Translate this page into:
Introduction to the second edition of ‘Diagnostic Cytopathology of Serous Fluids’ as CytoJournal Monograph (CMAS) in Open Access

*Corresponding author: Vinod B. Shidham, MD, FIAC, FRCPath, Department of Pathology, Wayne State University School of Medicine, Karmanos Cancer Center and Detroit Medical Center, Detroit, Michigan, United States. vshidham@med.wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Shidham VB. Introduction to the second edition of ‘Diagnostic Cytopathology of Serous Fluids’ as CytoJournal Monograph (CMAS) in Open Access. CytoJournal 2021;18:30.
Abstract
Serous fluids are excessive accumulation of fluids in a serous cavity as effusion. However, traditionally this area also covers cytopathologic evaluation of washings of these cavities including pelvic/peritoneal washing.
This is the introductory review article in series on this topic with the application of simplified algorithmic approaches. The series would be compiled finally as a book after minor modifications of individual review articles to accommodate the book layout on the topic as second edition of ‘Diagnostic Cytopathology of Serous Fluids’ book.
The approach is primarily directed towards detection of neoplastic cells based on morphology alone or with the help of various ancillary tests, including commonly applied immunocytochemistry to be interpreted as second foreign population with application of SCIP (subtractive coordinate immunoreactivity pattern) approach in effusion fluid tapings. As the role of molecular pathology tests is increasing, this component as ancillary testing will also be covered as applicable. Because a picture and sketches are worth a thousand words, illustrations and figures are included generously even at the risk of moderate repetition.
The clinically important serous cavities include peritoneal cavity, pericardial cavity, and two pleural cavities. The primary topic of this series is specimens from these cavities as effusion fluids and washings including cytopathologic evaluation of peritoneal/pelvic washing.
It is expected that some readers may not read the entire series or the final book from beginning to end, but refer to the individual review articles and chapters sporadically during their clinical practice. Considering this practical limitation, some brief repetition may be observed throughout the book. Some of the important themes will be highlighted as italicized and bolded text for quick reference.
Dedicated articles/chapters are assigned for technical and other reference material as appendices. Tables, algorithms, sketches, and combination of pictures are included generously for quick reference. Most of the illustrations are attempted to be labeled appropriately with arrows and other indicators to avoid equivocation, especially for beginners in the field.
This introductory review article describes general details under the following three broad headings:
Histology and general cytology of serous cavity lining
Effusion (general considerations)
Ancillary techniques in brief.
Keywords
Serous fluid
diagnostic cytopathology
effusion
tapping
paracentesis
immunohistochemistry
IHC
molecular pathology
SCIP approach
CellBlockistry
cell-block
cellblock

PREAMBLE
Effusion in this series refers to the accumulation of an excessive amount of fluid in a serous cavity. As reflected in the title, the final book is a compilation of various review articles embarking on a diagnostic approach to cytopathologic evaluation of serous cavity effusions and washings. Simplified algorithmic approach for interpretation of cells in effusions is practical for all readers from beginner in the field to the experts. The discussion will be primarily directed towards the detection of neoplastic cells based on morphology alone, or with the help of various ancillary tests, including commonly applied immunocytochemistry/immunohistochemistry.
The major serous cavities are [Figure 1] the peritoneal cavity, the pericardial cavity, and the two pleural cavities. Effusions from these cavities and related cytology specimens is the subject of the final book. Cytopathologic evaluation of peritoneal washings is referred to periodically with a dedicated article/chapter on the topic.
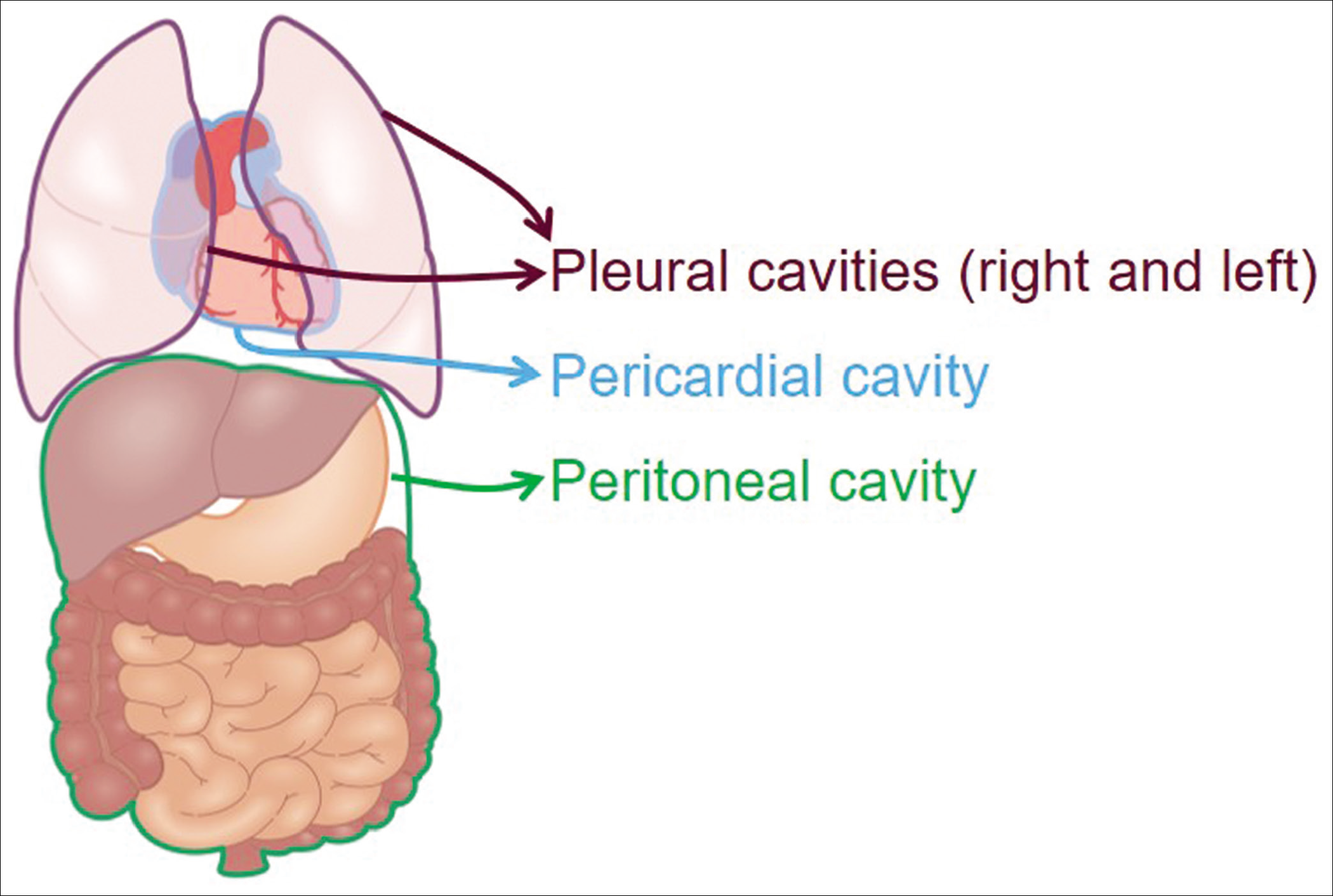
- Four major serous cavities.
The final book is predominantly focused on diagnostic application of cytomorphology with or without ancillary support by other methods such as immunocytochemistry.
A separate article/chapter will be dedicated to an overview of molecular and other special techniques including general technical and other reference material related to effusions.
It is expected that some readers may not read the series/ book from beginning to end, and choose to refer to the individual articles/chapters periodically during their clinical practice. Keeping this in mind, some of the themes and features will be repeated now and then in brief. To emphasize their significance, some of these important themes will be highlighted as italicized and bolded text for quick reference.
The concepts and information are compiled in tables, algorithms, sketches, and combination pictures as a quick reference guide for readers. Most of the illustrations are labeled with arrows and other indicators to avoid equivocation, especially for beginners in the field.
This introductory article/chapter describes general details under the following three headings:
Histology and general cytology of serous cavity lining
Effusion (general considerations)
Ancillary techniques in brief.
HISTOLOGY AND GENERAL CYTOLOGY OF SEROUS CAVITY LINING
The histologic and cytomorphologic features associated with various serous cavities and related fluids are similar without any site-specific characteristics.
Histology
The mesothelium forms a parietal and visceral layer in each cavity, where it is reflected over the organs therein. It consists of a flat monolayer of mesothelial cells, which have a tendency to undergo hypertrophy and hyperplasia secondary to various stimuli, usually resulting in a somewhat cuboidal appearance [Figure 2]. Such ‘reactive’ mesothelial cells frequently exfoliate into serous effusions. Although derived from mesoderm, mesothelial cells possess many of the morphologic and biologic features of epithelial cells [see Figure 2].
![Histology of serous lining (inguinal hernia sac). The mesothelial cells lining the fibrous tissue are flat (1). Focal reactive changes are seen as hypertrophy of some cells, which assume a cuboidal contour (2,3). [a–d, HE stain (a, 10×; b–d, 100×).]](/content/105/2021/18/1/img/Cytojournal-18-30-g002.png)
- Histology of serous lining (inguinal hernia sac). The mesothelial cells lining the fibrous tissue are flat (1). Focal reactive changes are seen as hypertrophy of some cells, which assume a cuboidal contour (2,3). [a–d, HE stain (a, 10×; b–d, 100×).]
Underlying the mesothelial cells of each serous cavity is a thin layer of fibrous connective tissue with a varying proportion of adipose tissue, small blood vessels, and lymphatics. The lymphatic vessels open on to the surface lining of the serous cavities through gaps (stoma) between the mesothelial cells, which provides continuity between the lymphatic system and the serous cavities.1,2 The lymphatics are a significant component of the system for absorption of fluid in serous cavities. Any homeostatic imbalance in this system results in accumulation of fluid in serous cavities leading to the effusions. It is of interest to note that the recently reported immunomarkers for lymphatic endothelium, such as D2-40 and podoplanin, have been reported to be immunoreactive in mesothelial cells also.[3–5] This suggests a relationship between mesothelial cells and lymphatic endothelial cells.
The serous cavities may be affected by a variety of processes, including inflammation, hepatic cirrhosis, congestive heart failure and other hemodynamic aberrations, and metastatic neoplasms. These processes often elicit reactive changes in mesothelial cells. The damaged mesothelium can be replaced by differentiation of the mesenchymal cells from the underlying stroma [see Figure 2]. The reactive mesothelial cells are hypertrophied and appear somewhat cuboidal with enlarged nuclei and conspicuous nucleoli [see Figure 2]. They may show variation in nuclear size and shape, multinucleation, and increased nuclear/cytoplasmic ratios. All these features are best observed in cytologic preparations Figure 3 and 4].
![Mesothelial cells (peritoneal fluid): show outer faintly stained ectoplasm (1) with inner denser endoplasm (2) rich in intermediate filaments. The nucleus is usually central or near central (b), but may be eccentric (c). Nucleoli are readily observed. The vacuolation generally begins at the periphery in ectoplasm (1). [b,c, PAP-stained Autocyte Prep smear (b,c, 100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g003.png)
- Mesothelial cells (peritoneal fluid): show outer faintly stained ectoplasm (1) with inner denser endoplasm (2) rich in intermediate filaments. The nucleus is usually central or near central (b), but may be eccentric (c). Nucleoli are readily observed. The vacuolation generally begins at the periphery in ectoplasm (1). [b,c, PAP-stained Autocyte Prep smear (b,c, 100× zoomed).]
![Mesothelial cell (pleural fluid): shows outer ectoplasm, which is denser than the inner endoplasm. The nucleus is central to slightly eccentric but not touching the cell periphery. The cell margin shows blebs and is ruffled. [DQ-stained Cytospin smear (100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g004.png)
- Mesothelial cell (pleural fluid): shows outer ectoplasm, which is denser than the inner endoplasm. The nucleus is central to slightly eccentric but not touching the cell periphery. The cell margin shows blebs and is ruffled. [DQ-stained Cytospin smear (100× zoomed).]
General cytology (with Papanicolaou and Diff-Quik stains)
Serous effusions may contain a variety of non-neoplastic cells, including mesothelial cells, macrophages, and other blood-derived cells [Table 1], together with other entities such as psammoma bodies and various incidental cellular and non-cellular elements [Table 2].
| Cells | Diff-Quik stain | Papanicolaou stain |
|---|---|---|
| Red blood cells | Eosinophilic, anucleated, round, with central pallor | Eosinophilic |
| Biconcave disks | ||
| Anucleated | ||
| Neutrophils | Nucleus: basophilic, multilobed with 2–5 lobes | Nucleus: basophilic, multilobed with 2–5 lobes |
| Cytoplasm: faintly eosinophilic with fine granularity | Cytoplasm: faintly cyanophilic, granular | |
| Eosinophils | Nucleus: basophilic, bilobed (may have up to four lobes) | Nucleus: basophilic, bilobed (may have up to four lobes) |
| Cytoplasm: numerous, coarse, eosinophilic granules | Cytoplasm: light pink to light green with coarse granularity | |
| Basophils | Nucleus: central, rounded to irregularly shaped | Nucleus: central, rounded to irregularly shaped Cytoplasm: coarsely granular, light pink to light green |
| Cytoplasm: coarse, basophilic granules which may overlap the nucleus | ||
| Histiocytes | Nucleus: reniform (kidney or bean shaped), central to eccentric | Nucleus: reniform (kidney or bean shaped), central to eccentric |
| Cytoplasm: vacuolated, foamy, without distinct ectoendoplasmic staining pattern, and without peripheral cytoplasmic blebs. Hemosiderin appear as gray to blue granular material in Diff Quik stained preparations | Cytoplasm: foamy, may contain phagocytosed material such as hemosiderin pigment, which appear as yellow to brown granular material in Pap stained preparations. | |
| Megakaryocytes | Morphology similar to that in bone marrow smears Nucleus: large, multilobed nuclei Multilobation may not be distinct in all cells Cytoplasm: variable amount |
Large, multilobed nuclei Cytoplasm: variable amount. Megakaryocytes with high N/C ratio may be misinterpreted as neoplastic or viral cytopathic effect, especially in PAP-stained preparations |
| Cartilage fragments |
| Cholesterol crystals |
| Ciliary tufts6 |
| Collagen balls7 |
| Colonic mucosa |
| Curschmann’s spirals8 |
| Endometrial cells as reflux |
| Endometriosis cells |
| Endosalpingiosis cells9 |
| Esophageal mucosa |
| Fallopian tube epithelium |
| Fecal matter |
| Fibroadipose tissue fragments |
| Hemosiderin |
| Hematoidin crystals |
| Bile pigment |
| Liver cells or fragments |
| Lung parenchyma fragments |
| Müllerian inclusions10 |
| Ovarian cyst contaminant |
| Psammoma bodies |
| Skeletal muscle fibers |
| Skin tags |
| Starch granules |
| Vegetablematter |
| Lubricant-like debris |
Mesothelial cells
After exfoliation, mesothelial cells round up and appear polyhedral due to the surface tension of the surrounding effusion fluid. They may be seen as singly scattered cells [Figures 3 and 4] or in small cohesive clusters and sheets [Figures 6, 8, 11]. The cells are of various sizes and may be round to oval. The morphology of mesothelial cells can be evaluated in Papanicolaou (PAP) and Diff-Quik (DQ) stained smears. In general, the PAP stain allows better evaluation of nuclear details, while the DQ stain highlights cytoplasmic details [Table 3].
![Mesothelial cells (a -c) versus adenocarcinoma cells (d -f) with eccentric nuclei. A thin rim between nuclear border and cell border (1) is seen in mesothelial cells. In comparison, the nuclear border in adenocarcinoma cells touches the cell border without a significant cytoplasmic rim (2). [PAP-stained Direct smear (b, e, 100× zoomed); PAP-stained SurePath Prep smear (c, f, 100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g005.png)
- Mesothelial cells (a -c) versus adenocarcinoma cells (d -f) with eccentric nuclei. A thin rim between nuclear border and cell border (1) is seen in mesothelial cells. In comparison, the nuclear border in adenocarcinoma cells touches the cell border without a significant cytoplasmic rim (2). [PAP-stained Direct smear (b, e, 100× zoomed); PAP-stained SurePath Prep smear (c, f, 100× zoomed).]
![Mesothelial cells versus adenocarcinoma cells. (a) Mesothelial cells with central to eccentric nuclei. A thin cytoplasmic rim separates the nuclear border from the cell border (red arrow 1). (b) In comparison, the adenocarcinoma cells with eccentric nuclei appose the cell border (blue arrow 2). [a,b, DQ-stained Cytospin smear (a,b, 100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g006.png)
- Mesothelial cells versus adenocarcinoma cells. (a) Mesothelial cells with central to eccentric nuclei. A thin cytoplasmic rim separates the nuclear border from the cell border (red arrow 1). (b) In comparison, the adenocarcinoma cells with eccentric nuclei appose the cell border (blue arrow 2). [a,b, DQ-stained Cytospin smear (a,b, 100× zoomed).]
![Multinucleated mesothelial cell: a mesothelial cell with three nuclei (arrows), which may vary in size. [PAP-stained SurePath Prep smear (100×).]](/content/105/2021/18/1/img/Cytojournal-18-30-g007.png)
- Multinucleated mesothelial cell: a mesothelial cell with three nuclei (arrows), which may vary in size. [PAP-stained SurePath Prep smear (100×).]
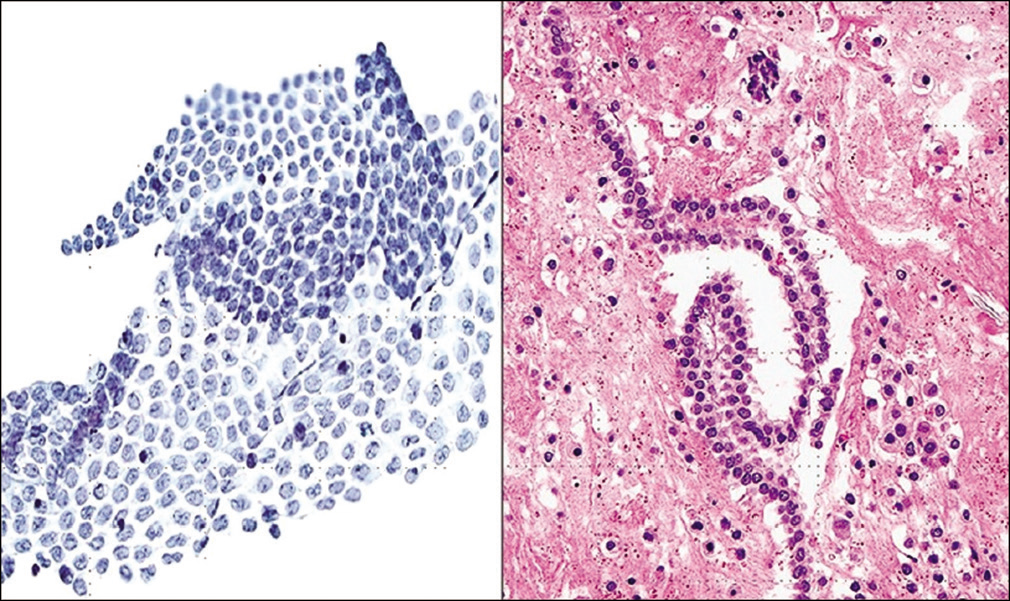
- Monolayer of mesothelial cells in pelvic washings which may fold on itself (Pap stain, ×200). (Right) Strip of mesothelium seen as a “string of pearls” in a cell-block preparation (Cell-block section H and E, ×200).
![Mesothelial windows (arrows): reactive mesothelial cells, pleural fluid. [DQ-stained Cytospin smear (a–d 100μ; inset of b, 100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g009.png)
- Mesothelial windows (arrows): reactive mesothelial cells, pleural fluid. [DQ-stained Cytospin smear (a–d 100μ; inset of b, 100× zoomed).]
![Mesothelial windows (arrows): reactive mesothelial cells, pleural fluid. [PAP-stained SurePath Prep (100×; inset, 100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g010.png)
- Mesothelial windows (arrows): reactive mesothelial cells, pleural fluid. [PAP-stained SurePath Prep (100×; inset, 100× zoomed).]
![Monolayered flat sheet of mesothelial cells: may resemble squamous metaplastic cells. The spaces between mesothelial cells, mesothelial windows, are common (red arrows). Microvilli prevent the adjacent cells from apposing their cell borders with each other. Depending on many variables, the mesothelial windows may be subtle to very wide (peritoneal washing). [PAP-stained Cytospin smear (100×).]](/content/105/2021/18/1/img/Cytojournal-18-30-g011.png)
- Monolayered flat sheet of mesothelial cells: may resemble squamous metaplastic cells. The spaces between mesothelial cells, mesothelial windows, are common (red arrows). Microvilli prevent the adjacent cells from apposing their cell borders with each other. Depending on many variables, the mesothelial windows may be subtle to very wide (peritoneal washing). [PAP-stained Cytospin smear (100×).]
| Feature | Romanowsky stains (RWS)* | Papanicolaou stain |
|---|---|---|
| Cell size and shape | Size and shape of cells: the cells are flat as they collapse during air- drying, making them slightly larger in dimension along the plane of the slide | Size and shape of cells: slightly shrunken. The cell thickness is greater in wet-fixed smears due to its fixation in three dimensions closer to its natural form |
| Cytoplasmic details | The cytoplasm is well demonstrated by RWS—thus highlighting even the scant amount of cytoplasm (such as in lymphocytes, small cell carcinoma, etc.), cytoplasmic vacuoles (renal cell carcinoma, macrophages, etc.), cytoplasmic blebs (mesothelial cells), different zones in the cytoplasm (mesothelial cells), etc. The details of cell groups are poorly visualized |
Cytoplasm: is rendered transparent which improves nuclear details In general cytoplasmic details are diminished. However, this improves the morphologic evaluation of cell groups, including three- dimensional clusters |
| Nuclear details | The details of nuclear chromatin to evaluate chromatin clumping and parachromatin clearing are not clear However, RWS are excellent for evaluating nuclear details of hematopoietic cells, as chromatin clumping and parachromatin clearing are not that significant for evaluating hematopoietic malignancies Nucleoli: are not as crisp as with the Papanicolaou stain, but they can be seen as pale structures Thus in brief, RWS does not allow evaluation of chromatin clumping and parachromatin clearing, but it allows evaluation of N/C ratio, nuclear size, shapes, nuclear pseudoinclusions, and nucleoli. Most of these are adequate for interpretation of hematopoietic lesions |
Nuclear details are excellent with crisp chromatin staining facilitating evaluation of chromatin clumping and parachromatin clearing, which are some of the most important features evaluated for interpretation of malignancy. |
| Nucleoli: well discerned | ||
| Extracellular material | Excellent staining of extracellular materials such as mucin, colloid, pseudocartilagenous and cartilagenous matrix, lymphoglandular bodies in lymphoproliferative processes, etc. | These extracellular materials are poorly stained |
In cytologic preparations, mesothelial cells are usually about 15–30 µm in diameter (1.5–2 times the size of neutrophils), but they may vary significantly and may range up to 50 µm in diameter. They appear larger in DQ-stained air-dried smears than the wet-fixed shrunken cells in PAP-stained smears [see Figure 13]. In PAP-stained preparations, the perinuclear zone, with its higher density of intermediate filaments, shows relatively dense staining endoplasm with a surrounding narrow zone of pale ectoplasm associated with microvilli [see Figure 3]. In DQ-stained preparations, the endoplasm is lightly stained with peripheral darker ectoplasm [see Figure 4]. The cell borders are round to oval but usually have ruffled surfaces with blebs. • In general, the two-zone staining characteristic of mesothelial cells is discerned better in DQ-stained preparations.
![Macrophages: mesothelial and histiocytic macrophages show morphological overlap. a–d are morphologically suggestive of mesothelial macrophages; e and f favor histiocytic macrophages (pleural fluid). [a–f, DQ-stained Cytospin smear (100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g012.png)
- Macrophages: mesothelial and histiocytic macrophages show morphological overlap. a–d are morphologically suggestive of mesothelial macrophages; e and f favor histiocytic macrophages (pleural fluid). [a–f, DQ-stained Cytospin smear (100× zoomed).]
![Reactive mesothelial cell (RM) with inflammatory cells: lymphocyte (L), neutrophil (N), and eosinophil (E) (pleural fluid). [a, PAP-stained SurePath Prep; b, DQ-stained Cytospin smear (a,b, 100×; L, N, E, 100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g013.png)
- Reactive mesothelial cell (RM) with inflammatory cells: lymphocyte (L), neutrophil (N), and eosinophil (E) (pleural fluid). [a, PAP-stained SurePath Prep; b, DQ-stained Cytospin smear (a,b, 100×; L, N, E, 100× zoomed).]
Although this typical appearance of mesothelial cells helps to distinguish them from other cells in effusions, including malignant cells, it is not specific for mesothelial cells. Nonmesothelial neoplasms such as malignant melanoma and well-differentiated adenocarcinomas of the breast and ovary may demonstrate some morphologic overlap with reactive mesothelial cells [Figures 5].
The nuclei of mesothelial cells are usually centrally placed or slightly off center, but may be distinctly eccentric. Even when they are eccentric, their nuclear membranes do not touch the cell border. Careful examination shows a narrow rim of cytoplasm adjacent to the eccentric nucleus [see Figure 5]. This narrow rim, which is better highlighted in DQ-stained preparations, is related to the microvilli on the surface of mesothelial cells [see Figure 6].
Binucleation and multinucleation of reactive mesothelial cells is frequent, especially in non-malignant effusions. It is not uncommon to see variation in the sizes of multiple nuclei in the same cell [Figure 7]. Nucleoli are usually seen and may be prominent [see Figure 3]. However, huge macronucleoli equal to one-third the size of the nuclear diameter associated with some malignancies such as melanoma, hepatocellular carcinoma, germ cell tumors, and prostatic adenocarcinoma are rarely found in reactive mesothelial cells. The chromatin is usually finely granular (powdery) with various degrees of chromasia. Many of these nuclear details can also be seen in DQ-stained smears, but malignancy-related hyperchromasia and chromatin details cannot be evaluated properly in DQ-stained smears [see Figure 4].
With DQ staining, mesothelial cell cytoplasm may not always show the two zones. As the mesothelial cells imbibe water from the effusion fluid, their cytoplasm may acquire a foamy macrophage phenotype with pale vacuolated cytoplasm [Figures 12]. The degree of vacuolization is directly proportional to the duration that the cells remain in the fluid medium. As the effusion becomes chronic, the cytoplasmic vacuoles become larger. The cytoplasmic vacuoles of mesothelial cells are usually small and located at the periphery of the cells, but they may be randomly distributed or even be central with nuclear overlap [see Figure 12]. A single, large, cytoplasmic vacuole displacing the nucleus may cause a mesothelial cell to resemble a signet ring cell of adenocarcinoma.
Differences between macrophage-like mesothelial cells and histiocytic macrophages, although not of great clinical significance, are difficult to identify by morphology alone. Nuclear morphology may help to make some distinction. Mesothelial cells have round to oval nuclei with smooth contours, whereas histiocytic macrophages typically show bean-shaped (kidney-shaped, reniform) nuclei or with slightly irregular contours [see Figure 12].
The surfaces of mesothelial cells have numerous, long, slender microvilli, which impart a peripheral rim of pallor in PAP-stained preparations. Microvilli are best seen by electron microscopy (EM). This characteristic feature of mesothelial cells has been applied to distinguish them from other cells such as carcinoma cells.[11–13] Although the microvilli cannot be seen directly under the light microscope, their presence may be inferred from a thin rim of cytoplasm by the side of an eccentric nucleus [see Figure 5]. This feature, although observed in both types of staining, is more easily recognized with DQ stain. • The swollen microvilli impart ruffled borders and peripheral blebs in DQ preparations [see Figures 4]. In contrast, if the nucleus is eccentric in histiocytic macrophages, it touches to the cytoplasmic membrane without rim of cytoplasm (comparable to that in adenocarcinoma cells with nuclear features of malignancy, better evaluated in PAP stained preparations [Figure 3]).
The microvilli of mesothelial cells may prevent adjacent cells from completely apposing each other, thereby creating a gap between the adjacent cell membranes, which gives rise to the appearance of a space referred to as a mesothelial window [see Figures 9-11]. Depending on many variables, these windows may be subtle or very wide in cytology smears. Such spaces are not specific for mesothelial cell only, but they may be seen in the cell groups of some metastatic cancers in effusions.
The cytoplasm stains light green with a variable degree of intensity and vacuolization in PAP-stained preparations.
PAP-stained preparations do not highlight the cytoplasm well, giving the impression of less cytoplasmic volume compared with that observed in DQ-stained smears [Figure 10]. The cytoplasm in PAP-stained preparations is less distinct and more transparent. The non-highlighting of cytoplasm associated with PAP staining is enhanced further due to cellular shrinkage in wet fixed preparations; although less distinct, mesothelial windows [Figure 10] and cytoplasmic vacuoles may still be evident in PAP-stained smears.
Nuclear details, however, are better seen in PAP-stained preparations [see Figure 3]. Usually, centrally placed nuclei are typically round to oval with smooth contours (even malignant mesothelial cells may continue to demonstrate round nucleus with regular smooth outlines). As their cytoplasm becomes vacuolated due to phagocytic activity or degenerative changes, the nuclei of these mesothelial cells may be displaced to the periphery of the cell. On close inspection, in most of the cells the nuclei do not touch the outer margin of the cell [see Figure 5a,b,c]. As mentioned previously, this is secondary to the slender bushy microvilli along the periphery of the mesothelial cells . This feature may be applied to distinguish mesothelial cells from histiocytic macrophages and carcinoma cells, which characteristically show peripherally located nuclei touching the cell membrane [see Figure 5d,e,f].
Although mesothelial cells do not proliferate in effusion fluid after exfoliation, they may complete an already started mitotic division. The presence of mitotic figures in effusion cytology may reflect a process in the serosal lining that is capable of causing significant proliferative activity of mesothelial cells in response to the factors responsible for the effusion.
As is applicable to cytopathologic evaluations in general, the presence of nucleoli and mitotic figures should not mislead the interpreter into making a false interpretation of malignancy. Other general morphologic features of malignancy should be applied to arrive at such an interpretation. Once correctly interpreted as malignant, then the nucleoli and mitotic figures may be considered for further categorization and grading of a neoplasm.
Mesothelial cells produce hyaluronic acid, which, if present, may be seen as magenta-colored intracytoplasmic or extracytoplasmic material in the DQ-stained preparations or as light gray streaks in the background of PAP-stained preparations. This may also be present in the center of small groups of mesothelial cells and may resemble a mucin-containing acinus of adenocarcinoma. Hyaluronic acid demonstrates positive staining with periodic acid–Schiff (PAS) and Alcian blue stains. This staining is lost if the slides with tissue sections are treated with hyaluronidase prior to staining. Hyaluronic acid is not the substrate for diastase and is not digested by it. Consequently, PAS positivity of hyaluronic acid in the sections stained with the commonly used PAS staining method (i.e. PAS staining after treatment with diastase) is not lost.
‘Atypical’ mesothelial cells
Apart from two major morphologic categories of mesothelial cells—with or without cytoplasmic vacuoles—reactive mesothelial cells have an extremely versatile morphologic spectrum leading to many ‘faces’. However, it is generally possible to trace a relationship between these cells and prototypic mesothelial cells to recognize their true nature. The morphologic spectrum of both nuclear and cytoplasmic appearances overlaps with cells of malignant mesotheliomas and various other well to moderately differentiated neoplasms. • Mesothelioma without a qualifier in this book refers to epithelioid mesothelioma, which is always malignant, diffuse, and has an epithelioid pattern of growth.
The cells of mesothelioma in most of the cases demonstrate morphologic overlap with cells in reactive effusions. On the other hand, reactive mesothelial cells may show features overlapping with the cells of well to moderately differentiated ovarian and mammary adenocarcinomas. • Effusions associated with malignancies may just be a reactive response to an underlying neoplasm without the presence of any malignant cells. Such cases might well contain only floridly reactive mesothelial cells. The possible pitfall in such cases is that the clinical details may tempt the interpreter to an erroneous false-positive interpretation. Although it is prudent to avoid the terminology of ‘atypical’ mesothelial cells, markedly reactive mesothelial cells showing extreme morphologic overlap with malignant cells may occasionally be referred to by some as ‘atypical’ mesothelial cells in some rare cases. In such cases, based on the clinical scenario if mesothelioma is not suspected and these cells are definitely mesothelial (based on cytomorphology and/or immunoprofile), these cells should be reported as floridly reactive mesothelial cells. However, if these cells are nonmesothelial with atypical features, then such cells may be reported as atypical cells with appropriate comment related to further evaluation and management.
Mesothelial cells in washings and lavages
Peritoneal washings and lavages are commonly submitted as part of staging procedures for gynecologic cancers.[2,14] In addition to this, other indications for peritoneal washings include staging of non-gynecologic malignancies (such as Pancreatic, gastric), ruling out occult cancer, and evaluate response to therapy (the “second-look” procedure). Recently peritoneal washings are performed in increasing numbers prior to the prophylactic ovarian and fallopian tube resections in cases with BRCA mutation carriers. Cancers were detected in 2–10% of specimens which may be missed during pathologic evaluation even after processing of the entire specimen15,16. As recommended by Society of Gynecologic Oncologists Clinical Practice Committee, although not required as the part of risk-reducing prophylactic resection procedures currently, peritoneal washing with cytologic evaluation is considered reasonable until more data on the topic is accumulated[9c].
In these specimens, the mesothelial cells have a different appearance than those in effusions, which are naturally exfoliated with reactive changes. The mechanically dislodged cells fall into a free-floating configuration in the fluids and are seen as monolayered flat sheets that may resemble sheets of squamous metaplastic cells [see Figure 8 and 11]. Individual mesothelial cells arranged in a jigsaw puzzle pattern have well-defined cytoplasmic borders with distinct slits between some cells. The cells are not round but have angulated rhomboidal/trapezoidal shapes with centrally placed bland nuclei. Some of these sheets of cells may curl and fold onto themselves and give a three-dimensional appearance. Careful evaluation of these aggregates along the periphery helps to decide the true nature of these folded sheets and distinguish them from the three-dimensional balls of proliferation spheres [Figure 17] of neoplastic cell groups. At least some areas along the edges of the group help to confirm the monolayered nature of these aggregates of mechanically stripped sheets of mesothelial cells related to the washing procedure. Contaminants such as collagen spheres,[17] Müllerian inclusions[10b] and atypical papillary proliferations[18] may lead to serious false positive error in the cytologic interpretation of peritoneal/pelvic washings.[19]
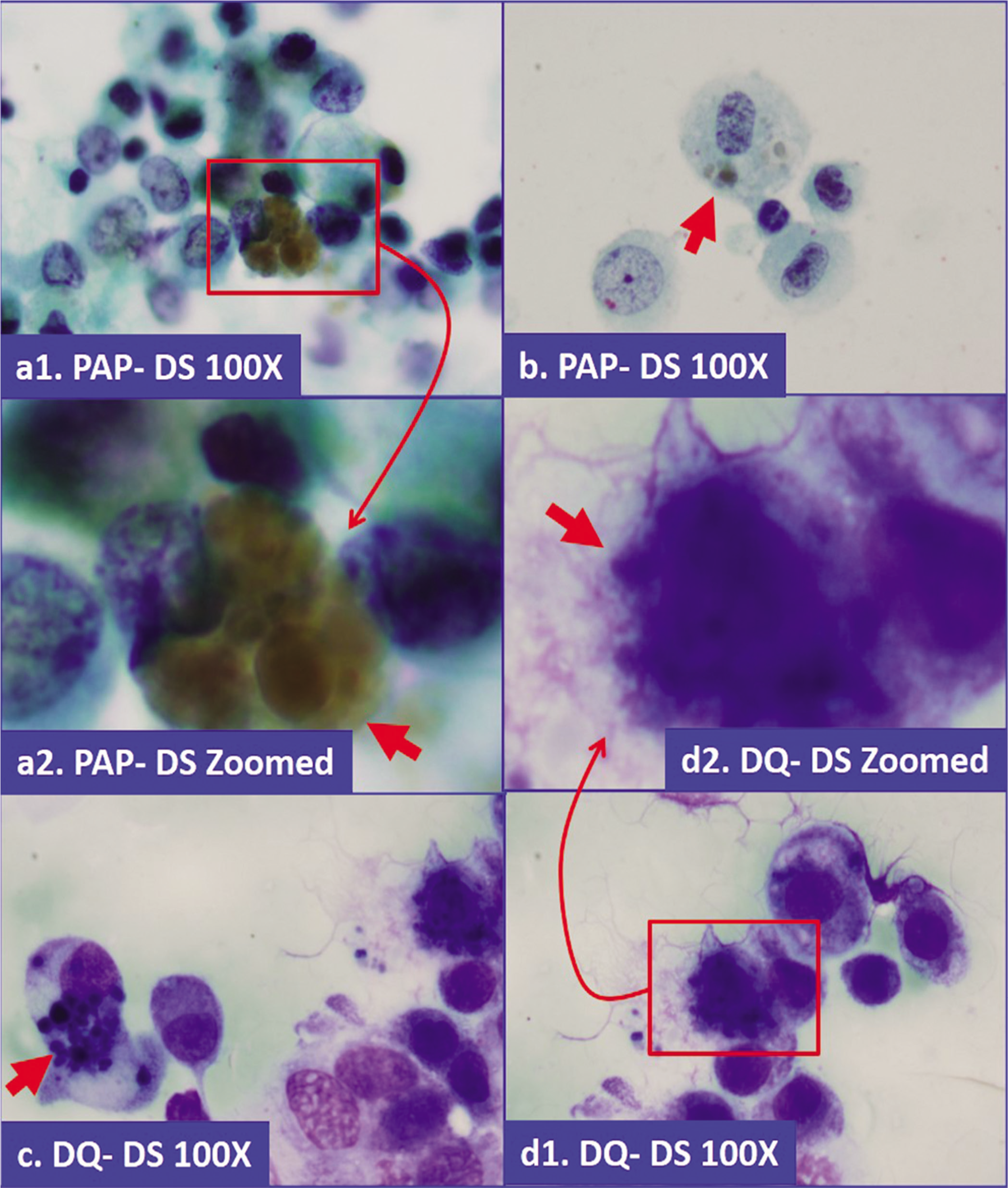
- Hemosiderin in macrophages. Coarsely granular, brown pigment, which is blue-gray in Diff-Quick stained preparation (a,b: Papanicolaou stained direct smear; c,d: Diff-Quick stained direct smear).
![Megakaryocyte (1) with adjacent mesothelial cell (2) in hemorrhagic pleural fluid. [DQ-stained Cytospin smear, 100×].](/content/105/2021/18/1/img/Cytojournal-18-30-g015.png)
- Megakaryocyte (1) with adjacent mesothelial cell (2) in hemorrhagic pleural fluid. [DQ-stained Cytospin smear, 100×].
![Psammoma body: round acellular bodies with concentric lamination (peritoneal fluid). [PAP-stained SurePath Prep (100× zoomed).]](/content/105/2021/18/1/img/Cytojournal-18-30-g016.png)
- Psammoma body: round acellular bodies with concentric lamination (peritoneal fluid). [PAP-stained SurePath Prep (100× zoomed).]
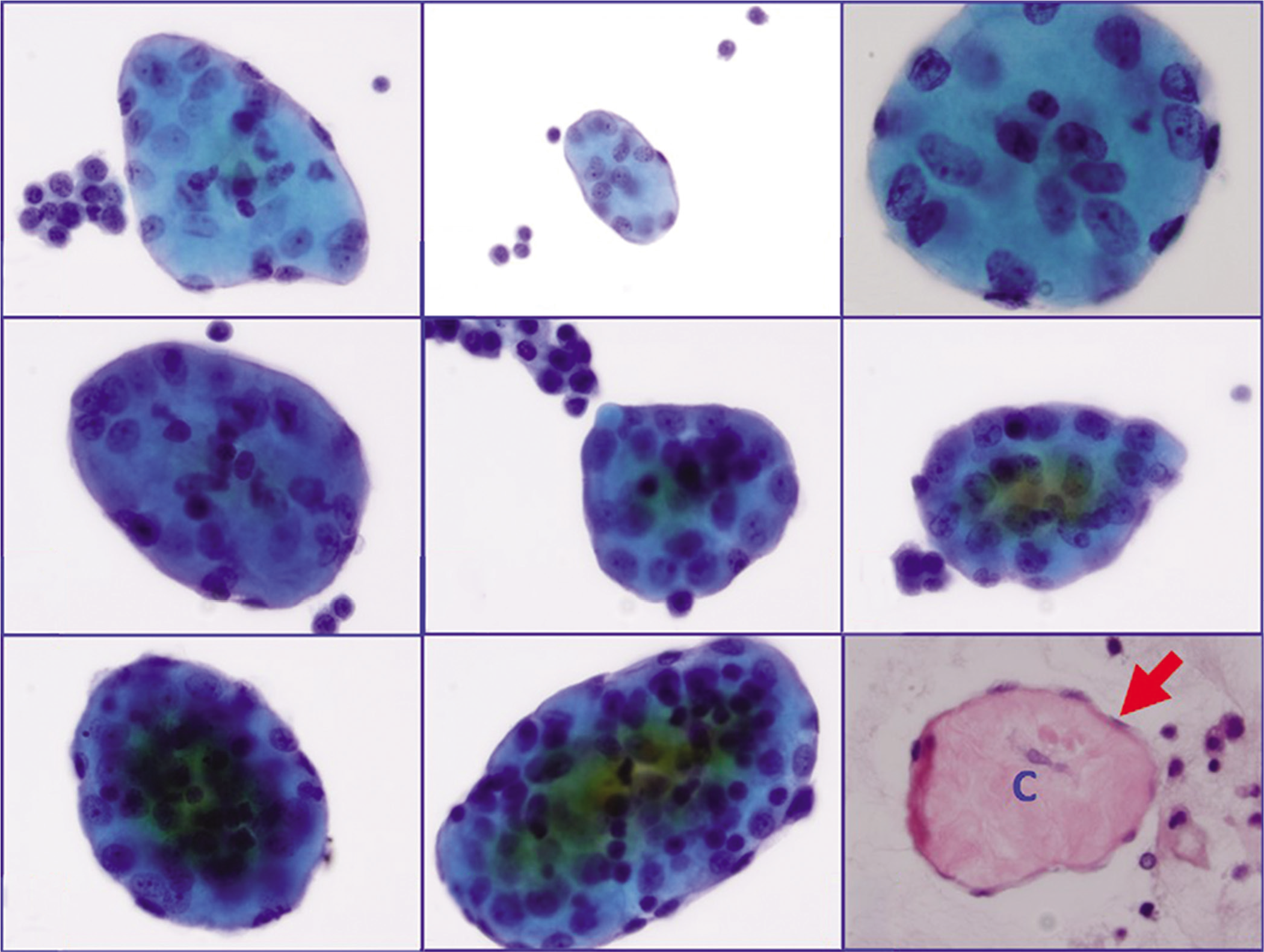
- a through h: Collagen globules (Pap stained ThinPrep, in increasing order of cellularity for the wrapping mesothelial cell layer) i: Collagen globule section (HE stained cell-block section) showing central collagen (c) with peripheral layer of mesothelial cells (arrow).
Macrophages
Macrophages in effusions may be mesothelial or histiocytic. They may be the predominant cell population in some effusions and have highly variable morphologic appearances in DQ-stained preparations [see Figure 12]. • Mesothelial and histiocytic macrophages are difficult to distinguish from each other by morphology alone. Although usually clinically irrelevant and generally not indicated, immunocytochemistry can distinguish them. Mesothelial macrophages demonstrate immunoreactivity for cytokeratin 7, calretinin (nuclear), D2-40, and membranous immuno-reactivity for HBME-1. Histiocytic macrophages, on the other hand, show immunoreactivity for CD68 (PG-M1 and KP1), CD163, and LCA. Generally, macrophages without a qualifier (as histiocytic or mesothelial) are considered to be histiocytic macrophages.
The histiocytic macrophages are round, with well- to ill-defined cell borders. They are non-cohesive and singly scattered, but may be seen in small, ill-defined, loose clusters with irregular peripheral contours. The nuclear margin of the eccentric nucleus may be closely approximated to the cell membrane. Generally, relatively normochromatic nuclei are kidney-shaped and nucleoli are indistinct [Figure 12 e,f]. Some cells may be multinucleated. In PAP stained preparations, the variably cyanophilic cytoplasm is pale and homogeneous to extensively vacuolated. Some macrophages may show intracytoplasmic waste products or material such as hemosiderin associated with endometriosis, melanin with malignant melanoma, and other components such as erythrocytes, inflammatory cells, nuclear particles, or microorganisms, etc.
Hemosiderin and melanin appear as yellow to brown granular material in Pap stained preparations. However, these pigments appear as gray to blue granular material in Diff Quik stained preparations [Figure 14]. In contrast to this, hematoidin and bile pigment are yellow in both Pap stained and Diff Quik stained preparations.
In comparison, mesothelial macrophages may also have overlapping morphologic features with some subtle differences such as round to oval nuclei (in contrast to reniform nuclei in histiocytic macrophages) and centrally positioned nuclei (rather than peripheral or eccentric nuclei as in histiocytic macrophages) [Figure 14]. If the nuclei of mesothelial macrophages are eccentric, the nuclear margin is usually not in close approximation to the cell border, but shows a narrow rim due to numerous long slender microvilli (in contrast to close approximation to the cell border in histiocytic macrophages). The cell borders are well defined with blebs (in contrast to ill-defined in histiocytic macrophages). Mesothelial macrophages are seen as cohesive groups with a distinct knobby contour (in contrast to non-cohesive ill-defined small loose groups with irregular outlines of histiocytic macrophages; Table 4].
| Feature | Mesothelial macrophage | Histiocytic macrophage |
|---|---|---|
| Nucleus | Round to oval | Reniform (bean-, kidney-shaped) |
| Nuclear location | Central (most frequently) | Eccentric (most frequently) |
| Nuclear border close to the cell border | Absent | May be |
| Cell border | Sharp, smooth | Not well defined |
| Cytoplasmic blebs | Present | Absent |
| Cohesive sheets with distinct cell borders | Present | Absent |
| Margins of cell aggregates | Knobby | Ill-defined, irregular |
| Immunocytochemistry | Calretinin: nuclear or nuclear and cytoplasmic immunoreactivity Cytokeratin 7: cytoplasmic immunoreactivity D2-40: cytoplasmic immunoreactivity HBME-1: membranous immuno- reactivity along cell membrane |
Immunoreactivity for CD45 (LCA), CD68 (PG-M1 and KP1), and CD163 |
Extensive vacuolization with peripheral displacement of nuclei in some macrophages may resemble adenocarcinoma cells with secretory vacuoles. The mucicarmine stain for mucin on the cell-block sections may help. Macrophages will not demonstrate positive staining, whereas adenocarcinoma cells may show mucin positivity [Figure 22]. However, as a histochemical stain, mucicarmine may show variation in interlaboratory reproducibility, which may compromise sensitivity and specificity of this otherwise simple, economical ancillary test.
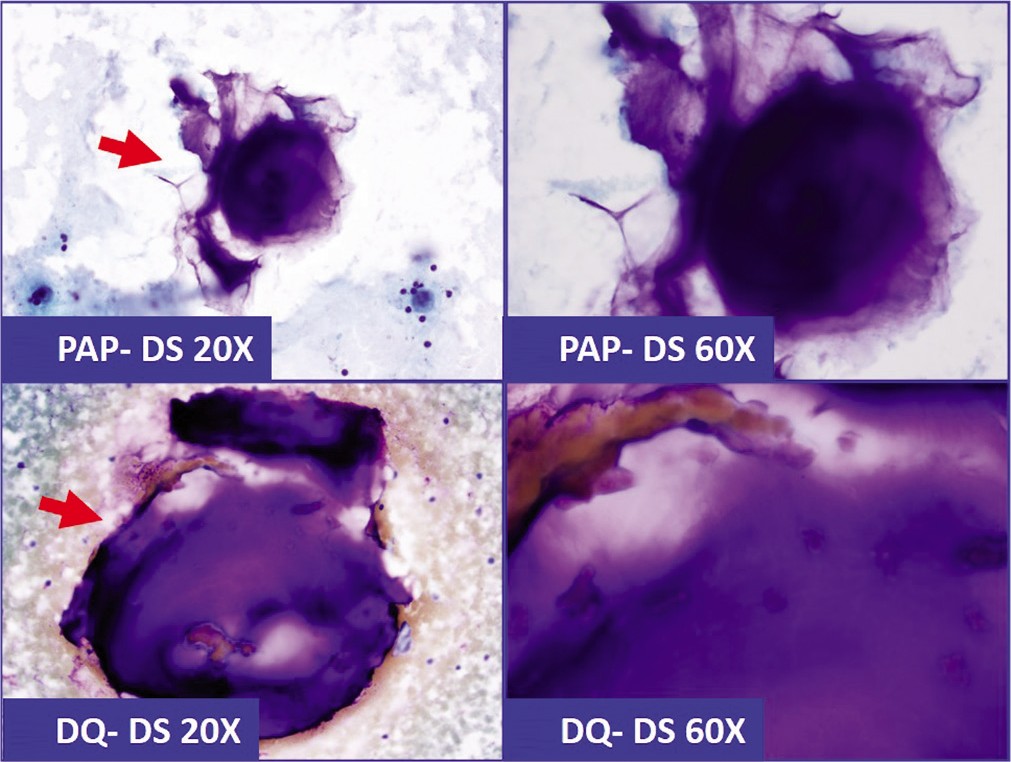
- Because of superficial morphological resemblance, lubricant-debris (arrows) in effusion specimen could be confused with mucin (a&b: Pap stained direct smear; c&d: Diff-Quick stained direct smear). This pit-fall may deepen further with histochemical testing with positivity for Mucicarmin stain (c&d in Figure 20).
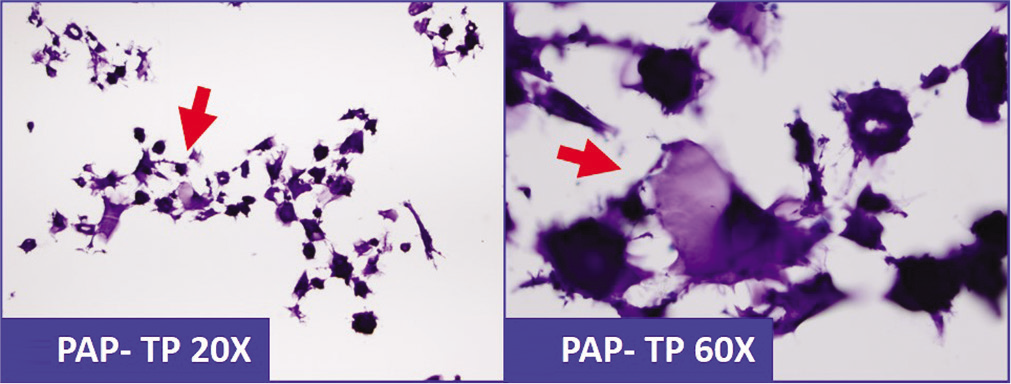
- Abundant lubricant-debris (arrows) in peritoneal washing (should not be confused with mucin).
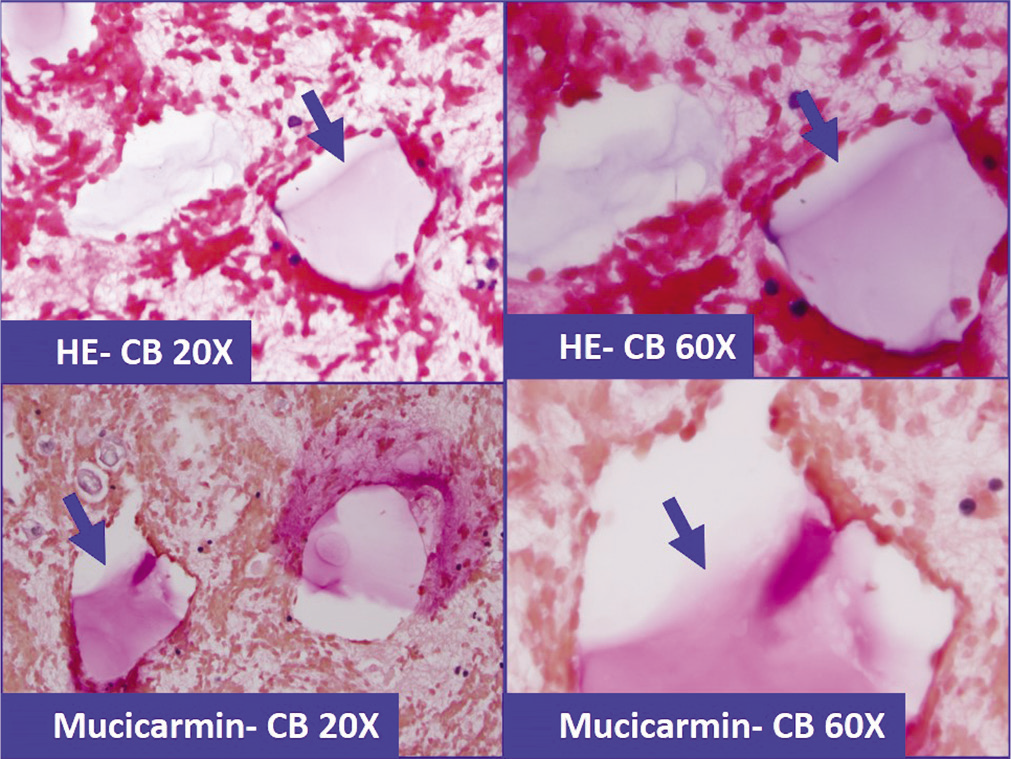
- Lubricant-debris (arrows) in effusion specimen (a&b: HE stained cell-block section), which because of superficial morphological resemblance could be confused with mucin. This pitfall may deepen further with histochemical testing with positivity for Mucicarmin stain (c&d).
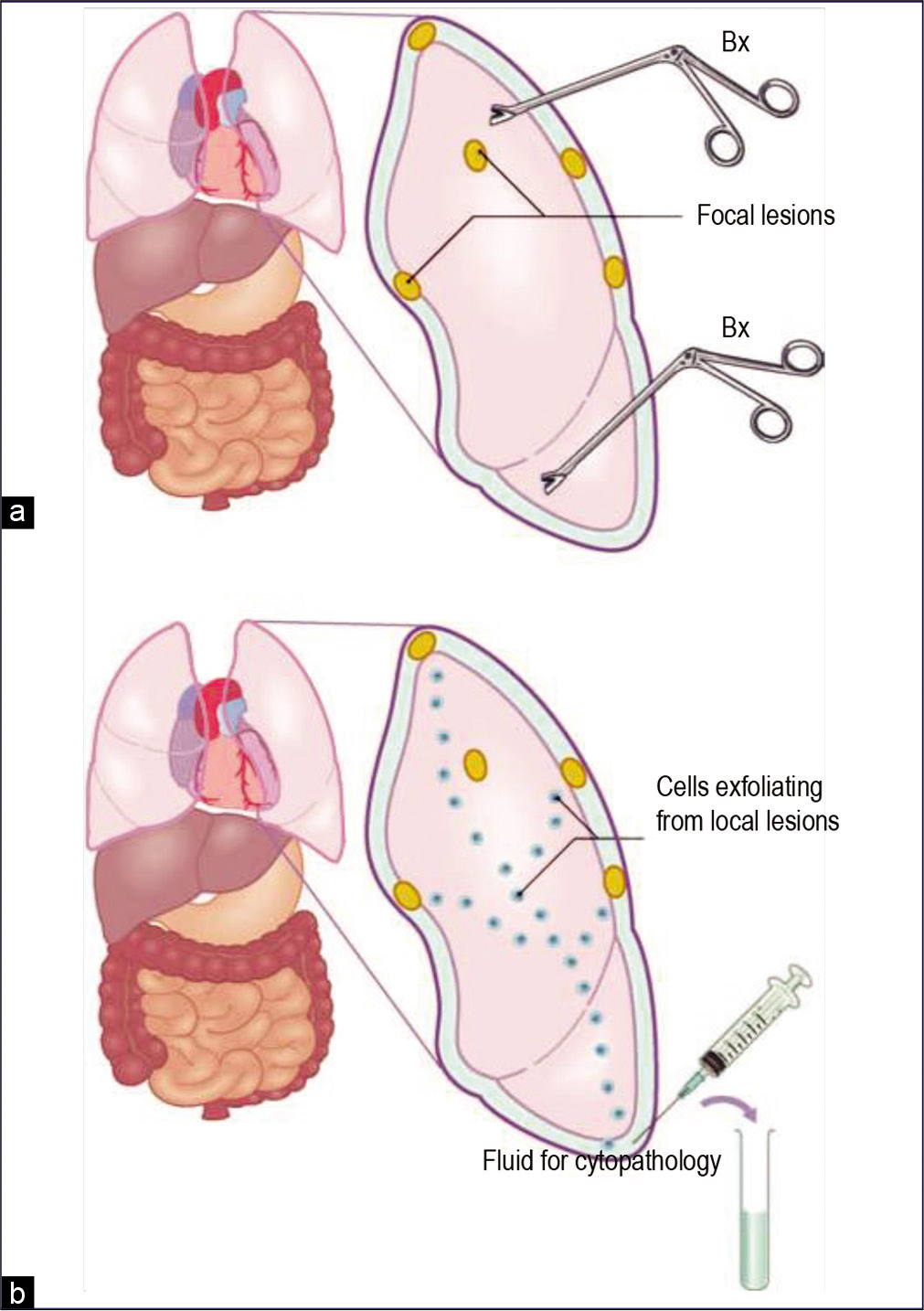
- Sampling of pleural lesions: biopsy (a) versus effusion cytology (b). Focal lesions may be missed by biopsy. However, cells exfoliated from any of these focal lesions are pooled in the fluid and should be present in a related effusion or washing (Bx, biopsy).
![Mucicarmine stain: adenocarcinoma cells are mucicarmine positive (arrow) (peritoneal fluid). [cell-block section, 100×.]](/content/105/2021/18/1/img/Cytojournal-18-30-g022.png)
- Mucicarmine stain: adenocarcinoma cells are mucicarmine positive (arrow) (peritoneal fluid). [cell-block section, 100×.]
Blood-derived cells
Depending on the extent of peripheral blood contamination and cause of an effusion, red blood cells, lymphocytes, neutrophils, eosinophils, basophils, histiocytes, and megakaryocytes [see Figure 13, Table 1] may be present in effusions. The morphology of these cells is similar to that in DQ-stained smears of peripheral blood and bone marrow. Due to the conventional approach of evaluating these cells with Romanowsky stains, they are best interpreted in DQ- rather than PAP-stained preparations.
Lymphocytes
Various types of lymphocytes may be present in effusions. A polymorphic lymphoid population with spectrum of lymphoid cells usually correlates with reactive process. However, depending on the clinical scenario, some effusions may contain a few monomorphic lymphoma cells mixed with non-neoplastic polymorphic reactive lymphocytes, which may be misinterpreted as reactive. Contrary to this, some effusions with lymphomas showing a polymorphic lymphoid population may resemble a reactive process. Such effusion specimens may have to be evaluated further with immunophenotyping by suitable methods such as flow cytometry. Predominance of obviously atypical lymphocytes in a monomorphic lymphoma and high grade lymphoma, including primary effusion lymphoma, are usually straightforward to be interpreted as lymphoma cells, especially in DQ-stained preparations.
Other blood derived cells, include neutrophils, eosinophils, and basophils. Neutrophils are 10–14 μm in diameter with multilobed nuclei [see Table 1, Figure 13]. A heavy predominance of neutrophils is seen in purulent inflammation.
Eosinophils are 12–15 μm in diameter with bilobed nuclei and coarse eosinophilic granules in DQ-stained preparations [see Figure 13]. The pink granularity is not well reproduced in PAP-stained preparations. Long-standing specimens may show pyknotic nuclei without the usual bilobed pattern. Numerous eosinophils are relatively rare in pericardial and peritoneal effusions; however, they are not uncommon in pleural effusions. Pleural effusions with a high eosinophil count are usually secondary to pneumothorax and repeated tappings. A significant proportion may be idiopathic. If pneumothorax is excluded, the other most common associations with high eosinophil counts in pleural fluid include neoplasms, pneumonia, pul-monary infarct, and various hypersensitivity reactions such as those caused by parasitic infection.[20] Usually, it is difficult to identify a single pathologic mechanism for high eosinophil counts in a pleural effusion. These effusions may be acute (because of an allergic cause or due to trauma)[21] or chronic.[22] In peritoneal effusions, increased eosinophils may be associated with neoplasms, parasitic infestation, allergic conditions, eosinophilic gastroenteritis, and chronic peritoneal dialysis.[20]
Occasional basophils and mast cells are found in effusions. Basophil counts are rarely increased in effusions but have been reported to rise up to 27% in chronic myeloid leukemia.[20] These cells are easily recognized with DQ stain, but not with the PAP stain.
Histiocytes
Histiocytes have been discussed previously under macrophages.
Megakaryocytes
Megakaryocytes in effusions resemble those in bone marrow aspirates. They are large cells with a variable amount of cytoplasm and dark multilobed nuclei. Multilobation may not be distinctly visible in all cells [Figure 15]. They are rare in effusions and, if present, are usually associated with a myeloproliferative disorder or with extramedullary hematopoiesis secondary to conditions such as extensive bone marrow replacement by metastatic carcinoma. Bleeding from the pulmonary microvasculature may lead to the presence of megakaryocytes in a pleural effusion.[23] Megakaryocytes in effusions may be misinterpreted as malignant cells or as a viral cytopathic effect [see Figure 15].
Other entities
In addition to mesothelial cells and inflammatory cells, some structures such as psammoma bodies, collagen balls, and detached ciliary tufts may be present, but usually in washing and cul-de-sac fluids.
Psammoma bodies
Psammoma (psammos [‘sand’] + oma [‘tumor’]) bodies are calcific spherules with a concentric laminations [Figure 16]. They are encountered in effusion fluids in up to 3.7% of cases.[24] In smears they have a tendency to be cracked and may be surrounded by cells. They may appear cyanophilic (blue-green) to acidophilic (pink) in PAP-stained preparations [Figure 16].
In fine-needle aspirates, they are usually associated with various papillary neoplasms and usually have diagnostic significance for neoplasms such as papillary thyroid carcinoma, ovarian papillary serous carcinoma, meningioma, and others. • In pleural and pericardial effusions, psammoma bodies are usually associated with malignancy. However, in some peritoneal effusion fluids, they may also be associated with benign conditions. • In peritoneal fluids they may be associated with benign processes in up to one-third of cases. In the absence of malignancy, the reported benign processes associated with psammoma bodies in peritoneal effusions include papillary mesothelial hyperplasia, endometriosis, endosalpingiosis, and ovarian cystadenoma/ cystadenofibroma.[24]
Collagen globules
These are globular micro-fragments of collagen covered with mesothelial cells [see Figure 17]. They have been reported in 4–29% of peritoneal washings, with higher prevalence in specimens submitted as pelvic washings (5.8%) than those submitted as peritoneal washings (1.6%) under the term ‘collagen balls’.[7,25] It is important not to misinterpret these non-neoplastic structures as a component of a papillary or mucinous gynecologic neoplasm.[26] Although collagen globules have been reported in ascitic fluid from a man with encapsulating peritonitis,[27] they are usually restricted to specimens from females, where they appear to originate most probably from the surface of the ovaries Figure 17.[7]
Collagen globules, especially the cellular ones with many reactive crowded mesothelial cells may be confused with proliferation spheres associated with metastatic carcinoma.
Detached ciliary tufts
Detached ciliary tufts derived from the ciliated epithelium lining of the fallopian tubes may be present in fluid from the pouch of Douglas and in peritoneal washings.[28,29] They are ciliated, non-nucleated fragments of cells. When fresh, the cilia may still demonstrate linear, rotating, jerky motility. In toluidine blue-stained wet preparations, they may be misinterpreted as parasites.[30] They are difficult to find in PAP-stained direct smears. However, liquid based cytology methods such as ThinPrep and SurePath may concentrate them with higher chance of detection. Most likely, they represent cyclic physiologic shedding of the tips of ciliated cells of the fallopian tubes in the luteal phase of the menstrual cycle.[6] They do not have any pathologic significance.
Curschmann’s spirals
Curschmann’s spirals have been reported in smears and cell-block preparations in spontaneous pleural and peritoneal effusions.[8] Although generally smaller, they were similar to those seen in sputum and bronchial washings.[31,32] Some spirals are believed to be formed from mucus secreted by mucus-producing adenocarcinoma cells. In non-neoplastic conditions, it is believed that they are the result of submesothelial connective tissue mucosubstance passing into the effusion fluid through the serosal lining due to increased permeability caused by inflammation.[8] In general, they are of less clinical significance.
Extraneous entities and non-cellular material in serous cavity effusions and washings [see Table 2]
Extraneous entities
Depending on the trajectory of the aspiration needle through neighboring structures, different types of extraneous tissues may be observed in effusions [see Table 2]. Similarly, various specimens collected with potential for mechanical dislodgement during some procedures such as washings, lavages, and scrapings and rarely some physiologic mechanisms may result in spontaneous appearance of such extreneous elements in effusions. These may create a challenge, especially for the inexperienced interpreter with inadequate clinical details:
Fragments of fibroadipose tissue may be present as contaminants either during washings or as fragments of tissue dislodged by a needle aspirating an effusion.
Normal hepatocytes in peritoneal and pleural effusions may be confused with mesothelial cells and well-differentiated cells of hepatocellular carcinoma. So-called dysplastic hepatocytes may be misinterpreted as neoplastic cells. Singly scattered liver cells associated with lipofuscin may resemble macrophages with hemosiderin, and hepatocytes with lipid vacuoles may be misinterpreted as vacuolated macrophages or vacuolated adenocarcinoma cells.
In long-standing effusions, degenerated cells from the female genital tract may accumulate in the cul-de-sac via the fallopian tubes. This reflux may be secondary to menstruation, particularly in association with an IUD. Endometriosis and endosalpingiosis implants[9] may be present in peritoneal effusions. These cells, especially with degenerative nuclear hyperchromasia, may be misinterpreted as malignant cells. Müllerian inclusions in peritoneal washings may lead to a similar pitfall.[10,19]
Cells from ectopic pancreas in the jejunum have been reported to be the cause of a false-positive interpretation in a patient with a history of ovarian adenocarcinoma.[33]
Non-cellular material
Vegetable matter as contaminating food particles or fecal matter may be present in ascitic fluid or cul-de-sac aspirate secondary to bowel perforation or penetration of the intestine by the aspirating needle.
Cholesterol crystals may be present in long-standing effusions, especially those of rheumatoid pleuritis.
Hematoidin crystals and hemosiderin-laden macrophages may be present in serosanguinous effusions.
Lubricant-like debris [Figures 18-20] may be confused as mucin [Table 2]. This pitfall may lead to the misdiagnosis, such as pseudomyxoma peritonei, especially in cases with history of adenocarcinoma. The lubricant-debris may be Mucicarmine positive and may further the pitfall [Figure 20].
EFFUSIONS (GENERAL CONSIDERATIONS)
Under physiologic conditions, the parietal and visceral layers of serosa are in close apposition, with a narrow gap of 5–10 µm between them. The accumulation of fluid in a serous cavity, with widening of this gap, is termed ‘effusion’. • All effusions, irrespective of their cellular composition, are pathologic. Blockage of lymphatics, vasodilatation, increased vascular permeability, hemodynamic imbalance of the microcirculation, and breakdown of small blood vessels may result in extravasation of fluid, leading to an effusion. High levels of vascular endothelial growth factor (VEGF), leading to increased vascular permeability, have been reported in association with malignant effusions.[34] • The pleural cavity can hold up to 3 liters of fluid in each cavity, and the peritoneal cavity can accumulate up to 15–20 liters. The pericardial cavity, however, cannot hold more than 0.6 liters without adversely affecting heart function, leading to cardiac tamponade.
Types of effusions
From the point of view of cytopathology, effusions may be non-neoplastic (secondary to collagen diseases, circulatory system disorders, trauma, inflammation, infection, etc.) or malignant (related to cancers).[35] Pathophysiologically,[36] effusions in serous cavities may be categorized as transudates, exudates, or chylous [Table 5].
| Transudate | Exudate | Chylous | |
|---|---|---|---|
| Biochemical features | Accumulation of fluid as an ultrafiltrate of plasma | Associated with increased permeability of the capillaries leading to exudation of protein- rich fluid | Leakage of lymphatic fluid secondary to trauma or the obstructed thoracic duct or cisterna chyli, caused by malignant neoplasms including lymphomas and carcinomas |
| a. Total protein 3.0 g/dL (30 g/L) or lower | a. Total protein 3.0 g/dL (30 g/L) or more | a. Milky white fluid | |
| b. Specific gravity 1.015 or less | b. Specific gravity 1.015 or more | b. Wet preparation shows usually small free fat droplets | |
| c. Ratio of fluid lactic dehydrogenase to serum lactic dehydrogenase less than 0.6 | c. Ratio of fluid lactic dehydrogenase to serum lactic dehydrogenase more than 0.6 | ||
| d. Does not coagulate | d. May coagulate on standing | ||
| Cytologic features | Hypocellular smears Mostly mesothelial cells | Hypercellular smears Predominantly inflammatory cells with reactive mesothelial cells with or without malignant cells | The smears are rich in lymphocytes and some lipid- laden macrophages |
| Causes | 1. Congestive heart failure | 1. Malignant neoplasms | 1. Metastatic cancer |
| 2. Pulmonary atelectasis | 2. Infections, including bacterial pneumonia, lung abscess, tuberculosis, fungal infections, viral infections, and parasitic diseases | 2. Trauma, including blunt trauma and operative trauma | |
| 3. Nephrotic syndrome | 3. Collagen vascular diseases, including systemic lupus erythematosus and rheumatoid pleuritis | 3. Retroperitoneal cancers and lymphoma | |
| 4. Postpartum effusion | 4. Pulmonary embolism/infarction | 4. Tuberculosis | |
| 5. Peritoneal dialysis | 5. Some abdominal diseases, including pancreatitis, subphrenic abscess, esophageal rupture, and hepatic abscess | 5. Congenital lymphatic anomalies | |
| 6. Superior vena cava obstruction | 6. Radiotherapy | ||
| 7. Portal vein hypertension secondary to cirrhosis, schistosomiasis, and diffuse metastatic neoplasm in liver | 7. Bile peritonitis | ||
| 8. Postoperative abdominal surgery | 8. Trauma | ||
| 9. Meigs’ syndrome | 9. Myocardial infarction and post- myocardial infarction syndrome | ||
| 10. Chronic renal diseases with impaired renal function | 10. Aortic dissection | ||
| 11. Inferior vena cava hypertension | 11. Cardiac rupture | ||
| 12. Coagulation disorder and anticoagulant therapy |
An effusion confirmed as a transudate usually does not require diagnostic evaluation for malignant cells. But exu-dates generally need cytologic evaluation to determine their cause. In experienced hands, cytologic examination of effusions is better than the biopsy of the serous lining for the diagnosis of malignancy[35,37–40] [Figure 21]. Because of the focal distribution of the lesions, they may be missed during biopsy of a serous surface. In contrast, an effusion contains cells exfoliated from the entire surface of a serous cavity [see Figure 21]. If consecutive effusions from cancer patients are evaluated, the detection rate for cancer cells is increased further.[41–43] • Wide variations in morphologic features of the highly versatile mesothelial cells overlap with those of malignant cells. This is one of the main challenges during interpretation of effusion cytopathology. Degenerative changes and poor cellular preservation, superimposed on other technical problems frequently associated with these specimens, add to the difficulty.
Pitfalls associated with cytomorphologic evaluation of nonneoplastic effusions, especially those associated with hepatic cirrhosis, pulmonary infarction, and acute pericarditis, may lead to diagnostic errors. As the reactive changes in the mesothelial cells associated with these conditions overlap significantly with malignant cells, the floridly reactive mesothelial cells in such cases may be misinterpreted as malignant. A conservative approach is recommended when interpreting effusion cytology with such a clinical history.
Malignant effusions
Most recurrent and hemorrhagic effusions are caused by cancer. • A massive bloody effusion in the absence of trauma (and without coagulopathy) is almost always due to cancer. Malignant neoplasms of almost any site of origin, perhaps with the exception of the central nervous system, can cause effusions in serous cavities by direct invasion or metastasis. However, a rare case of involvement of the peritoneal cavity secondary to diffuse leptomeningeal gliomatosis in a patient with a ventriculoperitoneal shunt has been reported.[44]
In men, carcinoma of lung is the most common cause of malignant pleural effusions, followed by carcinomas of the gastrointestinal tract and pancreas. In women, it is carcinoma of the breast, followed by lung and ovary. Cancers of the gastrointestinal tract, ovary, and pancreas predominate as causes of effusions in the peritoneal cavity.
• In comparison with non-malignant effusions, a malignant effusion will re-accumulate rapidly. However, if the effusion is non-malignant, it may not recur or may take a longer time to do so. If the initial cytologic specimen is suspicious but not conclusive for malignancy, a repeat specimen is easy to obtain. It may lead to a definitive diagnosis. Therefore, cytologic findings that appear worrisome but not diagnostic, it is recommended not to rush and make a definitive diagnosis. Instead, a repeat tap should be requested when the effusion recurs. Adequate quantity (preferably more than 100 ml, up to 1000 ml) of such a repeat specimen should be submitted fresh immediately after collection to avoid the artifacts secondary to degenerative changes, which are usually the predominant cause of initial indeterminate interpretation. Relevant clinical details should be available. • As the number of neoplastic cells in recurrent malignant effusions often increase, with many cohesive clusters the cytologic interpretation of repeat specimen is usually definitive.
Neoplasms associated with malignant effusions may be of epithelial or non-epithelial type. Epithelial neoplasms may be metastatic carcinoma or malignant mesotheliomas. Nonepithelial neoplasms include hematologic neoplasms, melanoma, and sarcomas. With the exception of hematologic neoplasms such as lymphoma, non-epithelial neoplasms are rare in effusions.
Pediatric malignant effusions
A series of 226 effusions from 146 patients under the age of 18 years over a 40-year period showed that pleural effusion is the most frequent malignant effusion in children:[45] 47% (66/139) of pleural fluids were positive for neoplastic cells, followed by 23% (15/65) of ascitic fluids, 27% (4/15) of peritoneal washings, and 43% (3/7) of pericardial fluids. Lymphoma and leukemia (52%) were the commonest associated primary neoplasms, followed by neuroblastoma (14%), Wilms’ tumor (9%), gonadal and extragonadal germ cell neoplasms (8%), bone and soft tissue sarcomas (7%), epithelial neoplasms (5%), Ewing’s sarcoma (2%), and other neoplasms (3%).[45]
Another study[46] with similar results reported that:
most pediatric effusions are benign
malignant pediatric effusions are usually secondary to small round cell neoplasms, mostly lymphoma and leukemia
distinguishing neoplasms of the small cell type from mononuclear inflammatory cells is the major diagnostic pitfall
the role of peritoneal washings in the pediatric group is similar to that in adults.
WASHINGS, LAVAGES, BRUSHINGS, SCRAPINGS, AND TOUCH IMPRINTS
Although this series is predominantly about the cytopathology of serous effusions and washings, sometimes other types of specimens are submitted from the serous cavities for cytologic evaluation. Since they are evaluated in a manner similar to effusion fluids, with subtle specific modifications, these specimens will be referred to periodically. An entire review article in this series is dedicated to peritoneal washings.
It has been observed that, even without effusion, involvement of serosa by cancer cells correlates with poor prognosis. Upstaging of such cases has been recommended.[47–49] • The method used for evaluating cancer cells in this subset of cancer patients, without effusion at the time of presentation, involves retrieval of irrigated physiologic saline as washings or lavages.Peritoneal washing (peritoneal lavage, pelvic washing) is routinely performed for staging of gynecologic cancers[26] and other indications.[14,15] Cul-de-sac (pouch of Douglas) specimens may be submitted as aspirate[50,51] or as intraoperative collections. [52] Similarly, pleural lavages have been recommended for staging of lung cancer[14,26,47-49] and esophageal cancer.[53] Other methods such as pleural brushings[54] and intraoperative touch imprint cytology of visceral serosa[55] have also been reported. Other scrapings and brushings from serous cavities such as diaphragmatic scrapings have been submitted for evaluation of cancer spread and staging.[56] Rarely, a cytopathology laboratory may receive peritoneal dialysis fluid that frequently manifests cytologic atypia.[57] This warrants caution with peritoneal dialysis fluid specimens to avoid a potential pitfall of malignant misinterpretation.
ANCILLARY TECHNIQUES IN BRIEF
Ancillary techniques, including cell-block preparations for histochemistry [Figure 22] and immunocytochemistry introduce objectivity to the cytologic interpretation of effusions. Other applicable ancillary techniques include: electron microscopy (EM); flow cytometry, cytochemistry, immunocytochemistry on smears; fluorescent in-situ hybridization (FISH) and chromogenic in-situ hybridization (CISH); cytogenetics; DNA cytometry;[58,59] digitized imaging;[60] genetic molecular tests including polymerase chain reaction (PCR), including reverse-transcriptase PCR (RT-PCR); and other tests such as suppressive subtractive hybridization, laser capture micro-dissection, proteomics with surface-enhanced laser desorption ionization mass spectrometry (SELDI-MS), matrix-assisted laser desorption/ ionization mass spectrometry (MALDI-MS). Many of these techniques are not in clinical use at present, but they may be applied routinely in the future.
Electron microscopy had been recommended for the diagnosis of some subtypes of mesothelioma.[11-13,61] However, the role of electron microscopy of effusions has been decreasing with continuous technical advances in immunocytochemistry. Electron microscopy has been used to demonstrate the characteristic long, slender, and numerous, bushy microvilli in epithelioid mesothelioma.[62] However, microvilli may vary in number and slenderness not only from one neoplasm to another but also within the same neoplasm.[63] Cell-blocks provide more flexibility and options for various ancillary studies as clinically indicated.[64,65]
Acknowledgement
The authors thank Janavi Kolpekwar for her proof-reading support.
LIST OF ABBREVIATIONS (In alphabetic order)
Bx – Biopsy
CISH – Chromogenic in-situ hybridization
CMAS – CytoJournal Monograph and Atlas Series
DNA – Deoxyribonucleic acid
DQ – Diff-Quik stain
EM – Electron microscopy
FISH – Fluorescent in-situ hybridization
g/L – Gram per liter
HE – Hematoxylin-eosin stain
IUD – Intrauterine device
MALDI-MS – Matrix-assisted laser desorption/ionisation mass spectrometry
MGG – May-Grunwald Giemsa
N/C – Nuclear/cytoplasmic
PAP – Papanicolaou stain
PAS – Periodic acid-Schiff stain
PCR – Polymerase chain reaction
RT-PCR – Reverse transcriptase-PCR
RWS – Romanowsky stain
SELDI-MS – Surface-enhanced laser desorption ionization mass spectrometry
VEGF – Vascular endothelial growth factor.
References
- Malignant Effusions: A Multimodal Approach to Cytologic Diagnosis New York: Igaku-Shoin; 1994.
- [CrossRef] [Google Scholar]
- Serous membranes In: Sternberg SS, ed. Histology for Pathologists (2nd edn). Philadelphia: Lippincott-Raven; 1997. p. :223-239.
- [Google Scholar]
- The diagnostic utility of immunohistochemistry and electron microscopy in distinguishing between peritoneal mesotheliomas and serous carcinomas: a comparative study. Mod Pathol. 2006;19:34-48.
- [CrossRef] [PubMed] [Google Scholar]
- D2-40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372-380.
- [CrossRef] [PubMed] [Google Scholar]
- Podoplanin as a marker for mesothelioma. Pathol Int. 2005;55:83-86.
- [CrossRef] [PubMed] [Google Scholar]
- Detached ciliary tufts in female peritoneal washings. A common finding. Acta Cytol. 1987;31:841-844.
- [Google Scholar]
- ‘Collagen balls’ in peritoneal washings. Prevalence morphology, origin and significance. Acta Cytol. 1992;36:466-470.
- [Google Scholar]
- Curschmann’s spirals in pleural and peritoneal effusions. Acta Cytol. 1990;34:474-8.
- [Google Scholar]
- Endosalpingiosis in female peritoneal washings: A diagnostic pitfall. Int J Gynecol Pathol. 1987;6:340-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mullerian inclusions in peritoneal washings. Potential source of error in cytologic diagnosis. Acta Cytol. 1986;30:271-276.
- [Google Scholar]
- Mesothelioma with clear cell features: an ultrastructural and immunohistochemical study of 20 cases. Hum Pathol. 2005;36:465-473.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphohistiocytoid mesothelioma: a clinical, immunohistochemical and ultrastructural study of four cases and literature review. Ultrastruct Pathol. 2004;28:213-228.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative pleural lavage: is it a valid prognostic factor in lung cancer? Ann Thorac Surg. 2005;79:254-257.
- [CrossRef] [PubMed] [Google Scholar]
- Gynecol Oncol. 2005;98:179-81.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal lavage cytology: An assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397-403.
- [CrossRef] [PubMed] [Google Scholar]
- Abdominopelvic washings: A comprehensive review. Cytojournal. 2013;10:7.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical papillary proliferation in gynecologic patients: a study of 32 pelvic washes. Diagn Cytopathol. 2005;32:76-81.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal washings in ovarian tumors. Potential sources of error in cytologic diagnosis. Acta Cytol. 1985;29:310-316.
- [Google Scholar]
- Pleural, peritoneal, and pericardial fluids In: Bibbo M, ed. Comprehensive Cytopathology (2nd edn). Philadelphia: WB Saunders; 1997. p. :551-621.
- [Google Scholar]
- Posttraumatic pleural-fluid and blood eosinophilia. JAMA. 1975;234:625-626.
- [CrossRef] [PubMed] [Google Scholar]
- Megakaryocytes in a hemorrhagic pleural effusion caused by anticoagulant overdose. Acta Cytol. 1986;30:163-165.
- [Google Scholar]
- Significance of psammoma bodies in serous cavity fluid: a cytopathologic analysis. Cancer. 2004;102:87-91.
- [CrossRef] [PubMed] [Google Scholar]
- Increase in the incidence of peritoneal collagen balls over a 10-year period. Acta Cytol. 2005;49:387-390.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic pitfalls of peritoneal washing cytology and the role of cell blocks in their diagnosis. Diagn Cytopathol. 2003;28:335-341.
- [CrossRef] [PubMed] [Google Scholar]
- Appearance of ‘collagen balls’ in ascitic fluid cytology with abdominal cocoon (encapsulating peritonitis) Diagn Cytopathol. 1997;16:469-470.
- [CrossRef] [Google Scholar]
- Detached ciliary tufts. Comparison with intestinal protozoa and a review of the literature. Am J Clin Pathol. 1990;93:541-545.
- [CrossRef] [PubMed] [Google Scholar]
- Detached ciliary tufts mistaken for peritoneal parasites: a warning. Rev Infect Dis. 1988;10:1044-1047.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory cytology In: Atkinson BF, ed. Atlas of Diagnostic Cytopathology (2nd edn). Philadelphia: WB Saunders; 2004. p. :273-356.
- [Google Scholar]
- Ectopic pancreas. A cause of false-positive peritoneal cytology. Acta Cytol. 1990;34:641-644.
- [Google Scholar]
- The role of angiogenesis in the accumulation of peritoneal fluid in benign conditions and the development of malignant ascites in the female. Gynecol Obstet Invest. 2000;50:217-224.
- [CrossRef] [PubMed] [Google Scholar]
- Pleural, pericardial, and peritoneal fluids In: Cibas ES, Ducatman BS, eds. Cytology Diagnostic Principles and Clinical Correlates (2nd edn). Philadelphia: WB Saunders; 2003. p. :119-144.
- [Google Scholar]
- Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507-513.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc. 1985;60:158-164.
- [CrossRef] [Google Scholar]
- Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991;4:320-324.
- [Google Scholar]
- Diagnostic value of pericardial biopsy: improvement with extensive sampling enabled by pericardioscopy. Circulation. 2003;107:978-983.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991;4:320-324.
- [Google Scholar]
- The malignant pleural effusion: a review of cytopathologic diagnosis of 584 specimens from 472 consecutive patients. Cancer. 1985;56:905-909.
- [CrossRef] [Google Scholar]
- Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20:350-357.
- [CrossRef] [Google Scholar]
- The value of multiple fluid specimens in the cytologic diagnosis of malignancy. Mod Pathol. 1994;7:665-668.
- [Google Scholar]
- Disseminated primary diffuse leptomeningeal gliomatosis: a case report with liquid based and conventional smear cytology. Cytojournal. 2005;2:16. Free full text is available at: http://www.cytojournal.com/content/2/1/16
- [CrossRef] [PubMed] [Google Scholar]
- Cytology of pleural, peritoneal and pericardial fluids in children. A 40-year summary. Acta Cytol. 1997;41:467-473.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology of fluids from pleural, peritoneal and pericardial cavities in children. A comprehensive survey. Acta Cytol. 1994;38:209-217.
- [Google Scholar]
- Pleural lavage cytology before and after lung resection in non-small cell lung cancer patients. Ann Thorac Surg. 2006;81:298-304.
- [CrossRef] [PubMed] [Google Scholar]
- Pleural lavage: a novel diagnostic approach for diagnosing exudative pleural effusion. Lung. 2000;178:371-379.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor cells in intraoperative pleural lavage. An indicator for the poor prognosis of bronchogenic carcinoma. Cancer. 1990;65:1801-1804.
- [CrossRef] [Google Scholar]
- Aspiration cytology from the pouch of Douglas at hysteroscopy. Cytopathology. 2001;12:44-47.
- [CrossRef] [PubMed] [Google Scholar]
- [Practical postgraduate training in pediatrics] Pediatriia. 1978;9:70-72. [in Russian]
- [Google Scholar]
- Clinical and cytological aspects of primary fallopian tube carcinoma. A report of ten cases. Acta Cytol. 1987;31:834-840.
- [Google Scholar]
- Pleural lavage cytology in esophageal cancer without pleural effusions: clinicopathologic analysis. Eur J Cardiothorac Surg. 2000;17:575-579.
- [CrossRef] [Google Scholar]
- Diagnosis of visceral pleural invasion by lung cancer using intraoperative touch cytology. Ann Thorac Surg. 2002;73:1552-1557.
- [CrossRef] [Google Scholar]
- Comparison of diaphragmatic wash and scrape specimens in staging of women with ovarian cancer. Gynecol Oncol. 2001;81:461-465.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic features of atypical mesothelial cells in peritoneal dialysis fluid. Diagn Cytopathol. 1990;6:22-26.
- [CrossRef] [PubMed] [Google Scholar]
- Benign and malignant cells in effusions: diagnostic value of image DNA cytometry in comparison to cytological analysis. Pathol Res Pract. 1998;194:791-795.
- [CrossRef] [Google Scholar]
- Analysis of pleural effusions using flow cytometry. Respiration. 1996;63:17-24.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of conventional microscopy and digitized imaging for diagnosis in serous effusions. Anal Quant Cytol Histol. 1997;19:202-206.
- [Google Scholar]
- The distinction of mesothelioma from adenocarcinoma in malignant effusions by electron microscopy. Acta Cytol. 1985;29:219-225.
- [Google Scholar]
- The immunohistochemical diagnosis of mesothelioma: Differentiation of mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 1989;13:276-91.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-blocks and other ancillary studies (including molecular pathology and proteomics) CytoJournal. 2021;18:4.
- [CrossRef] [PubMed] [Google Scholar]
- CellBlockistry 101 In: CytoJournal Monograph (CMAS) (1st ed). Cytopathology Foundation Inc; 2021.
- [Google Scholar]








