Translate this page into:
Inhibition of lysine-specific histone demethylase 1A suppresses adenomyosis through reduction in ectopic endometrial stromal cell proliferation, migration, and invasion

*Corresponding author: Shuping Zhao, Department of Gynecology, Qingdao Women and Children’s Hospital, Shandong University, Qingdao, China. woyaobsby@163.com
-
Received: ,
Accepted: ,
How to cite this article: Cui L, Sang C, Li R, Zhao S. Inhibition of lysine-specific histone demethylase 1A suppresses adenomyosis through reduction in ectopic endometrial stromal cell proliferation, migration, and invasion. CytoJournal. 2024;21;50. doi: 10.25259/Cytojournal_48_2024
Abstract
Objective:
Deep endometriosis is now referred to as adenomyosis externa, whereas adenomyosis is once known as endometriosis interna. Lysine-specific histone demethylase 1A (KDM1A, commonly LSD1) is a lysine demethylase that targets histone and non-histone proteins. This study aimed to assess how KDM1A affects the migration, invasion, and proliferation of adenomyosis-derived endometrial stromal cells (ESCs).
Material and Methods:
Immunocytochemistry staining was used to identify primary ectopic endometrial stromal cells (EESCs) and eutopic endometrial stromal cells (EuESCs) were isolated and purified from patients with complete hysterectomy for adenomyosis. Cell counting kit-8 assay, colony formation, wound scratch, and transwell assays were used to investigate the effect of silencing KDM1A on the inhibition cell viability, colony, migration, and invasion, respectively. Mechanistic investigations were carried out by Western blot and quantitative real-time polymerase chain reaction (qRT-PCR).
Results:
Vimentin staining was highly positive and cytokeratin staining was nearly negative in EESCs and EuESCs. KDM1A silencing reduced the ability of EESCs and EuESCs to proliferate (P < 0.001). The proliferation, motility, and invasiveness of EESCs and EuESCs were markedly reduced when KDM1A was silenced (P < 0.001). KDM1A silencing substantially downregulated invasion- and migration-related proteins or genes according to Western blot and qRT-PCR analysis (P < 0.05). EESCs and EuESCs with KDM1A silencing showed a higher reduction in these proteins than the control group (P < 0.05).
Conclusion:
In adenomyosis, silencing KDM1A can limit the motility, invasiveness, and proliferation of EuESCs and EESCs. These outcomes could potentially correlate with the decreased expression levels of matrix metalloproteinases (MMP)-2, MMP-9, Fascin, and Erzin proteins.
Keywords
adenomyosis
lysine-specific histone demethylase 1A
endometrial stromal cells
proliferation
metastasis migration
INTRODUCTION
The epidemiology and pathophysiology of adenomyosis (endometriosis interna), a benign uterine condition that is common in women worldwide, are not well known.[1-3] The condition manifests as subfertility, pelvic discomfort, and a soft, diffusely enlarged uterus with abnormal uterine bleeding.[4,5] About 20% of adenomyosis occurrences are documented in women under 40 years of age, with the remaining 80% occurring in women who are past the reproductive age (40–50 years).[6,7] Adenomyosis is defined as essentially having ectopic endometrium within the myometrium, and this definition is comparable to endometriosis, which is defined as having ectopic endometrium outside of the uterine cavity. Although their sites vary, the main causes of the two diseases – ectopic endometrium – remain the same. As a result, endometriosis interna is the term once used to describe adenomyosis,[8] presumably due to such a similarity. Unfortunately, few medications target adenomyopathy directly at present, making treatment difficult. Even though surgery is thought to be the best course of treatment for adenomyosis, it can be quite stressful for women who experience symptoms but are very desirous of becoming pregnant. Consequently, obtaining novel, efficient genes is critical to treat adenomyopathy. The histone lysine demethylase lysine-specific histone demethylase 1A (KDM1A), sometimes referred to as LSD1, was originally identified as demethylase H3K4me1/2 and H3K9me1/2 at target loci in a context-dependent manner.[9,10] KDM1A acts as a coactivator or corepressor in terms of substrate specificity.[11-13] However, the impact of KDM1A expression on the genesis of adenomyosis remains unknown. Numerous biological processes linked to the advancement of cancer have previously been demonstrated to require KDM1A, including proliferation,[14] epithelial-mesenchymal transformation,[15,16] senescence,[17] stem-cell pluripotency maintenance,[18,19] and multidrug resistance.[20] Literature reports on several KDM1A inhibitors, some of which are undergoing trials for small-cell lung cancer.[21,22] However, its role in advanced adenomyosis (endometriosis interna) is unknown. This study explored how KDM1A affects endometrial stromal cell (ESC) invasion, migration, and proliferation. The findings suggest a potential molecular pathway that controls KDM1A’s effects on cell invasion and migration. This research offers a potential course of treatment for advanced adenomyosis.
MATERIAL AND METHODS
Tissue collection
Human endometrial tissues were collected from 20 patients undergoing total hysterectomy for adenomyosis at Qingzhou People’s Hospital between November 2020 and October 2022. The median age of the patients was 44 years (range: 34–56 years). Histologic investigation verified the diagnosis, ruling out malignant cases. Each subject provided informed consent, and the Qingzhou People’s Hospital, approved the use of human tissues. This study was approved by the Ethics Committee of Qingzhou People’s Hospital, with approval No. 2023020. This study strictly adhered to the Helsinki Declaration.
Primary cell isolation and culture
Eutopic and ectopic endometrial tissues were extracted from the uterus of each patient and transferred to a cold mixture containing 1% penicillin–streptomycin antibiotics (15140148, Gibco, Rockville, MD, USA) and Dulbecco’s modified Eagle’s medium (DMEM; 11965092, Gibco, Rockville, MD, USA) in equal proportions. The tissues were initially rinsed with phosphate-buffered saline (PBS), minced into small pieces (1 mm3), and incubated at 37°C in a shaking water bath with 0.5% collagenase II for 1 h. The dispersed cells were filtered through a 100 μm mesh to remove any remaining tissue fragments. Centrifugation was conducted at 1000 rpm for 5 min to collect the eutopic and ectopic ESCs (eutopic endometrial stromal cells [EuESCs] and ectopic endometrial stromal cells [EESCs], respectively), followed by two to three washes with PBS. The EuESCs and EESCs were cultured in DMEM (12491015, Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; A5670701, Gibco, Rockville, MD, USA) and 1% penicillin-streptomycin antibiotic solution (15140148, Gibco, Rockville, MD, USA). The cells were maintained in culture dishes at 37°C in a humidified atmosphere containing 5% carbon dioxide (CO2) and 95% air. Cells from passages three to six were utilized for the subsequent experiments.
Cell characterization
A study has shown that cytokeratin and vimentin can be used to identify the characteristics of EuESCs and EESCs.[23] In summary, after cells were seeded onto chamber slides, they were fixed for 30 min at 4°C with 4% paraformaldehyde. Next, endogenous peroxidase activity was blocked for 10 min at room temperature using 3.0% hydrogen peroxide. Afterward, the cells were immunostained for 1 h using primary antibody vimentin (1:500, ab92547, Abcam, Cambridge, MA, USA) and cytokeratin (1:500, ab53280, Abcam, Cambridge, MA, USA) antibodies. The second antibody goat Anti-Rabbit IgG H&L (HRP, ab6721, Abcam, Cambridge, MA, USA) was incubated for 1h under light protection. Subsequently, 3,3’-diaminobenzidine (DAB, ab64238, Abcam, Cambridge, MA, USA) was used to visualize immunoreactivity. An immune response to vimentin and cytokeratin was detected in the cytoplasm, and cells with yellowish-brown staining were identified as positive. The cell lines used in this study underwent short tandem repeat authentication, and the mycoplasma test results were negative.
Cell transfection
GenePharma (Shanghai, China) created the small interfering RNAs (short hairpin RNAs [shRNAs]) specific to KDM1A (sh-KDM1A), the negative control (shNC), and the plasmids for KDM1A overexpression. The shRNA sequences 5’GGAAAGAATCAAGGAGG-3’ and 5’-GCTTGGAATGATCATCAAACA-3’ were used to target KDM1A and a non-targeting shRNA control (sh-NC), respectively. Lipofectamine 3000 (L3000150, Invitrogen, Carlsbad, CA, USA) was transfected into the EESCs and EuESCs. The transfection efficacy was assessed after 24 h using Western blot and quantitative real-time polymerase chain reaction (qRT-PCR).
Cell viability assay
Cell counting kit-8 (CCK-8) test (C0037, Beyotime, Shanghai, China) was utilized to assess the viability of EuESCs and EESCs. In a 96-well plate, 5 × 103 EuESCs or EESCs were planted. Following the prescribed intervention, 10 μL of CCK-8 reagent was added to each well to facilitate additional culture. Two hour later, the absorbance at 450 nm was measured using a microplate reader (iD5, SpectraMax, Molecular Devices, San Jose, California, USA).
Colony formation assay
First, EuESCs and EESCs were seeded at a low density of 500 cells per well into six well plates containing DMEM supplemented with 10% fetal bovine serum (FBS). The cells were incubated at 37°C in a humidified atmosphere with 5% CO2 until visible colonies formed, typically taking 1–2 weeks. Subsequently, the cells were fixed with 4% polyformaldehyde, followed by staining with 0.5% crystal violet solution for 15– 30 min. Images of the stained culture dishes were captured using an inverted light microscope (IX83, Olympus, Tokyo, Japan), and the number of colonies formed in each dish was counted.
Wound healing assay
The use of wound healing assay allowed for the detection of the migration potential of EuESCs and EESCs. In brief, six well plates were seeded with cells during the logarithmic phase, and the cells were then cultured until 95% cell fusion was reached. Next, the cell monolayer was gently scraped in a straight line using a disposable pipette tip. Following three rounds of washing in PBS, serum-free dulbecco’s modified eagle medium (DMEM) was used to culture EuESCs and EESCs at 37°C. Images of the wound site were taken using an optical microscope (IX83, Olympus, Tokyo, Japan) at 0–24 h. The width of the open area was then assessed right away using ImageJ (1.8.0, National Institutes of Health, Bethesda, MD, USA) to estimate motility.
Transwell assay
Matrigel invasion experiment was utilized to assess EuESC and EESC invasion. A Matrigel-coated upper chamber with 8 μm pore size was injected with resuspended EuESCs and EESCs in 200 μL serum-free media. DMEM with 10% FBS 600 μL, S9020, Solarbio, Beijing, China, was introduced to the lower chamber to serve as the chemoattractant. Following a 24-h incubation period, the cells in the invasion chamber’s bottom were fixed in 4% paraformaldehyde, dyed with crystal violet for 20 min, and captured on camera using a light microscope (IX83, Olympus, Tokyo, Japan).
qRT-PCR analysis
EuESCs and EESCs were treated with TRIzol reagent (15596026CN, Thermo Scientific, Wilmington, Massachusetts, USA) to extract the total RNA. Using the Reverse Transcription System Kit (4366597, Thermo Scientific, Wilmington, Massachusetts, USA), 1 μg of total RNA was used to create the first strand of cDNA. The QuantStudioTM5 RT-PCR System (Thermo Scientific) was used to detect the messenger RNA (mRNA) levels, and KAPA SYBR FAST UNI qPCR Kits (#KK4601, Kapa Biosystems, Boston, MA, USA) were used to quantify them. Every experiment was carried out in triplicate, with actin expression serving as the internal control. Calculated using the 2−△△CT method. The primer sequences are shown in Table 1.
| Name | Primer sequences |
|---|---|
| KDM1A | F:5’-GCTTGGCCAACCTCTCAGAA-3’ R:5’-GACAGTGTCAGCTTGTCCGTTG-3’ |
| Fascin | F:5’-CACAGGCAAATACTGGACGGT-3’ R:5’-CCACCTTGTTATAGTCGCAGAAC-3’ |
| Ezrin | F:5’-GCUCAAAGAUAAUGCUAUGTT-3’ R:5’-CAU AGCAUUAUCUUUGAGCTT-3’ |
| GAPDH | F:5’-GGAGCGAGATCCCTCCAAAAT-3’ R:5’-GGCTGTTGTCATACTTCTCATGG-3’ |
KDM1A: Lysine-specific histone demethylase 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, A: Adenosine, C: Cytosine, G: Guanin, T: Thymine
Western blot analysis
The total protein was extracted from the cells using radioimmunoprecipitation assay lysis buffer (P0013B, Beyotime, Shanghai, China). The Bicinchoninic Acid Assay (BCA) kit (P0009, Beyotime, Shanghai, China) was used to measure the protein concentration, and 40 μg of protein per lane was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis. Afterward, the proteins were placed on polyvinylidene fluoride membranes. The membranes were blocked for 2 h with 5% skim milk, and then, they were incubated for the entire night at 4°C with primary antibodies KDM1A (ab253727), Fascin (ab126772), Ezrin (ab40839), metalloproteinases (MMP)-2 (ab254516), MMP-9 (ab76003), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab8245). They were obtained from Abcam (Cambridge, MA, USA). The membranes were subjected to an incubation period using goat anti-rabbit secondary antibody (7074, CST, Ma, BSN, USA). Then, an enhanced chemiluminescence kit (BL520b, Biosharp Life Science, Hefei, Anhui, China) and BeyoImager™ 600 Chemiluminescent Imaging System (EI600, Beyotime Biotechnology, Shanghai, China) were used to observe the bands, and ImageJ software (version 3.0, NIH, Bethesda, MD, USA) was used for analysis.
Statistical analysis
Data analysis was performed using SPSS (version 16.0, IBM Corporation, New York City, NY, USA) statistical software. One-way analysis of variance with Tukey’s multiple comparison test was used for multiple comparisons. P < 0.05 indicated statistical significance.
RESULTS
EuESCs and EESCs’ phenotypic traits were determined
Vimentin and cytokeratin immunocytochemical staining was performed to determine if the separated cells were ESCs. The cytokeratin positive signal of EuESCs and EESCs was weak, while the mesenchymal cell marker vimentin positive signal was strong, indicating that these cells were ESCs [Figure 1a and b].
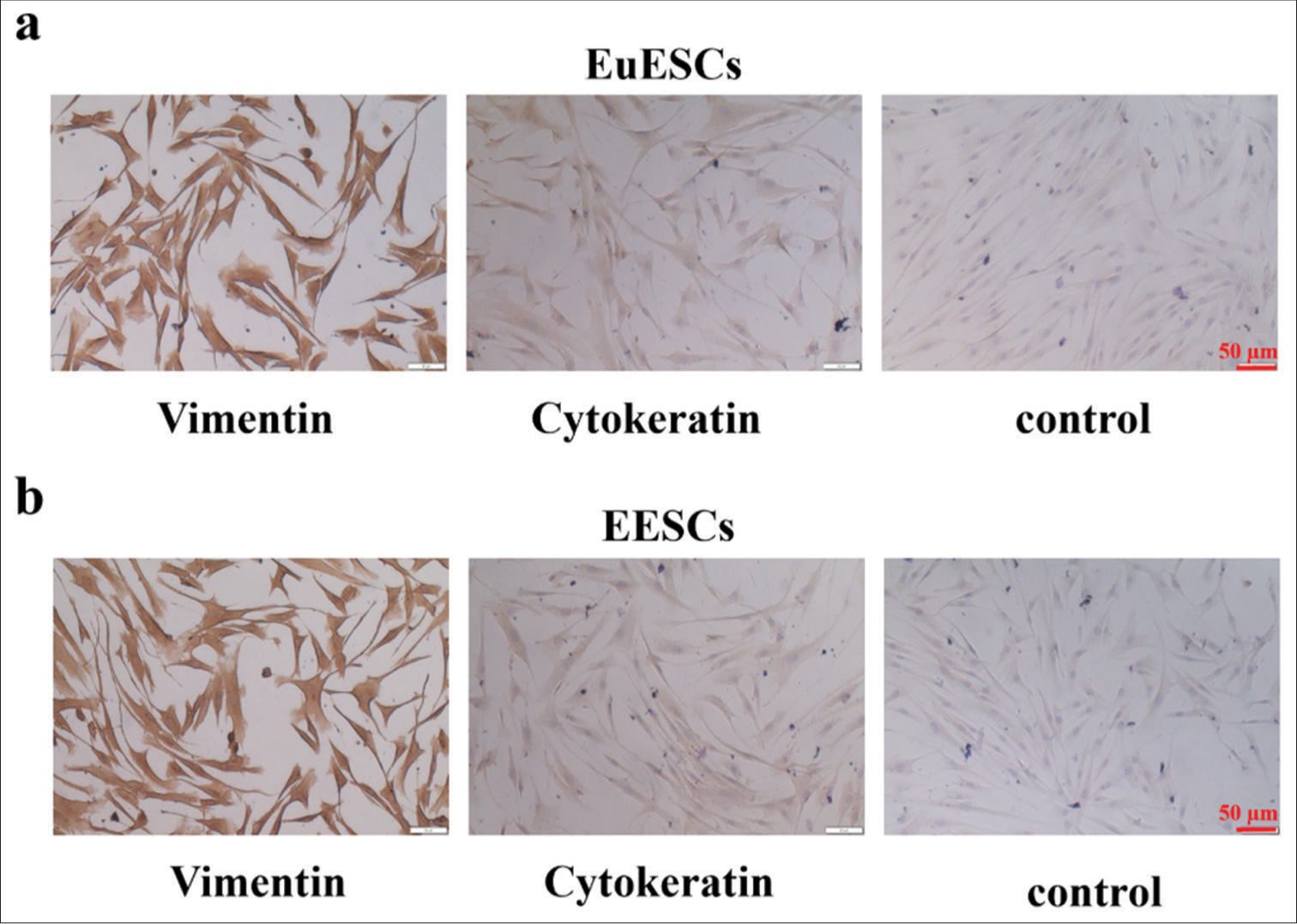
- EuESCs and EESCs’ phenotypic traits were determined. (a) EuESCs’ phenotypic traits of vimentin and cytokeratin. (b). EESCs’ phenotypic traits of vimentin and cytokeratin. n = 6. (EESCs: Ectopic endometrial stromal cells, EuESCs: Eutopic endometrial stromal cells.)
KDM1A expression was significantly increased in ectopic endometrial tissues
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to identify important histone methylation modifiers that regulate ectopic endometrium. The results showed that ectopic endometrial tissues had significantly greater levels of KDM1A mRNA expression than the adherent tissues [Figure 2a]. Therefore, KDM1A may be involved in the upkeep of ectopic endometrial illness. Next, Western blot was applied to detect KDM1A protein expression in ectopic endometrial tissues (12 cases) and normal tissues (11 cases). The ectopic endometrial disease tissues had higher levels of KDM1A protein expression than the normal tissues, as shown in Figure 2b and c. These findings showed that KDM1A is crucial to the development of ectopic endometrial disease (P < 0.01).
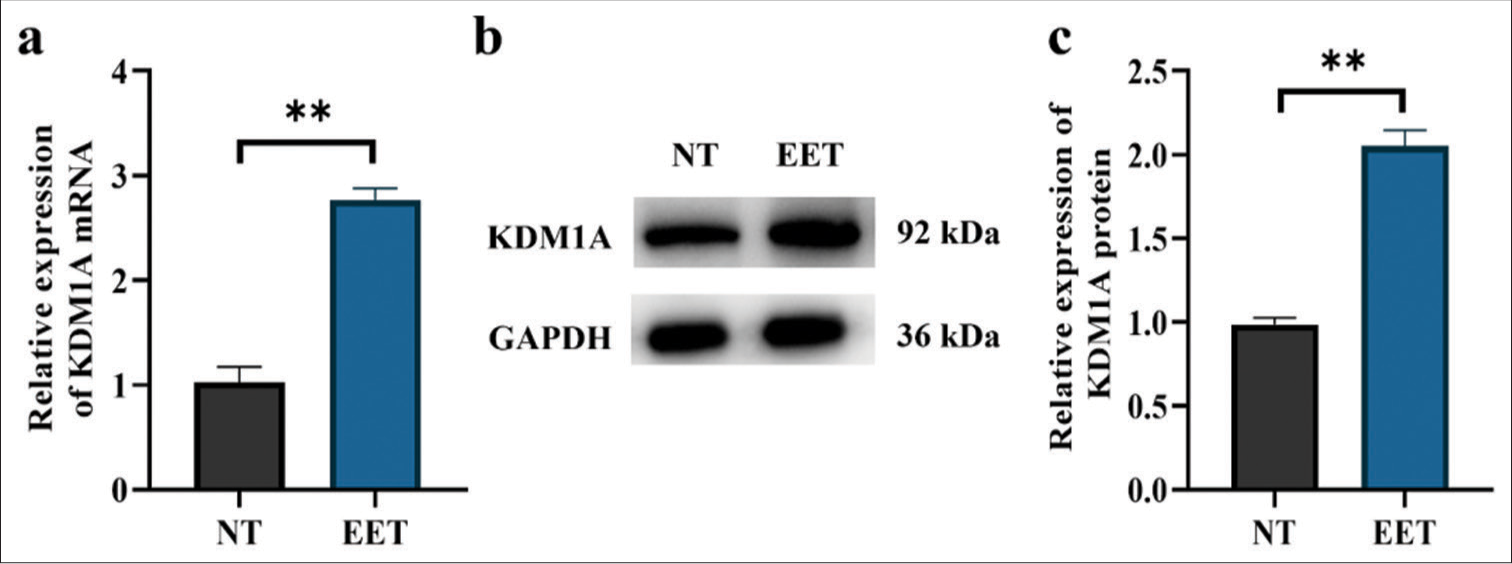
- KDM1A expression was significantly increased in ectopic endometrial tissues. (a) KDM1A expression in endometrium tissues. (b and c) KDM1A protein expression tested by Western blot. n = 20. **P < 0.01. (KDM1A: Lysine-specific histone demethylase 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, NT: Normal tissues, EET: Ectopic endometrial tissues)
KDM1A promoted the proliferation of EuESCs and EESCs
The overexpression of KDM1A in EuESCs and EESCs was determined. The Western blot and qRT-PCR results showed significantly higher expression of KDM1A in the OE-KDM1A group than in the vector (P < 0.001) [Figure 3a-f]. The enhanced cellular viability due to KDM1A overexpression in EuESCs and EESCs was validated. Figure 3g demonstrated that the KDM1A overexpression group had markedly increased cell viability as compared with the vector in EuESCs (P < 0.001). The effects of KDM1A on the proliferation of EuESCs were evaluated using colony formation assay. Compared with vector, KDM1A overexpression significantly enhanced colony formation (P < 0.001) [Figure 3h-i]. In EESCs, cell viability and colony formation showed the same trend as in EuESCs (P < 0.001) [Figure 3j-l].
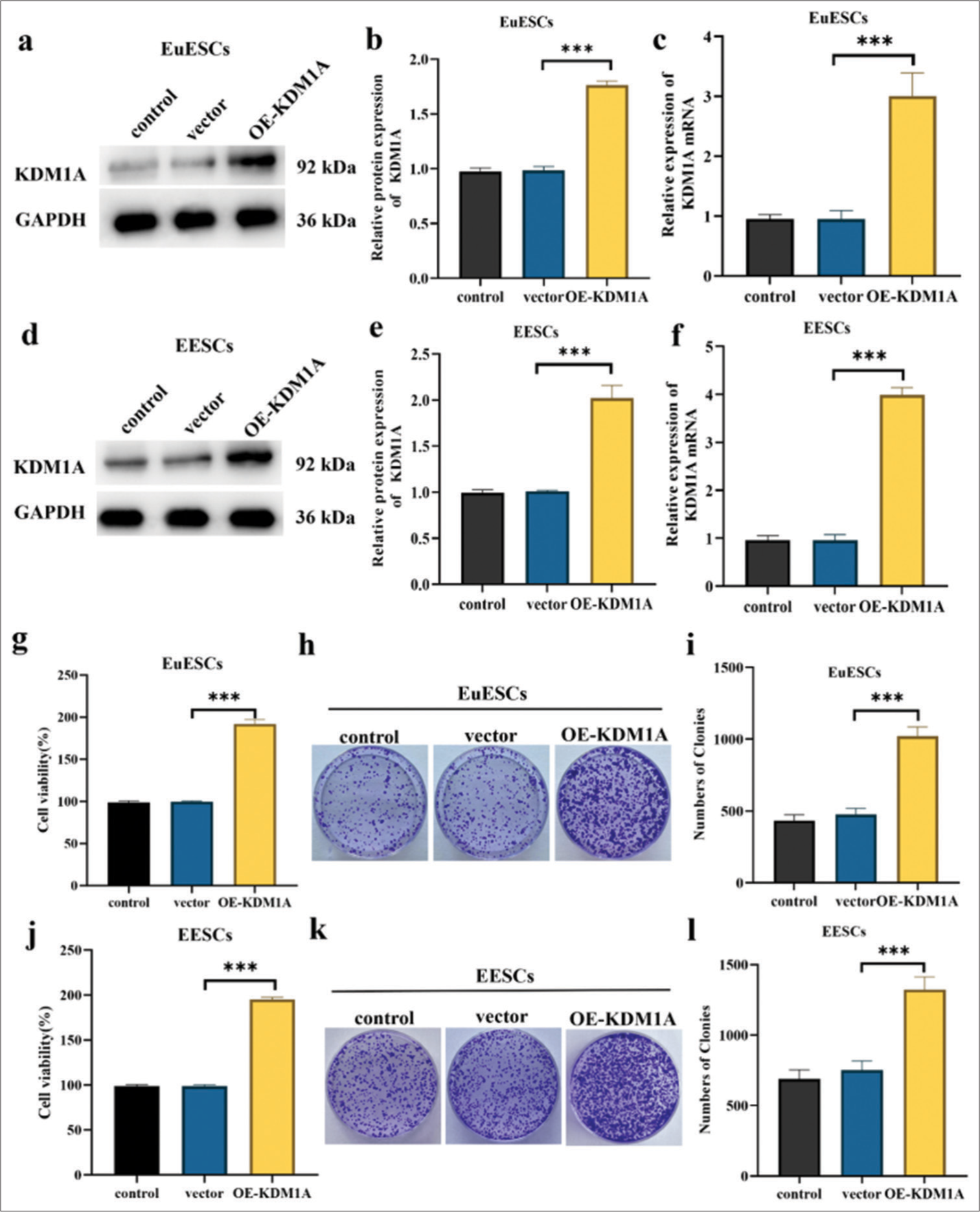
- KDM1A promoted proliferation of EuESCs and EESCs. (a-c) Western blot and qRT-PCR determination of the mRNA and protein expression levels of KDM1A in EuESCs. (d-f) Western blot and qRT-PCR determination of the mRNA and protein expression levels of KDM1A in EESCs. (g) Cell viability of EuESCs determined by CCK-8 assay. Control group 100% cell viability as control. (h-i) Colony formation capacity of EuESCs. (j) Cell viability of EuESCs determined by CCK-8 assay. Control group 100% cell viability as control. (k-l) Cell viability of EESCs determined by CCK-8 assay. n = 6. ***P < 0.001. (KDM1A: Lysine-specific histone demethylase 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, EESCs: Ectopic endometrial stromal cells, EuESCs: Eutopic endometrial stromal cells.)
KDM1A promoted the migration and invasion of EuESCs and EESCs
The effects of KDM1A on the proliferation of EuESCs and EESCs were determined by scratch and transwell assays. Figure 4a-h shows that the overexpression of KDM1A significantly increased the migration and invasion abilities of EuESCs and EESCs (P < 0.01, P < 0.001).
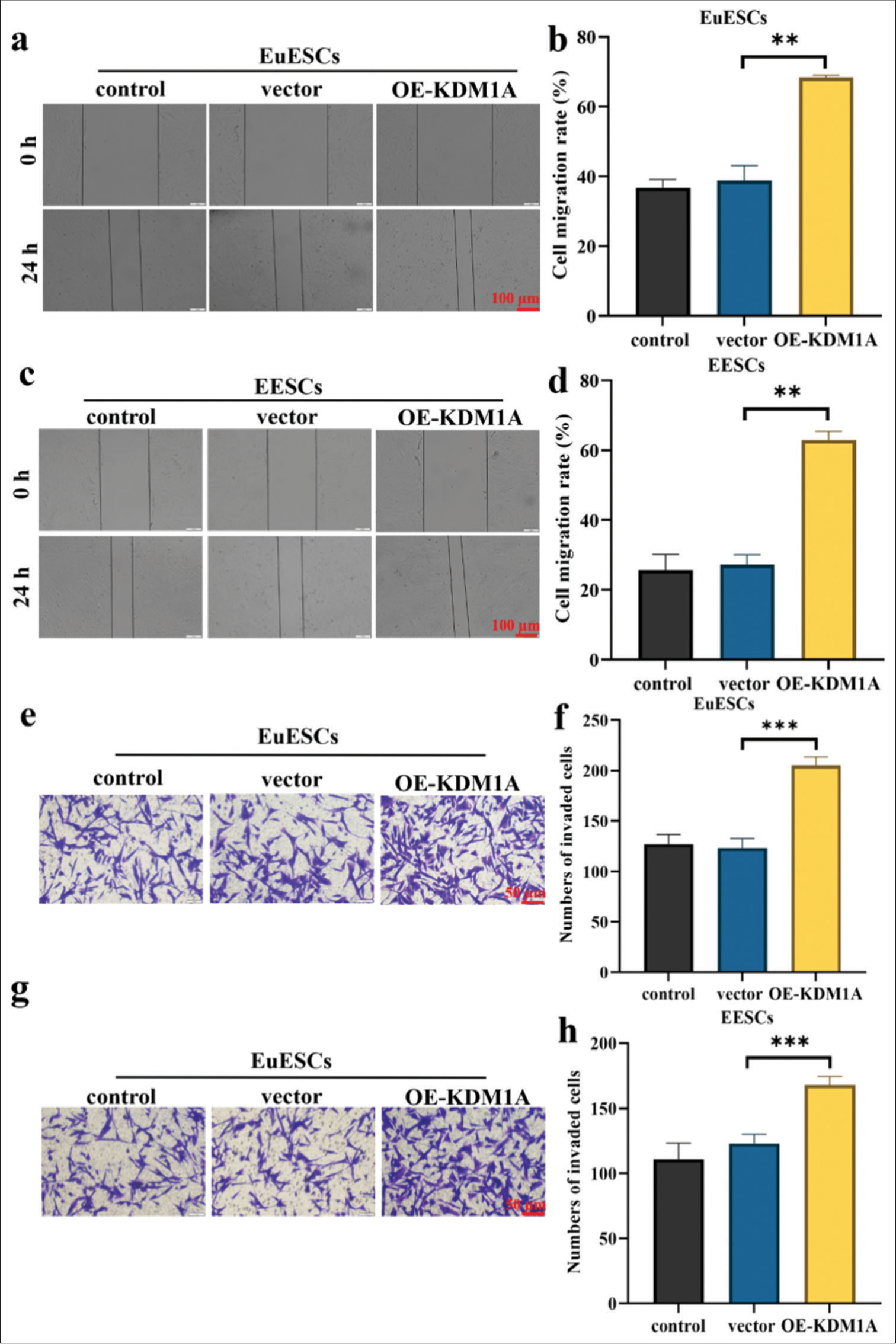
- KDM1A promoted migration and invasion of EuESCs and EESCs. (a-d) Cell migration assay. (e-h) Cell invasion assay. n = 6. **P < 0.01, ***P < 0.001. (KDM1A: Lysine-specific histone demethylase 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, EESCs: Ectopic endometrial stromal cells, EuESCs: Eutopic endometrial stromal cells.)
Silenced KDM1A inhibited the proliferation of EuESCs and EESCs in vitro
KDM1A expression was suppressed in EuESC and EESC lines using shRNAs to corroborate the control of KDM1A. The KDM1A knockdown group showed a decrease in KDM1A expression in EuESCs and EESCs according to qRT-PCR and Western blot (P < 0.05) [Figure 5a-f]. Figure 5g-l illustrates how the KDM1A silence group’s EuESC and EESC activity and proliferation were much lower than those of the sh-NC group (P < 0.001). The findings implied that silencing KDM1A can inhibit the proliferation of EESC and EuESC.
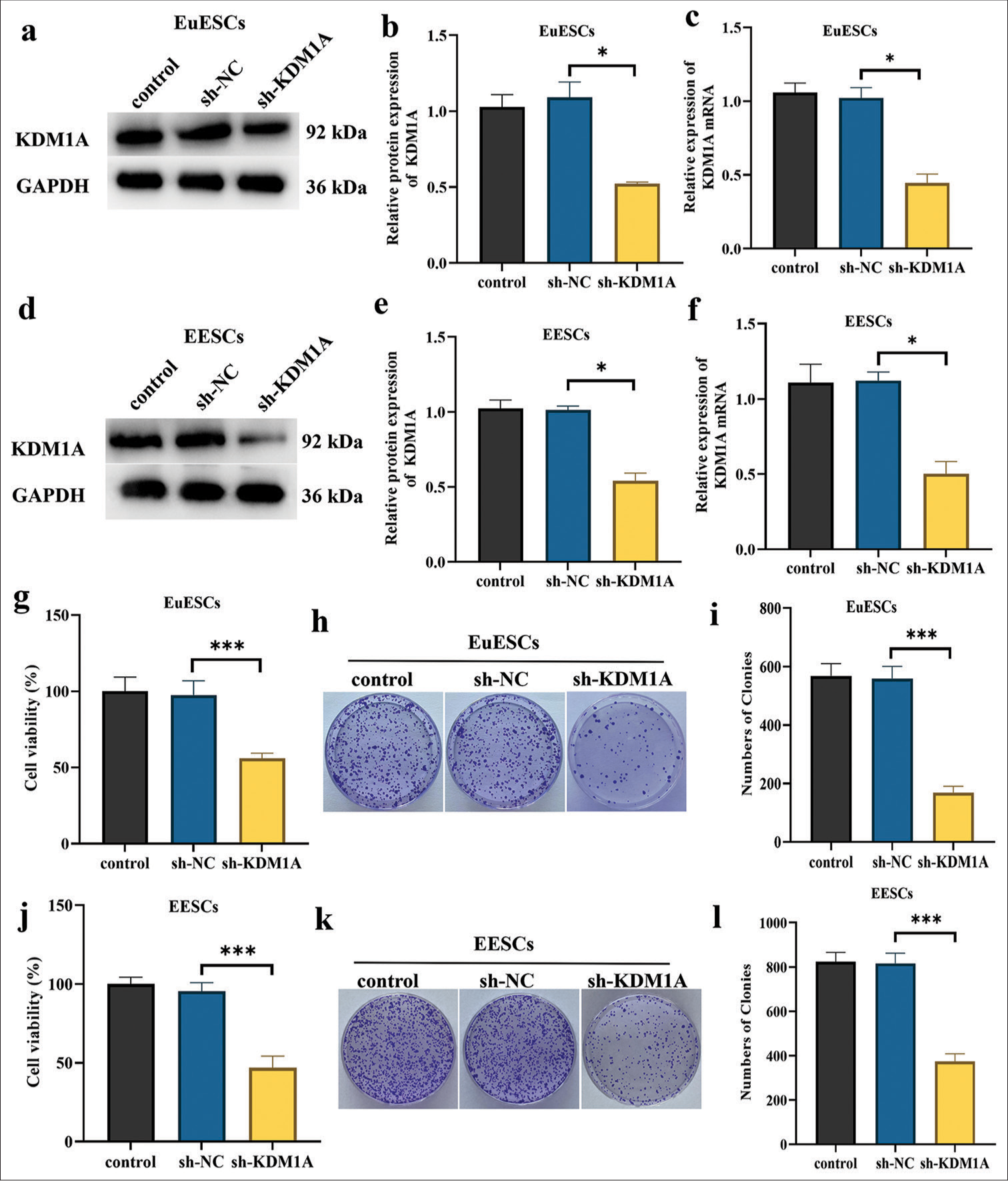
- Silencing KDM1A inhibited the proliferation of EuESCs and EESCs in vitro. (a-c). Western blot and qRT-PCR determination of the mRNA and protein expression levels of KDM1A in EuESCs. (d-f). Western blot and qRT-PCR determination of the mRNA and protein expression levels of KDM1A in EESCs. (g). Cell viability of EuESCs determined by CCK-8 assay. Control group 100% cell viability as control. (h-i). Cell migration assay of EuESCs. (j). Cell viability of EuESCs determined by CCK-8 assay. Control group 100% cell viability as control. (k-l) Cell viability of EESCs determined by CCK-8 assay. n = 6. *P < 0.05, ***P < 0.001. (KDM1A: Lysine-specific histone demethylase 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, EESCs: Ectopic endometrial stromal cells, EuESCs: Eutopic endometrial stromal cells, qRT-PCR: quantitative real-time polymerase chain reaction)
KDM1A silencing suppressed the migration of EuESCs and EESCs
The EuESCs and EESCs were transfected with sh-KDM1A, control, and sh-NC for 24 h before the cell experiment. The results of wound healing assay demonstrated that compared with the sh-NC, the migration of EuESCs and EESCs was significantly reduced in the sh-KDM1A group (P < 0.001) [Figure 6a-d]. As shown in Figure 6e-j, qRT-PCR and Western blot analysis suggested that KDM1A silencing reduced the expression levels of Fascin and Ezrin proteins in EuESCs and EESCs (P < 0.05). Research indicated that the expression levels of Fascin and Ezrin proteins are positively correlated with the metastasis of non-small cell lung cancer.[24] These findings showed that KDM1A silencing had a greater suppressive effect on the migration of EuESCs and EESCs than sh-NC treatment.
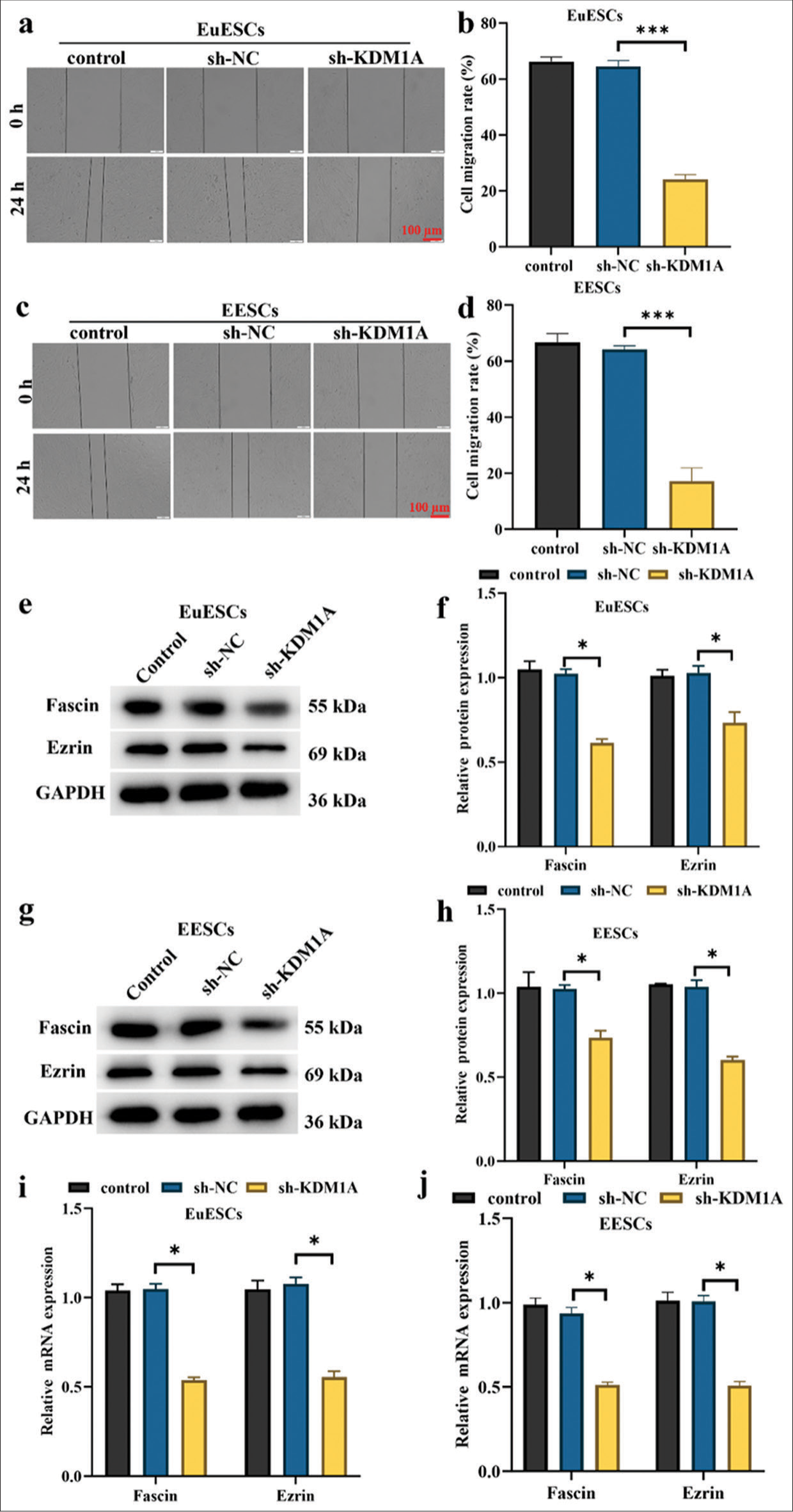
- KDM1A silencing suppressed the migration of EuESCs and EESCs. (a-d) Cell migration assessed by wound healing assay. (e-h) Western blot determination of the expression levels of Fascin and Ezrin. (i and j) qRT-PCR evaluation of Fascin and Ezrin. n = 6. *P < 0.05, ***P < 0.001. (KDM1A: Lysine-specific histone demethylase 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, EESCs: Ectopic endometrial stromal cells, EuESCs: Eutopic endometrial stromal cells.)
KDM1A silencing inhibited the invasion in EuESCs and EESCs
The findings demonstrated that KDM1A silencing exerted a higher inhibition of invasion on EuESCs and EESCs than control treatment (P < 0.001, [Figure 7a-d]). This suggests that after KDM1A silencing treatment, the number of invaded cells in both EuESCs and EESCs was greatly reduced (P < 0.001). After EuESCs and EESCs were transfected with sh-KDM1A, the expression levels of MMP-2 and MMP-9 proteins were significantly decreased (P < 0.05, [Figure 7e-h]. Xu et al.’s[25] study suggested that the upregulation of MMP-2 and MMP-9 protein expression levels is positively correlated with the migration and invasion of EESCs. These findings suggest that KDM1A silencing inhibited the invasion of EuESCs and EESCs.
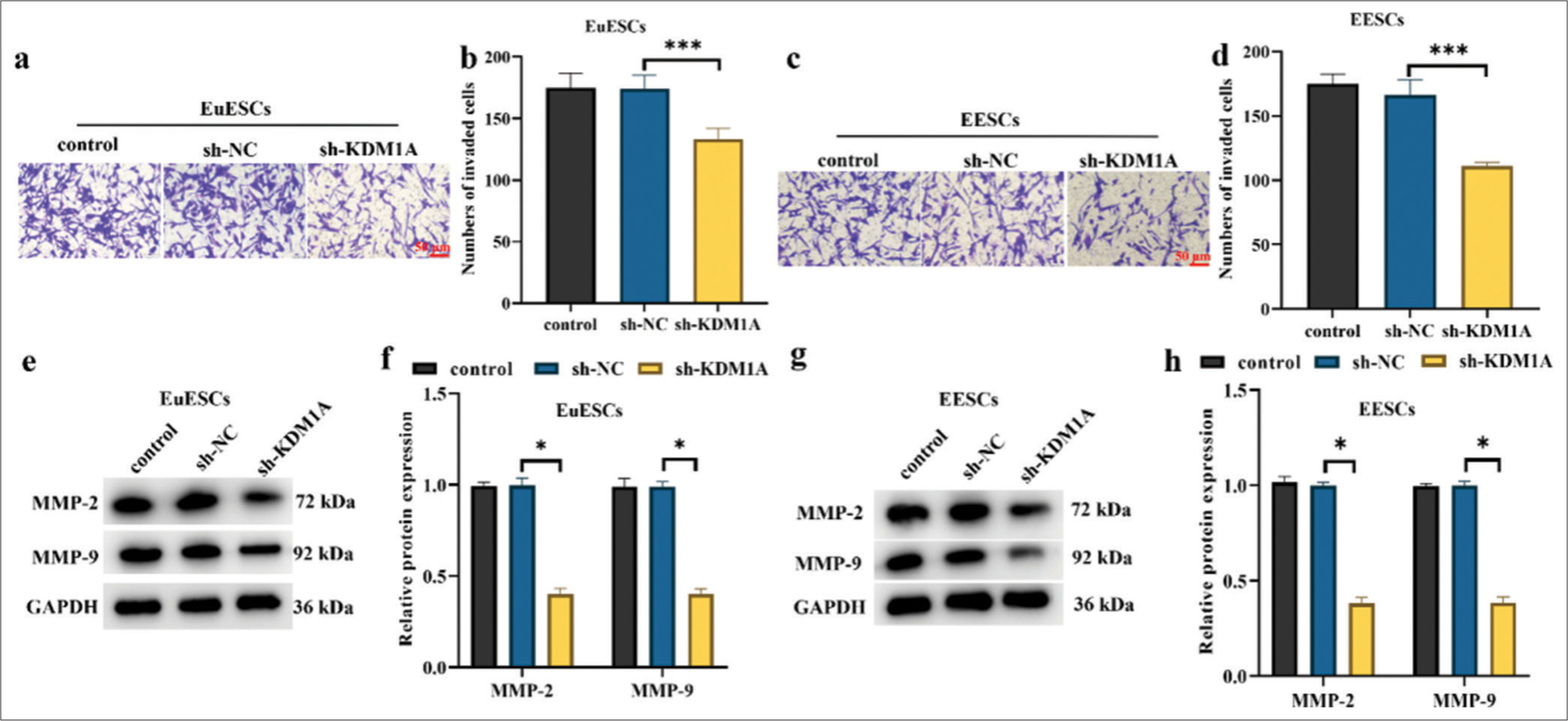
- KDM1A silencing inhibited the invasion in EuESCs and EESCs. (a-d) Representative images and bar graphs for Tanswell invasion assay in EuESCs and EESCs. (e-h) Western blot detection of invasion markers in EuESCs and EESCs. n = 6. *P < 0.05, ***P < 0.001. (KDM1A: Lysine-specific histone demethylase 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, EESCs: Ectopic endometrial stromal cells, EuESCs: Eutopic endometrial stromal cells.)
DISCUSSION
This work showed that patients with ectopic endometrial disease had overexpressed KDM1A. The proliferation, migration, and invasion of EuESCs and EESCs were facilitated by KDM1A upregulation. KDM1A also showed a favorable correlation with the expression levels of invasion and migratory proteins. However, the invasion, migration, and proliferation of EuESCs and EESCs were inhibited by KDM1A silencing.
Among the gynecological disorders that frequently coexist are adenomyosis and endometriosis.[26] Some studies estimated that 80–90% of patients with endometriosis have adenomyosis.[27] Less than half of the participants with endometriosis in other investigations had adenomyosis.[28] The authors presented evidence of a variation in the occurrence of endometriosis based on the myometrial location of the adenomyosis lesion.[29,30] In vivo, adenomyotic Eutopic endometrium stromal fibroblasts (eSFs) exhibited reduced caveolin (CAV1) expression, and eSF and eEC depletion of CAV1 exhibited markedly increased proliferation, migration, and invasion.[31] The enlargement of the nearby ectopic myometrium indicated that cell proliferation in adenomyosis tissues is a frequent phenomenon.[30] EuESCs and EESCs isolated from each patient’s ectopic and ectopic endometrial tissues were chosen in the present study.
KDM1A is essential for multiple oncogenic processes, including epithelial-mesenchymal transition; chemoresistance; and invasion, migration, proliferation, and survival of cells.[32] KDM1A is significantly expressed in many tumor types and closely linked to the development of malignancies.[33] The present work investigated the critical role that KDM1A plays in the development of adenomyosis. The results showed that ectopic endometrial tissues had a high expression of KDM1A. Additional mechanism exploration studies revealed that KDM1A overexpression may enhance the malignant biological characteristics of EuESCs and EESCs by promoting proliferation, migration, and invasion. Research demonstrated that KDM1A silencing enhanced the effectiveness of anti-CD47/PDL1 immunotherapy in the treatment of cervical cancer.[34] The present study showed that KDM1A silencing inhibited cell proliferation, migration, and invasion, consistent with previous reports.
The histone demethylase activity of KDM1A can activate or repress genes involved in adipocyte differentiation.[35] By demethylating H3K4me1/2, KDM1A can inhibit the transcription of genes that suppress adipogenesis. KDM1A may interact with key adipogenic transcription factors, such as peroxisome proliferator-activated receptor gamma and CCAAT/enhancer-binding protein alpha, by modulating their activity and promoting the expression of adipogenic genes.[36] Besides histones, KDM1A can demethylate non-histone proteins, affecting various signaling pathways and protein–protein interactions that are crucial for adipocyte differentiation and lipid metabolism.[37] KDM1A-mediated histone demethylation can lead to epigenetic reprogramming of adipocyte precursor cells, thereby enhancing their differentiation into mature adipocytes and impacting cell identity and function.[38]
This study has several limitations. First, the primary EESCs and EuESCs were isolated from patients undergoing complete hysterectomy for adenomyosis, which may limit the generalizability of the findings to all patients with adenomyosis. Second, while in vitro assays provide valuable insights, they may not fully replicate the complex in vivo environment. Therefore, the observed effects of KDM1A silencing on cell proliferation, migration, and invasion need to be validated in animal models or clinical settings. Third, the study primarily focused on KDM1A’s role in regulating specific proteins, such as MMP-2, MMP-9, Fascin, and Erzin, potentially overlooking other pathways and mechanisms that may contribute to the observed cellular behavior. Finally, the sample size for primary cell cultures may be limited, which could impact the statistical power and reproducibility of the results. Future research should aim to address these limitations by expanding the scope and depth of investigation. Larger and more diverse patient cohorts should be included to enhance the generalizability of the findings. In vivo studies using animal models of adenomyosis could provide a more comprehensive understanding of KDM1A’s role in disease progression and its potential as a therapeutic target. Further mechanistic studies should explore additional pathways and molecular interactions affected by KDM1A to uncover a broader spectrum of its biological functions. Investigating the long-term effects of KDM1A inhibition in vivo could be crucial to determine its potential therapeutic efficacy and safety. Finally, exploring combinatorial therapeutic approaches that target KDM1A, along with other key molecules involved in adenomyosis, could yield more effective treatment strategies for this condition.
SUMMARY
KDM1A expression was upregulated in EuESCs and EESCs, promoting invasion, migration, and proliferation. However, more in vivo research is needed to assess KDM1A’s possible therapeutic impact and potential signaling pathways in adenomyosis.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
KDM1A – Lysine-specific histone demethylase 1A
ESCs – Endometrial stromal cells
EESCs – Ectopic endometrial stromal cells
EuESCs – Eutopic endometrial stromal cells
CCK-8 – Counting Kit-8
qRT-PCR – Quantitative real-time polymerase chain reaction
MMP – Matrix metalloproteinase
DMEM – Dulbecco’s modified Eagle’s medium
PBS – Phosphate-buffered saline
FBS – Fetal bovine serum
DAB 3 – 3’-diaminobenzidine
BCA – Bicinchoninic Acid Assay
SDS-PAGE – Sodium dodecyl sulfate polyacrylamide gel
PVDF – Polyvinylidene fluoride
ECL – Enhanced chemiluminescence
AUTHOR CONTRIBUTIONS
LMC and RQL: Designed the research study; SPZ and CMS: Performed the research; LMC and SPZ: Collected and analyzed the data. All authors have been involved in drafting the manuscript and all authors have been involved in revising it critically for important intellectual content. All authors give final approval of the version to be published. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the ethics committee of Qingzhou People’s Hospital, approval No. 2023020. This study obtained informed consent from the patients and strictly adhered to the Helsinki Declaration.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Recent advances in understanding and managing adenomyosis. F1000Res. 2019;8:F1000. Faculty Rev-283
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod Biomed Online. 2017;35:592-601.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil Steril. 2018;109:371-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and management of abnormal uterine bleeding. Med J Malaysia. 2022;77:374-83.
- [Google Scholar]
- Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril. 2018;109:380-8.e381.
- [CrossRef] [PubMed] [Google Scholar]
- Adenomyosis: Disease, uterine aging process leading to symptoms, or both? Facts Views Vis Obgyn. 2020;12:91-104.
- [Google Scholar]
- The pathogenesis of adenomyosis vis-à-vis endometriosis. J Clin Med. 2020;9:485.
- [CrossRef] [PubMed] [Google Scholar]
- Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941-53.
- [CrossRef] [PubMed] [Google Scholar]
- LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436-9.
- [CrossRef] [PubMed] [Google Scholar]
- KDM1A inhibition augments the efficacy of rapamycin for the treatment of endometrial cancer. Cancer Lett. 2022;524:219-31.
- [CrossRef] [PubMed] [Google Scholar]
- KDM1A microenvironment, its oncogenic potential, and therapeutic significance. Epigenetics Chromatin. 2018;11:33.
- [CrossRef] [PubMed] [Google Scholar]
- LSD1: Biologic roles and therapeutic targeting. Epigenomics. 2016;8:1103-16.
- [CrossRef] [PubMed] [Google Scholar]
- Long intergenic non-protein-coding RNA 01446 facilitates the proliferation and metastasis of gastric cancer cells through interacting with the histone lysine-specific demethylase LSD1. Cell Death Dis. 2020;11:522.
- [CrossRef] [PubMed] [Google Scholar]
- Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene. 2010;29:4896-904.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibiting interactions of lysine demethylase LSD1 with snail/slug blocks cancer cell invasion. Cancer Res. 2013;73:235-45.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting the senescence-overriding cooperative activity of structurally unrelated H3K9 demethylases in melanoma. Cancer Cell. 2018;33:322-36.e328.
- [CrossRef] [PubMed] [Google Scholar]
- The deubiquitinase USP28 stabilizes LSD1 and confers stem-cell-like traits to breast cancer cells. Cell Rep. 2013;5:224-36.
- [CrossRef] [PubMed] [Google Scholar]
- Lysine specific demethylase 1 inactivation enhances differentiation and promotes cytotoxic response when combined with all-trans retinoic acid in acute myeloid leukemia across subtypes. Haematologica. 2019;104:1156-67.
- [CrossRef] [PubMed] [Google Scholar]
- Lysine-specific demethylase 1 promotes the stemness and chemoresistance of Lgr5(+) liver cancer initiating cells by suppressing negative regulators of b-catenin signaling. Oncogene. 2015;34:3188-98.
- [CrossRef] [Google Scholar]
- Therapeutic potential of targeting LSD1/KDM1A in cancers. Pharmacol Res. 2022;175:105958.
- [CrossRef] [PubMed] [Google Scholar]
- LSD1/KDM1A, a gate-keeper of cancer stemness and a promising therapeutic target. Cancers (Basel). 2019;11:1821.
- [CrossRef] [PubMed] [Google Scholar]
- Establishment of an immortalized endometriotic stromal cell line from human ovarian endometrioma. Reprod Sci. 2020;27:2082-91.
- [CrossRef] [PubMed] [Google Scholar]
- Increases in mRNA and protein levels of the genes for the actin-binding proteins profilin, fascin, and ezrin promote metastasis in non-small cell lung cancer. Mol Biol (Mosk). 2020;54:285-92.
- [CrossRef] [PubMed] [Google Scholar]
- Quercetin inhibits adenomyosis by attenuating cell proliferation, migration and invasion of ectopic endometrial stromal cells. Drug Des Devel Ther. 2020;14:3815-26.
- [CrossRef] [PubMed] [Google Scholar]
- Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666-82.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic signs of adenomyosis are prevalent in women undergoing surgery for endometriosis and may suggest a higher risk of infertility. Biomed Res Int. 2017;2017:8967803.
- [CrossRef] [PubMed] [Google Scholar]
- How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012;27:3432-9.
- [CrossRef] [PubMed] [Google Scholar]
- Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207:114.e111-7.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32:1393-401.
- [CrossRef] [PubMed] [Google Scholar]
- The expression and functionality of stromal caveolin 1 in human adenomyosis. Hum Reprod. 2013;28:1324-38.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting lysine-specific demethylase 1 (KDM1A/LSD1) impairs colorectal cancer tumorigenesis by affecting cancer cells stemness, motility, and differentiation. Cell Death Discov. 2023;9:201.
- [CrossRef] [PubMed] [Google Scholar]
- KDM1A and KDM3A promote tumor growth by upregulating cell cycle-associated genes in pancreatic cancer. Exp Biol Med (Maywood). 2021;246:1869-83.
- [CrossRef] [PubMed] [Google Scholar]
- LSD1 silencing contributes to enhanced efficacy of anti-CD47/PD-L1 immunotherapy in cervical cancer. Cell Death Dis. 2021;12:282.
- [CrossRef] [PubMed] [Google Scholar]
- Candidate biomarkers in brown adipose tissue for postmortem diagnosis of fatal hypothermia. Int J Legal Med. 2024;138:61-72.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic histone modulations of PPARg and related pathways contribute to olanzapine-induced metabolic disorders. Pharmacol Res. 2020;155:104703.
- [CrossRef] [PubMed] [Google Scholar]
- LSD1 mediates microbial metabolite butyrate-induced thermogenesis in brown and white adipose tissue. Metabolism. 2020;102:154011.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological inhibition of KDM1A/LSD1 enhances estrogen receptor beta-mediated tumor suppression in ovarian cancer. Cancer Lett. 2023;575:216383.
- [CrossRef] [PubMed] [Google Scholar]








