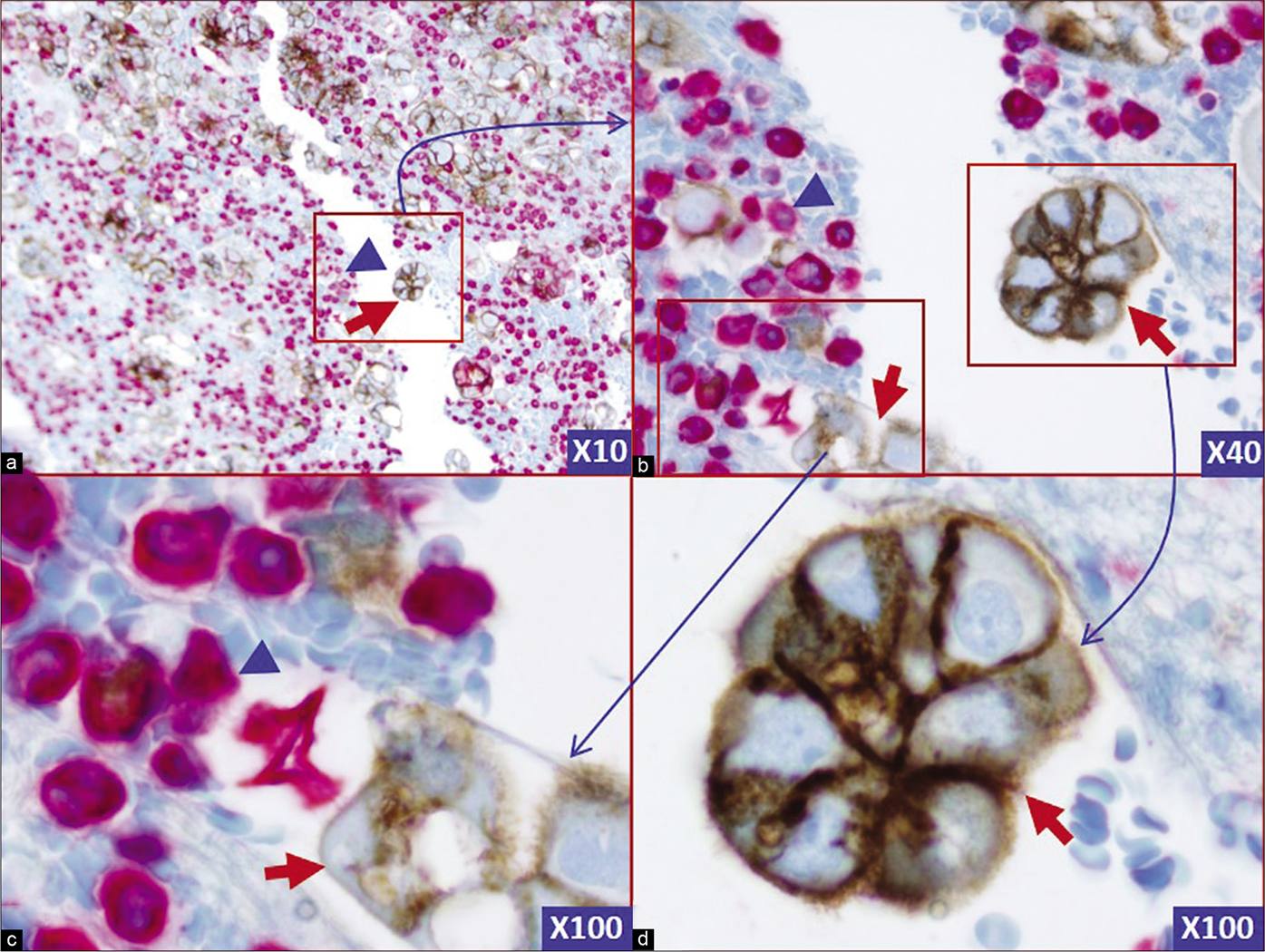
- Two color BerEP4 (Brown) with vimentin (Red): the neoplastic cells (red arrows) are immunoreactive for BerEP4 (Brown) and non-immunoreactive for vimentin (Red). The background inflammatory cells and rare reactive mesothelial cells are immunoreactive for vimentin (blue arrowheads). Two color immunostaining for BerEP4 with vimentin highlights the basic map of the effusion fluid cell-block section and facilitates detection of ‘second foreign population’ consistent with metastatic tumor cells. These would be followed in serial sections (Figure 1) to evaluate coordinate immunoreactivity for other immunomarkers used depending on the clinical scenario for the differential diagnosis of primary site. (Metastatic adenocarcinoma, pleural fluid, cell-block section).
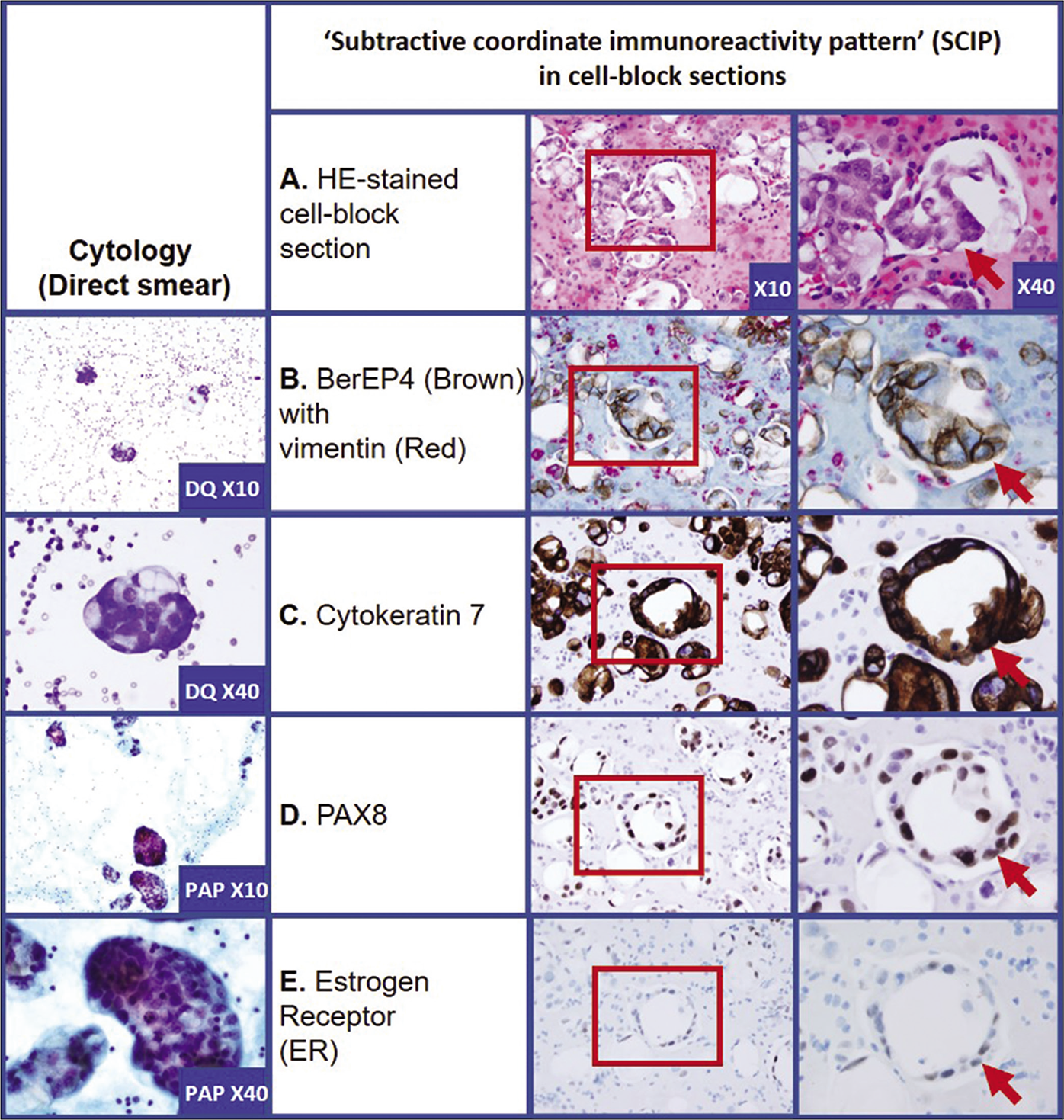
- Metastatic adenocarcinoma: Mullerian primary (pleural fluid). A. HE-stained cell-block section: Section shows of proliferation of the neoplastic cells (red arrow). B. Two color BerEP4 (Brown) with vimentin (Red): the neoplastic cells (red arrow) are immunoreactive for BerEP4 (Brown) and non-immunoreactive for vimentin (Red). The background inflammatory cells and rare reactive mesothelial cells are immunoreactive for vimentin (Red). C. Cytokeratin 7: the neoplastic cells (red arrow) show cytoplasmic immunoreactivity. D. PAX8: the neoplastic cells (red arrow) show nuclear immunoreactivity in most of the neoplastic cells. E. Estrogen Receptor (ER): the neoplastic cells (red arrow) show nuclear immunoreactivity in some neoplastic cells. Two color immunostaining for BerEP4 with vimentin highlights the basic map of the effusion fluid cell-block section and facilitates detection of ‘second foreign population’ consistent with metastatic tumor cells. These cells are followed in serial sections to evaluate coordinate immunoreactivity for other immunomarkers used depending on the clinical scenario for the differential diagnosis of primary site. In this case, the tumor cells showed immunoreactivity for Cytokeratin 7 with nuclear immunoreactivity for PAX8. The tumor cells also showed nuclear immunoreactivity for ER in some tumor cells. This immunoprofile is consistent with Mullerian primary (patient had history of ovarian carcinoma).
![Metastatic gastric adenocarcinoma, peritoneal fluid. The metastatic carcinoma cells are only immunoreactive for cytokeratin 7 (red) (brown arrows in b). Vimentin (brown) immunoreactive inflammatory cells (blue arrows in b) and the reactive mesothelial cells with calretinin nuclear immunoreactivity (brown) (blue arrow) are seen in the background (c). These inflammatory cells (blue arrows in b) and the reactive mesothelial cells (blue arrow) are negative for cytokeratin 7 (red) and BerEP4 (red) in b and c, respectively. Discussion in brief. Numerous isolated neoplastic cells as predominant population (a) suggested a differential diagnosis of high-grade large cell lymphoma and mesothelial cells with florid reactive changes. Immunoreactivity for cytokeratin 7 (brown arrow) in b excluded lymphoma and concurrent lack of vimentin (brown) immunostaining excluded mesothelial cells. The patient had a history of gastric carcinoma. The pattern was consistent with metastatic gastric carcinoma. A few vimentin immunoreactive (brown) inflammatory cells were present in the background (b). Rare reactive mesothelial cells with brown nuclear and cytoplasmic immunostaining (blue arrows) were also present in the background with inflammatory cells (c). The metastatic cells were also weakly immunoreactive for BerEP4 by one color immunostaining, but this weak immunoreactivity was not detectable in the dual color immunostained sections even after using the same titer of antibody dilution used for routine one color immunostaining (c) [30a] [a, HE-stained section; b, vimentin (brown) followed by cytokeratin 7 (red) (dual color immunostained cell-block section); c, calretinin (brown) followed by BerEP4 (red) (dual color immunostained cell-block section); (a–c, 40X).]](/content/105/2022/19/1/img/Cytojournal-19-3-g019.png)
- Metastatic gastric adenocarcinoma, peritoneal fluid. The metastatic carcinoma cells are only immunoreactive for cytokeratin 7 (red) (brown arrows in b). Vimentin (brown) immunoreactive inflammatory cells (blue arrows in b) and the reactive mesothelial cells with calretinin nuclear immunoreactivity (brown) (blue arrow) are seen in the background (c). These inflammatory cells (blue arrows in b) and the reactive mesothelial cells (blue arrow) are negative for cytokeratin 7 (red) and BerEP4 (red) in b and c, respectively. Discussion in brief. Numerous isolated neoplastic cells as predominant population (a) suggested a differential diagnosis of high-grade large cell lymphoma and mesothelial cells with florid reactive changes. Immunoreactivity for cytokeratin 7 (brown arrow) in b excluded lymphoma and concurrent lack of vimentin (brown) immunostaining excluded mesothelial cells. The patient had a history of gastric carcinoma. The pattern was consistent with metastatic gastric carcinoma. A few vimentin immunoreactive (brown) inflammatory cells were present in the background (b). Rare reactive mesothelial cells with brown nuclear and cytoplasmic immunostaining (blue arrows) were also present in the background with inflammatory cells (c). The metastatic cells were also weakly immunoreactive for BerEP4 by one color immunostaining, but this weak immunoreactivity was not detectable in the dual color immunostained sections even after using the same titer of antibody dilution used for routine one color immunostaining (c) [30a] [a, HE-stained section; b, vimentin (brown) followed by cytokeratin 7 (red) (dual color immunostained cell-block section); c, calretinin (brown) followed by BerEP4 (red) (dual color immunostained cell-block section); (a–c, 40X).]
| Immunomarker | Diagnostic immunomorphological (immunostaining) patterns | Remark | |||
|---|---|---|---|---|---|
 |
 |
 |
 |
||
| Nuclear | Cytoplasmic | Membranous | Microvillous | ||
| vimentin | X | ||||
| Ber-EP4 Claudin-4 |
X | X | X1 | ||
| Calretinin | X2 | ||||
| *I. Other nuclear immunomarkers | X* | See notes below | |||
| **II. Other cytoplasmic immunomarkers | X** | See notes below | |||
Other immunomarkers with immunostaining patterns: Bile canalicular immunostaining: polyclonal CEA (pCEA, not mCEA: monoclonal CEA) and CD10 in hepatocytes
PERSPECTIVE
Cell-blocks are the preferred choice for immunocytochemical evaluation of effusions. However, if only very scanty material is present, it is not advisable to perform immunocytochemistry on such material. In such a setting, an adequate additional specimen is recommended to be submitted. • Because malignant effusions usually reaccumulate quickly, acquiring a new sample is generally not a problem. It is not uncommon to submit only a small fraction of a large volume of effusion fluid collected.
Therefore, it should be specifically communicated in the final cytopathology report as a recommendation with a comment: ‘Recommend submission of most of the drained effusion fluid (up to 1000 mL). Larger specimen volume facilitates retrieval of adequate cellular material in cell-block sections for evaluation with ancillary studies including immunocytochemistry.’
Cell-blocks offer dual benefits including precise immunomorphologic interpretation and better standardization, with results comparable to those with surgical pathology specimens. Multiple sections from a single cell-block allow for multiple immunomarkers to be evaluated. Additionally, the cell-blocks may be archived and made available for other types of testing including molecular pathology and proteomics as indicated at later stage.[8,20]
The frequency of using cytology smears for immunocytochemical testing for different applications is increasing.[21-23]
With the advent of heat-induced epitope retrieval techniques, the quality of immunostained cytology smears has improved remarkably. Immunocytochemical evaluation of effusion smears has also been reported and discussed.[6,10,24] However, most experts do not recommend immunostaining of cytology smears of effusion fluids as a routine practice.[6,24]
On rare occasions, however, performing immunocytochemistry on effusion smears may be justified: e.g. patients with a known primary neoplasm showing a distinct immunoreactivity pattern. Immunostaining may be performed on cytology smears in such cases, if only a scant effusion specimen is available and cell-blocks are not possible. However, applicable limitations, such as the inability to evaluate the coordinate immunoreactivity pattern in cytology smears and possible lack of cross-verification should be weighted prior to final interpretation.
Straightforward positive–negative interpretation of immunostained sections is an artificial simplification, but it may serve its purpose in many situations. However, this approach needs to be refined in complex situations such as evaluation of cell-block sections of effusion fluids, which are affected by many variables not applicable to other specimens. Some of these variables are:
The cells to be evaluated in the cell-block sections may not be obvious. They usually have significant morphologic overlap with the reactive mesothelial cells which form the predominant background population in the cell-block sections of effusion fluids.
In addition to reactive mesothelial cells, a variety of inflammatory cells are also intricately intermingled with the cells under scrutiny.
It is challenging to follow the same cells in different cell-block sections on different slides for the evaluation of immunoreactivity for various immunomarkers to study the coordinate immunoexpression [see Figure 1]. This challenge is more apparent if the cells in the effusion are predominantly singly scattered cells.
The protein-rich effusion fluid may introduce some nonspecific immunoreactivity, not otherwise observed when the corresponding immunomarker is applied to routine surgical pathology tissue specimens.
-
The protocols for cell-block preparation may vary and introduce remarkable variations at different steps, such as:
the fluid may not have been submitted fresh but in a fixative
these fixatives themselves may be of different types, such as ethanol or other proprietary fixatives, without any data on the exact composition
transportation conditions: time and temperature variation
delayed processing of unfixed specimen after collection
variation in methods of cell-block preparation: agar gel method, picric acid method, thrombin clot, and so on. However, recently there is opportunity to standardize the methods. For example currently available ready to use NextGen CelBloking (NGCB kits)[25] has introduced processing comparable to surgical pathology specimens by allowing the fresh-unfixed specimen to be fixed directly in 10% formalin to generate formalin-fixed paraffin-embedded cell-block.[25-27]
Some of these variables are of less significance than others. For example, submission of effusions in fixative, delayed transportation at room temperature, and the picric acid method for cell-block preparation are deleterious for most of the immunomarkers.
For optimal results, the immunostained cell-block sections of effusion fluids should be evaluated immunohistomorphologically in a manner similar to histomorphological evaluation of hematoxylin and eosin (HE)-stained sections, where we consider multiple qualitative and quantitative morphologic features to reach a final interpretation. • All aspects of individual and complementary immunomarkers should be considered collectively [see Figures 1 and 5], rather than applying a reflexive positive–negative approach.
Categorization of effusion immunomarkers
Many immunomarkers can be applied to distinguish reactive and neoplastic mesothelial cells from metastatic tumor cells, which are predominantly adenocarcinomas. Immunomarkers for the evaluation of effusions secondary to lymphomas, melanomas, sarcomas, and unknown primaries are also discussed as dedicated articles in the series. More details about immunomarkers related to the evaluation of neoplasms associated with serous cavities may be found in specific reviews.[9,28-32]• Due to the continuous advances in this area with frequent updates, application of the basic principles described in this chapter to contemporary immunomarkers after relevant adjustments is recommended.
‘Positive’ mesothelial markers
Currently, calretinin, cytokeratin 5/6, and WT-1 appear to be the best immunomarkers for reactive and neoplastic mesothelial cells. Although calretinin and cytokeratin 5/6 are more sensitive than WT-1, immunoreactivity for all of these markers may be observed in a minority of carcinomas. WT-1 is slightly less sensitive, but is not expressed in lung adenocarcinomas and most other adenocarcinomas. WT-1 may be considered more specific in non-peritoneal settings. However, it is also immunoexpressed in ovarian/peritoneal carcinoma and desmoplastic small round cell tumor,[33] thus decreasing the specificity of WT-1 in peritoneal fluid.
D2-40 was initially reported as a lymphatic endothelial marker with a membranous immunostaining pattern. Recently, it has been reported to be as sensitive as calretinin and more sensitive than cytokeratin 5/6 and WT-1 for the differential diagnosis of malignant mesothelioma and adenocarcinoma.[34] The report concluded that D2-40 is a sensitive ‘positive’ immunomarker for cells of mesothelial origin.[30,35] Podoplanin is another immunomarker reported to be a specific positive marker for mesothelial cells.[31,36,37] However, recently it has been reported that a commercially available mouse monoclonal antibody, D2-40, specifically recognizes human podoplanin.[38-40] These immunomarkers have shown some overlap with other neoplasms, especially ovarian neoplasms.[41,42]
Because of the overlap between mesothelial and nonmesothelial cells, other immunomarkers such as thrombomodulin, mesothelin, HBME-1, N-cadherin, OV632, and CD44S, which were initially reported as mesothelial markers, are not recommended as a part of the routine diagnostic immunopanel for evaluation of effusion fluids. They are both less sensitive and less specific. Rabbit polyclonal antibody AMAD-2 with granular cytoplasmic immunoreactivity was reported to be specific for mesothelial cells without further significant updates.[43]
Vimentin and cytokeratin 7 are additional mesothelial immunomarkers with higher sensitivity but lower specificity. Further refinement and standardization of the aforementioned immunomarkers may allow for some of them to be used as reliable ‘positive’ immunomarkers for mesothelial cells when used in proper permutation and combinations of coordinate-immunoreactivity patterns with other immunomarkers.
‘Negative’ mesothelial immunomarkers
If there is a reliable immunomarker which is consistently negative in reactive and/or neoplastic mesothelial cells, it would be an ideal ‘negative’ mesothelial marker. mCEA, MOC-31, Ber-EP4, BG-8, B72.3, and Claudin-4.[44] are a few which may be included in this group. These appear to show a relatively improved sensitivity and specificity for the differential diagnosis of malignant mesotheliomas and adenocarcinomas. However, although rare, mesothelial cells may demonstrate unexpected cross-immunoreactivity with these immunomarkers. In case of rare false-positive immunoreactivity for these immunomarkers, the immunostaining pattern in mesothelial cells may show membranous/microvillous immuno-morphology.
Additional less-sensitive but relatively specific ‘negative’ mesothelial immunomarkers for the differential diagnosis of adenocarcinoma include various organ-specific immunomarkers such as thyroid transcription factor-1 (TTF-1), prostate-specific antigen (PSA), calcitonin, NKX3.1, PAX8, PAX2, GATA3, and estrogen receptor (ER). In specific clinical settings, they are critical for identifying unknown primary neoplasms [Table 2].
| Possible primary site (s) | Immunoreactivity pattern of second population |
|---|---|
| Immunomarkers suggestive of a subset of specific tumors | |
| Biliary tract, mucinous ovarian, and transitional cell ca CK | 20+, CK 7+ |
| Breast, lung, endometrial, and non-mucinous ovarian ca | CK 20-, CK 7+ |
| Thyroid, endometrial, and renal ca | Co-expression of Vim and CK |
| Breast and non-mucinous ovarian ca | ER/PR/Ar+ |
| Immunomarkers for specific tumors | |
| Breast[42] | CK7+, ER/PR/AR+ (even if ER/PR/CK7-), Mammaglobin+, GATA3 |
| Lung | CK7+TTF-1+, CK20- |
| Gastric | CK 7+, MUC5AC+, CK 20- |
| Small cell ca | Neuroendocrine (NE) markers+ (Synaptophysin, Chromogranin, CD56) |
| Merkel cell ca[43] | Globular CK20+, NE markers+ |
| Thyroid ca | Vim+, CK+, Tff-1, PAX8, Thyroglobulin+ |
| Medullary ca[23] | Calcitonin+TTF-1+ |
| Prostate ca | PSAP+, PSA+, NKX3.1, CEA- |
| Choriocarcinoma | HCG+ |
| Colon ca[46] | CK 20+, CK7-, CDX2+(nuclear), SATB2, villin, diffuse mCEA+, MUC5AC- |
| Hepatocellular ca | pCEA+canalicular pattern, AFP+, Arginase+,Albumin mRNA ISH, CD34 immunoreactive diffuse capillarization , Hep Par 1+, MUC5AC- |
| Cholangiocarcinoma | CK 7+, MUC5AC+, pCEA-/diffuse (non-canalicular)+, AFP- |
| Seminoma | PLAP+ |
| GIST | CD117+, DOG1, CD34+ |
| Desmoplastic small round cell tumor[33] | CK Globular+, Desmin Globular+, Vim+/- |
| Melanoma[30,41] | S100 protein +, Tyrosinase, Melan A, MART 1. MCW melanoma cocktail +, HMB45+, CK-, Calretinine- |
| Differential between two types of tumors | |
| Prostatic adenocarcinoma versus transitional cell ca[47] | PSA/PSA/Androgen receptor (AR)+, ERG (Ets-related gene product)+, NKX3.1 (Homeobox protein NKX3.1)+, AMACR (Alpha-methylacyl-CoA racemase)+versus CK 7 & 20+, CK 903 (24bE12)+ |
| Small cell ca lung versus Merkel cell ca | CK 7+, ER+, PAX8+, CK 20-, TTF-1+versus CK 20+ |
| Thyroid ca versus medullary ca of thyroid | Thyroglobulin+versus Calcitonin+ |
| Breast ca versus colon ca | ER/PR/AR/CK 7+versus CK 20+, CDX2+ |
| Ovarian mucinous ca versus appendiceal mucinous ca | CK 7+versus CK 20+, CDX2+ |
| Hepatocellular ca versus cholangiocarcinoma[46] | pCEA canalicular+, Hep Par 1+, Albumin mRNA (ISH)+, versus CK7+MUC1+CK 19+ |
| Mesothelioma versus lung ca | Calretinin+ (nuclear) versus TTF-1+(nuclear) |
| Endocervical ca versus endometrial ca[48] | p16 (INK4)+, mCEA+, Vim-versus p16(INK4)-, mCEA+/-, Vim+ |
| Stromal tumors versus leiomyosarcoma | CD10+versus SMA+ |
| Pancreatobiliary adenocarcinomas versus extra-pancreatobiliary nonmucinous adenocarcinomas[46] | MUC1+, CK19+versus MUC2/CDX2+ |
ca, carcinoma; CK, cytokeratin; pCEA, polyclonal carcinoembryonic antigen; PSAP, prostate-specifi c acid phosphatase; PSA, prostate-specific antigen; Vim, vimentin; HCG, human chorionic gonadotropin, GIST, gastrointestinal stromal tumors.
Other immunomarkers
Some immunomarkers are neither positive nor negative mesothelial markers, but they have typical immunostaining patterns [see Table 1]. A thick, membranous ruffled pattern highlighting microvilli favors mesothelial cells [see Figure 20]. Adenocarcinoma cells usually show diffuse, coarse cytoplasmic or flimsy, membranous immunostaining patterns [Figure 13]. The immunomarkers in this group are EMA and HBME-1 (and very rarely other non-mesothelial immunomarkers such as BerEP4). Without considering this immunomorphologic correlation of immunostaining patterns, these immunomarkers may lead to misinterpretation.
-
Immunomarkers highlighting various components of effusion fluid are:
Non-epithelial (and mesothelial) component by vimentin [including two color BerEP4 (brown) for adenocarcinoma with vimentin (Red) for mesothelial cells and inflammatory cells][45]
Epithelial component by pan-cytokeratin (e.g. mixture of AE1/AE3 and CAM5.2) or other cytokeratin, including coordinate application of cytokeratin 7 and cytokeratin 20
Inflammatory cell component by LCA (leukocyte common antigen, CD45) or PGM1 (CD68) or mixture of LCA and PGM1. LCA (CD45) is relatively strong and sensitive. It does not require an antigen retrieval step. As compared to this, PGM1 (CD68) needs an antigen retrieval step. If LCA and PGM1 are combined, then the titer of LCA antibody should be adjusted to accommodate the antigen retrieval used for PGM1. For selection of CD68 antibody, in our experience PGM1 usually lacks non-specific immunoreactivity, which is observed frequently with KP1, and so PGM1 may be preferred over KP1.
![Diffuse malignant mesothelioma of epithelial type (DMME), pleural fluid. Neoplastic cells are immunoreactive for EMA (a,b) and HBME-1 (c,d,e) with a membranous immunostaining pattern (arrows) highlighting long, slender microvilli (arrowheads). [a–e, Immunostained cell-block sections (a, 100X; b, 100X zoomed; c, 40X; c, 100X; e, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-3-g021.png)
- Diffuse malignant mesothelioma of epithelial type (DMME), pleural fluid. Neoplastic cells are immunoreactive for EMA (a,b) and HBME-1 (c,d,e) with a membranous immunostaining pattern (arrows) highlighting long, slender microvilli (arrowheads). [a–e, Immunostained cell-block sections (a, 100X; b, 100X zoomed; c, 40X; c, 100X; e, 100X zoomed).]
This group of immunomarkers creates a basic map for evaluation, localization, and identification of neoplastic cells [see Figures 1 and 5] by the SCIP approach (see below).
SCIP approach [Figures 1,6 -12]
For the evaluation of tissue specimens, a panel with at least four immunomarkers with two positive mesothelial immunomarkers and two negative immunomarkers (but positive for adenocarcinoma, such as BerEP4 and Claudin-4) would achieve the desired distinction between mesothelial cells and adenocarcinoma with improved sensitivity and specificity. In effusions, however, the challenge is more complex and has to be approached in a slightly different manner [see Figures 1 and 5]. Depending on the case, the interpreter may not even be sure about the presence of neoplastic cells.
The basic map of different components of the effusion fluid may be created by immunostaining for vimentin, pancytokeratin (such as a mixture of AE1/AE3 and CAM5.2), and LCA (CD45) or PGM1 (CD68) or a mixture of LCA and PGM1. This facilitates evaluation of different components by correlating the coordinate immunoreactivity and nonimmunoreactivity in concert with calretinin and WT-1 immunostaining to decide the nature of different cells in the effusion fluid [see Figure 1X]. Other immunomarkers in specific clinical situations may be added to further characterize the second population.
We call this approach—to localize, identify, and characterize the ‘second-foreign’ population in cell-block sections by immunocytochemistry —as SCIP approach. It can be simply summarized with the examples provided in [Figures 1, 6 through 12]. To simplify interpretation of immunostained cell-block sections, sequential serial sections are oriented identically on glass slides [see Figure 2]; the goal is to track the relative position of each cell and cell group in different serial sections and evaluate their coordinate immunoreactivity pattern with reference to other cellular components in the effusion and deduce the final interpretation using the SCIP approach.[32]
Recent update: SCIP with dual color immunostaining
Recently we evaluated[45,49] dual color immunostaining of cell-block sections of effusion fluids [Figure 14]. Based on various immunomarkers discussed above, different combinations of two immunomarkers applicable to effusion fluid immunocytochemistry are possible. Three combinations were selected and evaluated.[49]
BerEP4 (brown) followed by vimentin (red) [Figures 16].[45]
Vimentin (brown) followed by cytokeratin 7 (red) [Figures 14, 15, 18]
Calretinin (brown) followed by BerEP4 (red) [Figures 15, 18].[49]
These combinations facilitated easy evaluation by the SCIP approach, where the basic map of different components of the effusion fluids were evaluated in a single or relatively few sections. The study[45,49] concluded that dual color immunostaining facilitated easy objective identification of foreign populations of malignant cells in effusion fluids.
The dual-color immunostaining in combination A highlights all reactive components (reactive mesothelial cells and inflammatory cells) in the effusion fluids as vimentin immunoreactive red colored background as the basic map. In case metastatic vimentin nonimmunoreactive adenocarcinoma/carcinoma (vast majority of adenocarcinoma) cells are present in the effusion fluid, they will be immunoreactive for BerEP4 in most cases and seen as brown immunostained cells [Figure 16].[45]
In combination B, reactive mesothelial cells were brown or brown and red (vimentin and CK 7 immunoreactivity) and inflammatory cells were brown (vimentin immunoreactivity) [see Figure 14]. Any cells immunostaining exclusively as red color (without brown component) (CK 7 immunoreactivity only), or not immunostained at all, were consistent with metastatic disease [see Figure 14]. Depending on the primary site, these cells were red (breast, ovary, lung, etc.) or unstained (other carcinomas and other tumors). In metastatic adenocarcinoma, the corresponding cells in combination C were seen as red cells (BerEP4 immunoreactivity) amongst reactive mesothelial cells with brown nuclei due to calretinin immunoreactivity [see Figure 18]. Some of the examples with dual color immunostaining are shown in Figures 14 through 18). Based on our practice, combination A [BerEP4 (brown) followed by vimentin (red)] showed better results with better objective interpretation.[45]
THE ANTIBODY PANEL
• In general, monoclonal antibodies offer better specificity with less non-specific background staining. Recent availability of Rabbit monoclonal antibodies[50] for immunohistochemistry has further increased the sensitivity for many immunomarkers. As immunoexpression patterns may be heterogeneous, it is prudent to select at least two immunomarkers. Immunoprofiles of various neoplasms and lesions may overlap significantly. Consequently, it is advisable to use the optimum number of immunomarkers for appropriate objective interpretation. • Using a suboptimal restricted immunopanel may lead to spurious interpretation, with the possibility of undesirable consequences for patient care.
Depending on the institutional protocol and the algorithm used, immunocytochemical evaluation with different immunomarkers may be sequential (to economize) or concurrent (for rapid turnaround time) [see Figure 5].
If the presence of neoplastic cells (a ‘second-foreign’ population) is confirmed with the SCIP approach [see Figures 1 and6 through 12] in correlation with the cytomorphology, the next task is to characterize these neoplastic cells for their type and primary site as indicated by clinical scenario [see Figure 5].
Vimentin and two color BerEP4 (brown) with vimentin (red), Claudin-4, pan-cytokeratin (mixture AE1/AE3 and CAM5.2), and LCA (CD45) or PGM1 (CD68) or preferably a mixture of LCA and PGM1 help to create the basic map for SCIP approach for identifying a second-foreign population [see Figures 1, 6A-C, 7A,B, 8A-C, and 9A-C].
The addition of calretinin and WT-1 (or cytokeratin (CK) 5/6, CK 7), as positive mesothelial markers, identifies both reactive and neoplastic mesothelial cells in the basic map [Figures 6D,E, 7C, 8D, and 9C].
-
If, after microscopic subtractive assessment of serial sections, non-mesothelial/non-inflammatory cells are present as a second foreign population, they are usually consistent with a metastatic neoplasm.
If this population is immunoreactive for cytokeratin (rarely also for vimentin), it is consistent with carcinoma [see Figures 1B, 5B, 6B, 7B, 8B, and 9D]. This population is usually immunoreactive for one or all ‘negative’ mesothelial markers such as, BerEP4, B72.3, Claudin-4, CEA, and/or MOC-31 [see Figures 5, 7D, 9E].
If the second population is non-immunoreactive for cytokeratin but immunoreactive for vimentin, it may represent sarcoma, melanoma (immunoreactive for melanoma markers), or lymphoma (immunoreactive for LCA and other lymphoma markers, with flow cytometry or immunocytochemistry as indicated) [see Figures 5, 12]. Except for lymphoma, these are rare causes of malignant effusion.
In summary, the initial immunopanel should include two color BerEP4 (brown) with vimentin (red), cytokeratin 7, calretinine, and claudin-4 for evaluation of SCIP to detect or confirm an initial suspicion of a second population of neoplastic cells in cytology smears.
Modify the initial immunopanel depending on the clinical scenario:
Possible primary neoplasms known—add a short panel, such as TTF-1 for lung, PSA and NKX3.1 for prostate, GATA-3+ER+ mammaglobin+CRxA-01 for breast, CK 20+CDX2+SATB2+ for colon, PAX8 for Mullerian primary, CA IX for renal primary, appropriate lymphoma panel for lymphoma, S-100 protein and other melanoma markers for melanoma, and so on [see Table 2].[23,30,31,46,47,51-53]
If the primary neoplasm is unknown: add a suitable immunopanel (in concert with CK 7 with CK 20 for evaluation of CK7/CK20 coordinate immunoreactivity patterns) for many broad category of immunomarkers [see Table 2].[16,26,27,29,32,54]
In cases with numerous, solitary, scattered, non-mesothelial, malignant cells exhibiting negligible numbers of mesothelial cells, the second-foreign population of cells may be the only population with non-mesothelial immunoprofile (nonimmunoreactivity for nuclear calretinin and vimentin with immunoreactivity for BerEP4) [see Figure 8]. Depending on cytomorphology and clinical history, these cells may demonstrate an immunoprofile consistent with a carcinoma (cytokeratin+. usually vimentin-) [see Figures 8, 10], lymphoma (CD45+), sarcoma (vimentin+, cytokeratin−), or melanoma (S-100+ with one of the melanoma markers).[16,29,30,47,55,56]
• The availability of a past history of cancer remarkably improves the interpretation by proper modification of the basic immunopanel. The challenge is relatively complex in cases with malignant effusions secondary to neoplasms without a known primary site. In such cases, a wider range of site-specific immunomarkers may have to be included [see Table 2].[16,26,27,29,32,54]
Most metastases to serous cavities are secondary to adenocarcinoma and majority of which are vimentin nonimmunoreactive except a few listed in Table 3. Because of this, some cases may show immunoreactivity for both vimentin and BerEP4 with red and brown color immunostaining in the same metastatic tumor cells.
| Vimentin immunoreactive carcinomas |
| Renal cell carcinoma (except the chromophobe variant) |
| Adrenal cortical carcinoma |
| Endometrial adenocarcinoma (Endometrial endometrioid carcinomas immunostain strongly for vimentin, but endocervical carcinomas rarely stain (weak focal staining in up to 13% of endocervical carcinomas). |
| Malignant mixed Mullerian tumors, serous ovarian carcinomas |
| Large cell carcinoma of lung |
| Metaplastic carcinoma of breast |
| Poorly differentiated adenocarcinoma of stomach (6%) |
| Sarcomatoid carcinomas (spindle cell carcinomas) |
| Pleomorphic salivary gland tumors |
| “Basal-like” breast carcinomas |
| Follicular and anaplastic thyroid carcinomas |
| Epithelial and sarcomatoid mesotheliomas |
| Solid pseudopapillary neoplasm of the pancreas Vimentin expressing tumor epithelial cells in surgically resected carcinomas including pancreatic adenocarcinomas and hepatocellular carcinoma independently predicted a shorter postsurgical survival |
| Vimentin non-immunoreactive sarcoma |
| Alveolar soft part sarcoma |
Reproduced from open access publication Cytojournal. 2021 Jan 30;18:2.[8]
On similar lines, although a vast majority of adenocarcinomas are Ber-EP4 immunoreactive, a few listed in Table 4 may be Ber-EP4 non-immunoreactive. Other immunomarkers including Claudin-4 would cover many of the tumor types in this group.
| Immunoreactive | Non-immunoreactive |
|---|---|
| Adenocarcinomas (most, 50-100% in various studies) Neuroendocrine tumors, including small cell carcinoma Chromophobe renal cell carcinoma (75%), Papillary renal cell carcinoma (55%), Clear cell renal carcinoma (18%), Metastatic renal cell carcinoma (14%) Hepatocellular carcinoma Basal cell carcinomas Basosquamous carcinomas Synovial sarcoma |
Mesothelioma* Lymphoma Most soft-tissue sarcomas Adenomatoid tumor Renal oncocytoma |
Reproduced from open access publication Cytojournal. 2021 Jan 30;18:2.[8]
It is important to highlight that, even if immunocytochemistry does not demonstrate a second foreign population of nonmesothelial and non-inflammatory cells, mesothelioma is not excluded. The diagnosis of mesothelioma is based on qualitative and quantitative cytologic features in concert with a clinical and radiologic correlation (discussed in another review article in the series). Reports have suggested that p53 and OV632 are definitive markers for distinguishing between mesothelioma and reactive mesothelial cells, but such a magic immunomarker does not exist at this date.[57,58] However, after evaluating clones E29 and Mc5 of EMA antibodies, E29 has been reported to distinguish between reactive mesothelial cells and neoplastic mesothelial cells. In this study, 75% of malignant mesotheliomas showed immunoreactivity for EMA with clone E29, but reactive mesothelial cells were nonimmunoreactive in all cases.[59] Malignant mesothelioma demonstrates high Ki-67 index.[60] Mesothelioma cells demonstrate strong membranous GLUT-1 immunoreactivity with strong nuclear immunoreactivity for p53.[60] Lack of desmin immunoreactivity with EMA immunoreactivity favors malignant mesothelioma cells over reactive mesothelial cells, which usually show immunoreactivity for desmin with lack of EMA immunoreactivity.[60]
A few additional immunomarkers applied in a algorithmic manner along with molecular testing is suggested for distinguishing mesothelioma versus reactive mesothelial cells [Figure 19].[61-63] Loss of BAP1 immunoreactivity in mesothelial cells immunoreactive for mesothelial immunomarkers is consistent with mesothelioma. Some cases with BAP1 immunoreactive mesothelial cells with loss of MTAP cytoplasmic immunoreactivity and deletion on CDKNA FISH are consistent with mesothelioma. Other patterns with undetermined patterns should be tested further with molecular test/Next Gen Sequencing (NGS) [Figure 19].[61-63]
ADENOCARCINOMA VERSUS MESOTHELIOMA/ REACTIVE MESOTHELIAL CELLS
An increasing number of antibodies that could potentially be applied for the evaluation of effusions have been developed. Many of these are already commercially available and are applied directly to effusion cytology.[26,27,32,71] Those not listed may be added during the immunocytochemical evaluation of effusions by individual pathologists, depending on the clinical scenario, personal experience, and institutional resources.[6,28,31]
Historically, the interpretation of mesothelial cells (both reactive and neoplastic) has been based on their nonimmunoreactivity for immunomarkers such as BerEP4, Claudin-4, B72.3, monoclonal carcinoembryonic antigen (mCEA), and LeuM1. These immunomarkers have been labeled as ‘negative’ mesothelial markers.[4,18,19,72-75] However, unexpected immunoreactivity of mesothelial cells for these immunomarkers has been reported in a small percentage of cases.[65,76,77] The final interpretation in such cases can be reached if an optimum immunopanel is applied and the immunoreactivity patterns (such as cytoplasmic vs membranous due to bushy microvilli) are also taken into consideration [Figure 20]. Meanwhile, the search for additional, definitive immunomarkers for mesothelial cells continues. Some of the commonly used antibodies in the differential diagnosis of adenocarcinoma from mesothelial cells are listed in Table 5.
| Immunomarker* | Adenocarcinoma | Mesothelial cells§ |
|---|---|---|
| CK[17] | + | + |
| CK 7 | +/- (depending on primary—Table 2) | + |
| CK 20 | +/- (depending on primary—Table 2) | - |
| Vimentin[17] | - | + |
| BerEP4[64-65] | + | - |
| Calretinin[66] | - | + (nuclear or nuclear & cytoplasmic) |
| Claudin-4[44] | + | - |
| OV632[58] | - | + (in malignant mesothelioma) |
| p53[60] | + (variable) | +Nuclear (in malignant mesothelioma) |
| Desmin[60] | - | + Reactive mesothelial cells - Malignant mesothelioma |
| EMA[60-67] | + (strong cytoplasmic) | + (membranous highlighting microvilli) |
| mCEA[17-19] | +/- | - |
| LeuM1 (CD15)[6] | + | - |
| B72.3 | + | - |
| CA19.9[64-68] | + | - |
| E-cadherin[69] | + | - |
| WT-l[19•59] | - (ovarian-peritoneal ca, DSCRT +) | + |
| CK 5/6[19-59-70] | -/+ | + |
| HBME-1[68] | -/+ (may be fuzzy cytoplasmic) | + (membranous highlighting microvilli) |
| D2-40[34-36] | - | + membranous immunostaining |
| Podoplanin[36,37] | - | + membranous immunostaining |
§Mesothelial cells [(malignant mesothelioma (nuclear p53+ with high Ki-67; strong membranous GLUT-1 immunoreactivity; Lack of desmin immunoreactivity with EMA immunoreactivity; OV632 immunoreactivity) versus reactive mesothelial cells (desmin immunoreactive without EMA immunoreactivity)] (see also Figure 8).
+, positive; −, negative; +/−, variable usually positive; −/+, variable usually negative.
Ca, carcinoma; CK, cytokeratin; EMA, epithelial membrane antigen; mCEA, monoclonal carcinoembryonic antigen; WT-1, Wilms’ tumor-1; DSCRT, desmoplastic small round cell tumor.
Until recently, satisfactory immunomarkers for mesotheliomas were not available for their ‘positive’ identification. The mesothelioma cells were usually interpreted by a ‘negative’ immunoprofile, but this approach has been less reliable for the definitive identification of mesothelioma cells. Recently, some ‘positive’ mesothelioma markers such as calretinin and WT-1 have been described. Depending on the clinical differential diagnosis, these immunomarkers show promising results if properly interpreted with the help of the SCIP approach [see Figure 1]. The immunopanel may be more effective in cases with a known primary than in cases with an unknown primary neoplasm. Other immunomarkers, including CK 5/6, HBME-1, N-cadherin, OV632, thrombomodulin, mesothelin, CD44S, AMAD-2, and the recently reported D2-40, podoplanin,[31,34,36,37] have also been claimed as ‘definitive’ for mesothelial cells with variable reports.[17-19,41-43,57,64,66,69,70,73-76,78-97]
Sections immunostained with HBME-1 and EMA show thick, bushy, membranous immunostaining of mesothelial cells. This immunostaining pattern along the cell borders is due to the presence of slender microvilli, which are seen easily under an oil immersion lens [see Figure 20b,e, Table 1]. If adenocarcinoma cells are immunoreactive, they usually show a diffuse, coarse, cytoplasmic or flimsy, membranous immunostaining pattern [see Figure 13].[98] Proper understanding of the immunoreactivity pattern with these immunomarkers usually obviates the need for electron microscopy (EM) to demonstrate microvilli in malignant cells, which is difficult or impossible with effusion specimens. The most significant challenge is that the malignant cell(s) cannot be identified unequivocally in EM sections of effusion fluids to be selected for evaluation of microvilli. This may lead to misinterpretation of background reactive mesothelial cells with microvilli as mesothelioma cells.
The approach for immunocytochemical evaluation of malignant effusion for an unknown primary neoplasm is comparable to metastases to other sites and is summarized in Table 2.[8,29,54]
Although the basic approach with SCIP evaluation would be the same, the suggested combination of immunomarkers may be modified for a given laboratory, depending on the pathologist’s experience with a particular immunomarker, the quality of immunostaining, and the accessibility of resources with additional availability of improved immunomarkers in future.[6,31,99]
ABBREVIATIONS (IN ALPHABETIC ORDER)
DMEM - Diffuse malignant epithelioid mesothelioma
EM - Electron microscopy
HE - Hematoxylin and eosin
Neg – Negative
NGCB kits - NextGen CelBloking kits
Pos – Positive
SCIP - Subtractive coordinate immunoreactivity pattern
Acknowledgment
Authors thank Mir Khan, MD (Resident, Wayne State University School of Medicine, Detroit, MI, USA) for assisting the image acquisition for some of the figures.
In addition, we thank Farah Ahmad, MBBS, MPH (Research Assistant to Dr. Shidham, Wayne State University School of Medicine, Detroit, MI, USA) and Janavi Kolpekwar for copy-editing support.
References
- Effusions in the presence of cancer In: Koss' Diagnostic Cytology and Its Histopathologic Bases (5th edn). Philadelphia: Lippincott Williams & Wilkins; 2006. p. :949-1022.
- [Google Scholar]
- Pleural, peritoneal, and pericardial fluids In: Bibbo M, ed. Comprehensive Cytopathology (2nd edn). Philadelphia: WB Saunders; 1997. p. :551-621.
- [Google Scholar]
- Cytologic diagnosis of malignant mesothelioma, with particular emphasis on the epithelial noncohesive cell type. Diagn Cytopathol. 1999;20:57-62.
- [CrossRef] [Google Scholar]
- Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407-16.
- [CrossRef] [PubMed] [Google Scholar]
- Immunocytochemistry in effusion cytology: a contemporary review. Cancer Cytopathol. 2001;93:293-308.
- [CrossRef] [PubMed] [Google Scholar]
- CellBlockistry: Chemistry and art of cell-block making-a detailed review of various historical options with recent advances. Cytojournal. 2019;16:12.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol. 2002;26:61-66.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid transcription factor-1 immunocytochemical staining of pleural fluid cytocentrifuge preparations for detection of small cell lung carcinoma. Acta Cytol. 2004;48:635-640.
- [CrossRef] [PubMed] [Google Scholar]
- If cells could talk: the application of new techniques to cytopathology. Clin Lab Med. 1998;18:561-583.
- [CrossRef] [Google Scholar]
- Overview of the clinical immunohistochemistry laboratory: regulations and troubleshooting guidelines In: Javois LC, ed. Methods in Molecular Biology. 115: Immunocytochemical Methods and Protocols. Totowa, NJ: Humana Press; 1999. p. :405-414.
- [CrossRef] [PubMed] [Google Scholar]
- Department of Health and Human Services Health Care Financing Administration Clinical Laboratory Improvement Amendments of 1988 Final Rule. Federal Register 57, no. 40 (February 28 1992) :7001-288.
- [Google Scholar]
- (2000) Standards for Laboratory Accreditation Commission of Laboratory Accreditation Inspection Checklist, Sections 1 and 8 Northfield, IL: College of American Pathologists; 2000.
- [Google Scholar]
- Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunohistochem. 1995;3:99-107.
- [Google Scholar]
- Immunohistochemistry: diagnostic and prognostic applications In: Detrick B, Hamilton RG, Folds JD, eds. Manual Molecular and Clinical Laboratory Immunology (7th edn). Washington, DC: American Society for Microbiology; 2006. p. :408-413.
- [CrossRef] [Google Scholar]
- The immunohistochemical diagnosis of mesothelioma. Differentiation of mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 1989;13:276-291.
- [CrossRef] [Google Scholar]
- Malignant mesothelioma of the pleura: the reproducibility of the immunohistological diagnosis. Pathol Res Pract. 1997;193:759-765.
- [CrossRef] [Google Scholar]
- The immunohistochemical diagnosis of epithelial mesothelioma. Hum Pathol. 1999;30:313-323.
- [CrossRef] [Google Scholar]
- Cell-blocks and other ancillary studies (including molecular genetic tests and proteomics) Cytojournal. 2021;18:4.
- [CrossRef] [PubMed] [Google Scholar]
- Immunostaining of cytology smears: a comparative study to identify the most suitable method of smear preparation and fixation with reference to commonly used immunomarkers. Diagn Cytopathol. 2003;29:217-221.
- [CrossRef] [PubMed] [Google Scholar]
- Optimization of an immunostaining protocol for the rapid intraoperative evaluation of melanoma sentinel lymph node imprint smears with the 'MCW melanoma cocktail' Cytojournal. 2004;1:2. Free full text is available at: http://www.cytojournal.com/content/1/1/2
- [CrossRef] [PubMed] [Google Scholar]
- Expression of thyroid transcription factor-1 (TTF-1) in human C cells and medullary thyroid carcinomas. Hum Pathol. 2000;31:386-393.
- [CrossRef] [Google Scholar]
- Methods of cytology smear preparation and fixation: Effect on the immunoreactivity of commonly used anti-cytokeratin antibody AE1/AE3. Acta Cytol. 2000;44:1015-22.
- [CrossRef] [PubMed] [Google Scholar]
- Nano NextGen CelBloking™ Kit: AV Bio Innovation. Available from: https://www.avbioinnovation.com/product/nano-nextgencelbloking-kit
- [Google Scholar]
- CellBlockistry 101. 2021. (1st ed). United States: Cytopathology Foundation Inc.; Available from: https://www.amazon.com/-/es/Vinod-Shidham-ebook/dp/B098B65PSL https://www.cytojournal.com/eissues
- [Google Scholar]
- The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 2003;27:1031-1051.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory cytology In: Atkinson BF, ed. Atkinson Atlas of Diagnostic Cytopathology (2nd edn). Philadelphia: WB Saunders; 2004. p. :273-356.
- [Google Scholar]
- Improved immunohistochemical evaluation of micrometastases in sentinel lymph nodes of cutaneous melanoma with 'MCW melanoma cocktail'-a mixture of monoclonal antibodies to MART-1, Melan-A, and tyrosinase. BMC Cancer. 2003;3:15. Free full text is available at: http://www.biomedcentral.com/qc/1471-2407/3/15
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical diagnosis of epithelioid mesothelioma: an update. Arch Pathol Lab Med. 2005;129:1407-1414.
- [CrossRef] [PubMed] [Google Scholar]
- Serous effusions: Reactive, benign and malignant In: Gray W, Kocjan G, eds. Diagnostic Cytopathology Vol Ch. 3. (3rd ed). Netherlands: Elsevier; 2010.
- [CrossRef] [Google Scholar]
- Desmoplastic small round cell tumour: cytological and immunocytochemical features. Cytojournal. 2005;2:6. Free full text is available at: http://www.cytojournal.com/content/2/1/6
- [CrossRef] [PubMed] [Google Scholar]
- Utility of D240, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005;18:105-110.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of D2-40 immunostaining for malignant mesothelioma: A meta-analysis. Oncotarget. 2017;8:64407-616.
- [CrossRef] [PubMed] [Google Scholar]
- D2-40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372-380.
- [CrossRef] [PubMed] [Google Scholar]
- Podoplanin as a marker for mesothelioma. Pathol Int. 2005;55:83-86.
- [CrossRef] [PubMed] [Google Scholar]
- Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2, podoplanin) in human developing testis, testicular carcinoma in situ and germ-cell tumours. Virchows Arch. 2006;449:200-206.
- [CrossRef] [PubMed] [Google Scholar]
- Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913-921.
- [CrossRef] [Google Scholar]
- Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006;13:83-88.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of D2-40 in primary peritoneal serous carcinomas, ovarian serous carcinomas, uterine carcinomas and sex cord stromal tunors. Mod Pathol. 2006;19(Suppl1):1A-359A.
- [Google Scholar]
- Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913-921.
- [CrossRef] [Google Scholar]
- Newly marketed tissue markers for malignant mesothelioma: immunoreactivity of rabbit AMAD-2 antiserum compared with monoclonal antibody HBME-1 and a review of the literature on so-called antimesothelioma antibodies. Hum Pathol. 1997;28:929-937.
- [CrossRef] [Google Scholar]
- Usefulness of Claudin 4 in the cytological diagnosis of serosal effusions. Diagn Cytopathol. 2011;39:313-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficiency and effectivity of using dual-color immunostaining with a combination of BerEp4 and vimentin as compared to BerEp4 alone on serous effusions. J Am Soc Cytopathol. 2020;9:S5-6.
- [CrossRef] [Google Scholar]
- Mammaglobin and CRxA-01 in pleural effusion cytology: potential utility of distinguishing metastatic breast carcinomas from other cytokeratin 7-positive/cytokeratin 20-negative carcinomas. Cancer. 2004;102:368-372.
- [CrossRef] [PubMed] [Google Scholar]
- Effusion cytology of malignant melanoma. A morphologic and immunocytochemical analysis including application of the MART-1 antibody. Cancer. 1997;81:57-63.
- [CrossRef] [Google Scholar]
- Distinction of endocervical and endometrial adenocarcinomas: immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. Am J Surg Pathol. 2004;28:160-167.
- [CrossRef] [PubMed] [Google Scholar]
- Two color immunocytochemistry for evaluation of serous cavity fluids. Poster #20 96th Annual Meeting of United States and Canadian Academy of Pathology, March 24-30, 2007 San Diego, CA. Mod Pathol. 2007;20(Suppl 2) Abstract No. 285
- [Google Scholar]
- From rabbit antibody repertoires to rabbit monoclonal antibodies. Exp Mol Med. 2017;49:e305.
- [CrossRef] [PubMed] [Google Scholar]
- Merkel cell carcinoma in a malignant pleural effusion: case report. Cytojournal. 2004;1:5. Free full text is available at: http://www.cytojournal.com/content/1/1/5
- [CrossRef] [PubMed] [Google Scholar]
- Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- [CrossRef] [PubMed] [Google Scholar]
- Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766-3772.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemistry for diagnosis of metastatic carcinomas of unknown primary site. Cancers (Basel). 2018;10:108.
- [CrossRef] [PubMed] [Google Scholar]
- Melanoma-associated antigen recognized by T-cells (MART-1): the advent of a preferred immunocytochemical antibody for the diagnosis of metastatic malignant melanoma in fine-needle aspirations. Cancer (Cancer Cytopathol). 1999;87:37-42.
- [CrossRef] [Google Scholar]
- Monoclonal Mouse Anti-human Leukocyte Common Antigen Package Insert. Carpenteria, CA: Dako Corporation; 1998.
- [Google Scholar]
- OV632 as a possible marker for malignant mesothelioma: high expectations; low specificity. Diagn Cytopathol. 1995;12:81-82.
- [CrossRef] [PubMed] [Google Scholar]
- Immunocytochemistry of malignant mesothelioma: OV632 as a marker of malignant mesothelioma. J Pathol. 1991;165:137-43.
- [CrossRef] [PubMed] [Google Scholar]
- The value of epithelial membrane antigen expression in separating benign mesothelial proliferation from malignant mesothelioma: a comparative study. Diagn Cytopathol. 2005;32:156-159.
- [CrossRef] [PubMed] [Google Scholar]
- The use of immunohistochemistry to distinguish reactive mesothelial cells from malignant mesothelioma in cytologic effusions. Cancer Cytopathol. 2010;118:90-6.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of methylthioadenosine phosphorylase compared with BAP1 immunohistochemistry, and CDKN2A and NF2 fluorescence in situ hybridization in separating reactive mesothelial proliferations from epithelioid malignant mesotheliomas. Arch Pathol Lab Med. 2018;142:1549-53.
- [CrossRef] [PubMed] [Google Scholar]
- Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108-13.
- [Google Scholar]
- Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407-16.
- [CrossRef] [PubMed] [Google Scholar]
- Optimization of a battery using nine immunocytochemical variables for distinguishing between epithelial mesothelioma and adenocarcinoma. APMIS. 1997;105:889-894.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of nine newly derived mesothelioma cell lines. Ann Thorac Surg. 1995;59:835-844.
- [CrossRef] [Google Scholar]
- HBME-l: a monoclonal antibody useful in the differential diagnosis of mesothelioma, adenocarcinoma, and soft-tissue and bone tumors. Appl Immunohistochem. 1995;3:115-122.
- [Google Scholar]
- The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen. p53 platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology. 2003;43:231-238.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of BER-EP4 in the diagnosis of adenocarcinoma in effusions: an immunocytochemical study of 232 cases. Diagn Cytopathol. 1993;9:516-21.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of the antibodies CA 19-9, HBME-l, and thrombomodulin in the diagnosis of malignant mesothelioma and adenocarcinoma in cytology. Cancer (Cancer Cytopathol). 1998;84:101-108.
- [CrossRef] [Google Scholar]
- E-cadherin and calretinin: a useful combination of immunochemical markers for differentiation between mesothelioma and metastatic adenocarcinoma. Histopathology. 1998;32:209-216.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemistry for diagnosis of metastatic carcinomas of unknown primary site. Cancers (Basel). 2018;10:108.
- [CrossRef] [PubMed] [Google Scholar]
- Reactivity of six antibodies in effusions of mesothelioma, adenocarcinoma and mesotheliosis: stepwise logistic regression analysis. Cytopathology. 2000;11:8-17.
- [CrossRef] [PubMed] [Google Scholar]
- The immunohistochemistry of malignant mesothelioma. Pathol Annu. 1994;29(Pt 2):157-179.
- [Google Scholar]
- Role of immunohistochemistry in differentiating epithelial mesothelioma from adenocarcinoma. Am J Clin Pathol. 1999;112:75-89.
- [CrossRef] [PubMed] [Google Scholar]
- Glycogen-rich mesothelioma. Ultrastruct Pathol. 1999;23:401-406.
- [CrossRef] [PubMed] [Google Scholar]
- An optimized battery of eight antibodies that can distinguish most cases of epithelial mesothelioma from adenocarcinoma. Am J Clin Pathol. 2000;114:203-209.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of cancer cells in effusions from patients diagnosed with gynaecological malignancies. Evaluation of five epithelial markers. Virchows Arch. 1999;435:43-49.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic differential diagnosis among reactive mesothelial cells, malignant mesothelioma, and adenocarcinoma: utility of combined E-cadherin and calretinin immunostaining. Cancer. 2000;90:55-60.
- [CrossRef] [Google Scholar]
- N-cadherin distinguishes pleural mesotheliomas from lung adenocarcinomas: a ThinPrep immunocytochemical study. Cancer. 1999;87:83-86.
- [CrossRef] [Google Scholar]
- Value of calretinin immunostaining in differentiating epithelial mesothelioma from lung adenocarcinoma. Mod Pathol. 1998;11:929-933.
- [Google Scholar]
- Ecadherin, N-cadherin, and calretinin in pleural effusions: the good, the bad, the worthless. Diagn Cytopathol. 1999;20:125-130.
- [CrossRef] [Google Scholar]
- Differential expression of N-cadherin in pleural mesotheliomas and E-cadherin in lung adenocarcinomas in formalin-fixed, paraffin-embedded tissues. Hum Pathol. 1997;28:641-645.
- [CrossRef] [Google Scholar]
- MOC-31 aids in the differentiation between adenocarcinoma and reactive mesothelial cells. Cancer. 1999;87:390-394.
- [CrossRef] [Google Scholar]
- HBME-l, MOC-31, WT1, and calretinin: an assessment of recently described markers for mesothelioma and adenocarcinoma. Histopathology. 2000;36:341-347.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemistry in the differential diagnosis of mesothelioma and adenocarcinoma. Evaluation of 5 new antibodies and 6 traditional antibodies. Ann Pathol. 1998;18:460-465.
- [Google Scholar]
- Immunohistochemical panels for differentiating epithelial malignant mesothelioma from lung adenocarcinoma: a study with logistic regression analysis. Am J Surg Pathol. 2001;25:43-50.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronate binding probe and CD44 in the differential diagnosis of malignant effusions-disappointing results in cytology material. Diagn Cytopathol. 1998;18:473-474.
- [CrossRef] [Google Scholar]
- Value of the mesothelium-associated antibodies thrombomodulin, cytokeratin 5/6, calretinin, and CD44H in distinguishing epithelioid pleural mesothelioma from adenocarcinoma metastatic to the pleura. Mod Pathol. 2000;13:107-112.
- [CrossRef] [PubMed] [Google Scholar]
- Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol. 2000;24:598-606.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic diagnosis of malignant mesothelioma in serous effusions using an antimesothelial-cell antibody. Diagn Cytopathol. 1992;8:361-365.
- [CrossRef] [PubMed] [Google Scholar]
- A novel biotinylated probe specific for hyaluronate: its diagnostic value in diffuse malignant mesothelioma. Am J Surg Pathol. 1992;16:116-121.
- [CrossRef] [PubMed] [Google Scholar]
- Calretinin: a novel immunocytochemical marker for mesothelioma. Am J Surg Pathol. 1996;20:1037-1046.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic utility of calretinin immunohistochemistry in cytologic cell-block preparations. Cancer. 2000;90:312-319.
- [CrossRef] [Google Scholar]
- The value of anti-calretinin antibody in the differential diagnosis of normal and reactive mesothelia versus metastatic tumors in effusion cytology. Pathol Res Pract. 1998;194:759-764.
- [CrossRef] [Google Scholar]
- Calretinin staining pattern aids in the differentiation of mesothelioma from adenocarcinoma in serous effusions. Cancer. 2000;90:194-200.
- [CrossRef] [Google Scholar]
- Comparison of antibodies to HBME-l and calretinin for the detection of mesothelial cells in effusion cytology. Diagn Cytopathol. 2001;25:158-161.
- [CrossRef] [PubMed] [Google Scholar]
- Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418-1428.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic utility and limitations of electron microscopy in effusion fluid cytology smears. Diagn Cytopathol. 2003;29:46-48.
- [CrossRef] [PubMed] [Google Scholar]
- Cytopathologic Diagnosis of Serous Fluids Vol Ch. 15. (1st ed). United States: Elsevier, W. B. Saunders Company; 2007.
- [CrossRef] [Google Scholar]




![Algorithmic approach for diagnostic work-up for evaluation of mesothelial proliferations.[61-63]](/content/105/2022/19/1/img/Cytojournal-19-3-g020.png)




