Translate this page into:
Large lung mass: Cytopathological features
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
PERTINENT CLINICAL HISTORY
A Japanese woman in 50s presented with blood-tinged sputum. Chest X-ray and computed tomography scan revealed a large mass lesion in the lung. She had no asbestos exposure. It was considered to be a primary pulmonary malignant tumor because no tumorous lesions were found at any other sites, including the soft tissue. After the preoperative bronchial brushing cytology [Figure 1] was performed, she underwent lobectomy.
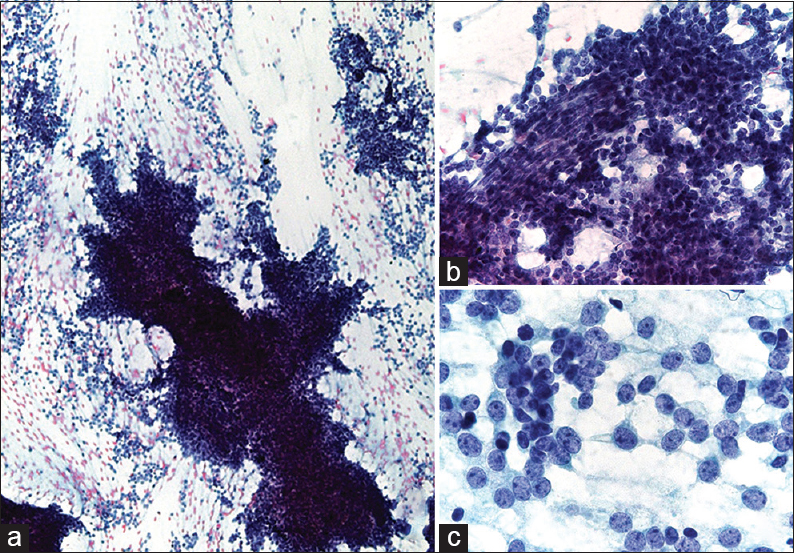
- (a) The cytologic smear is hypercellular and consists of cell clusters with frayed edges and numerous single cells. (b) The cluster is composed of loosely cohesive, small, round to oval, and short spindle to spindle cells surrounding the capillary. The small round cells have scant cytoplasm with hyperchromatic and inconspicuous or absent nucleoli. (c) Most tumor cells are uniformly small, round to oval with high nuclear-cytoplasmic ratio. The nuclear chromatin is finely granular with several small nucleoli
QUESTION
What is your interpretation?
-
Malignant mesothelioma
-
Carcinosarcoma
-
Synovial sarcoma (SS)
-
Malignant peripheral nerve sheath tumor.
ANSWER
The correct cytopathologic interpretation is C. Synovial sarcoma
BRIEF DISCUSSION
The resected tumors, measuring 11 cm × 7 cm, were well circumscribed and whitish-yellow in color with extensive hemorrhage and necrosis. Histopathologically, tumor cells showed small, round, oval to short-spindled cells with high nuclear-cytoplasmic (N/C) ratio proliferated in a vaguely intersecting fascicular pattern [Figures 2 and 3]. Focally, hemangiopericytomatous staghorn-like configurations were also observed [Figure 2]. Mitotic figures were frequent. In addition, it showed areas of small round cells in a solid sheet pattern. Pleural or vascular invasion was not detected.
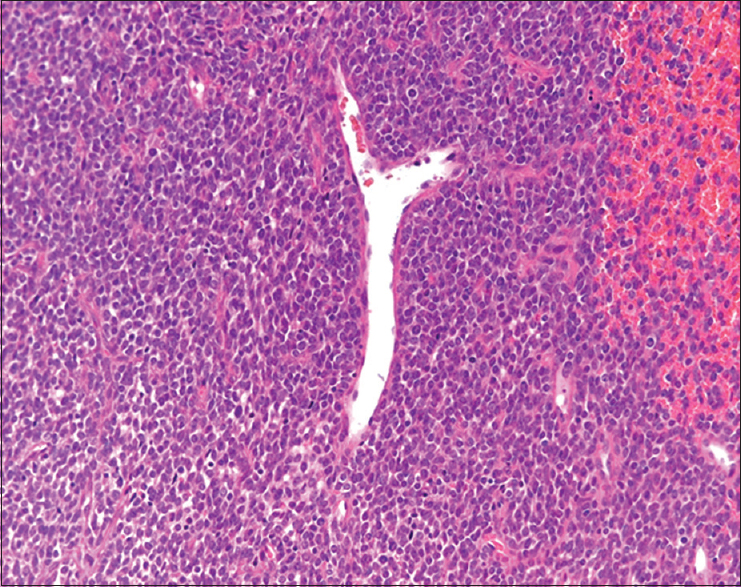
- The small round cells and short-spindled cells showed an intersecting fascicular pattern. Focally, hemangiopericytomatous staghorn-like configurations were observed
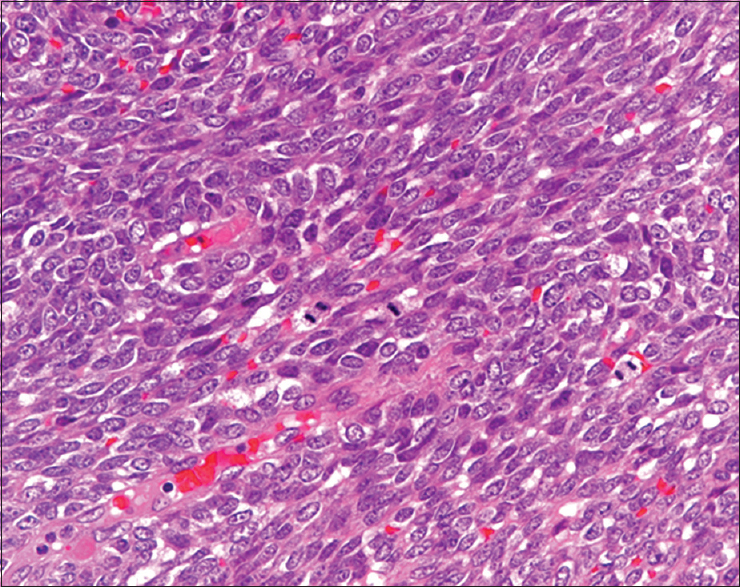
- Small, oval to short-spindled cells with high nuclear-cytoplasmic ratio proliferate in a vaguely intersecting fascicular pattern. Mitotic figures are observed
ADDITIONAL QUIZ QUESTIONS
Q1. Which of the following cytomorphologic and histopathological pattern is the most frequent for typical SS?
-
Biphasic
-
Monophasic fibrous
-
Monophasic epithelial
-
Poorly differentiated.
Q2. What is the most useful immunohistochemistry for diagnosis?
-
TLE1
-
STAS6
-
SOX10
-
NKX3.1.
Q3. What is the most characteristic gene translocation?
-
EWSR1-FLI1
-
NAB2-STAT6
-
ETV6-NTRK
-
SYT-SSX.
ANSWERS TO ADDITIONAL QUIZ QUESTIONS
Q1; B, Q2; A, Q3; D
Immunohistochemically, the tumor was positive for vimentin and MIC2 (CD99) and focally for epithelial membrane antigen but negative for CD34. Recently, although the immunohistochemical staining for TLE1 is specific for diagnosis, the SYT/SSX1 fusion gene was detected by real-time polymerase chain reaction and direct sequencing. Finally, she was diagnosed with monophasic fibrous SS, because the poorly differentiated component was focal.
BRIEF REVIEW OF THE TOPIC
SS is usually recognized as a malignant soft-tissue tumor of uncertain differentiation with a predilection for the extremities in adolescents and young adults.[12] However, SS rarely arises at other anatomic sites, including internal organs such as the lung, heart, kidney, liver, gastrointestinal tract, and others.[12] Because of the unusual location, SS of the internal organs is occasionally misdiagnosed.
Primary pulmonary SS should be differentiated from metastatic SS, which depends on whether primary SS at another site is present or not. Histologically, SS is classified into four subtypes: biphasic, monophasic epithelial, monophasic fibrous, and poorly differentiated. SS of the monophasic fibrous type is most common at all sites.[123456789101112] The present patient was diagnosed with monophasic SS, but small round cells suggestive of poorly differentiated SS of small-cell type were also presented. Including SS of both soft tissue and unusual sites, there are >10 reports describing cytologic features, only a few of which describe primary and metastatic pulmonary SS.[23569101112] Immunohistochemical staining for TLE1 is considered specific for diagnosis. In addition, SMARCB1/INI-1 expression is reduced in 69% of SS.[13]
Recently, reports of SS at unusual sites have been increasing with the advance of immunohistochemical and cytogenetic techniques. Especially, detection of SS-specific chromosomal translocation t(X; 18) and the subsequent SYT-SSX fusion gene is diagnostic.[1] Primary pulmonary SS is now well recognized, and about 60 cases had been reported in the English literature up to 2005.[3] The clinicopathologic findings of pulmonary SS are similar to those of soft-tissue SS, except for its location and site-specific symptoms.
There are no differences in histologic and cytologic features between SS of the soft tissue and the lung.[23456789101112] According to the previous reports, SS shows some common characteristic cytologic features, that is, high cellularity and admixture of branching or irregularly shaped clusters with frayed edges and single cells.[23456789101112] Individual cells are uniformly small to short spindled with high N/C ratio, scant cytoplasm, finely granular chromatin, and small nucleoli. In case, small round cells are mixed, so-called small–round-cell tumors such as small-cell carcinoma, Ewing's sarcoma should be excluded from the study.[46] Actually, small-cell carcinoma was cytologically suspected in the present case because of the small-sized cells and the patient's age (in her 50s). However, upon reviewing the smear, the tumor cells lacked the cytologic characteristics of small-cell carcinoma, that is, nuclear hyperchromasia, nuclear molding, and no nucleoli. A definite cytologic diagnosis of pulmonary SS may be difficult or impossible to make without immunohistochemical and cytogenetic examination, but recognition of this entity is important to avoid misdiagnosis.[6] In cytopathology, there are solitary fibrous tumor, malignant peripheral nerve sheath tumor, or so-called fibrosarcoma for the differential diagnosis of SS (especially monophasic fibrous) in pulmonary [Figure 4]. These tumors' feature is similar to SS, so the diagnosis of SS may be difficult by the morphological characteristics only. Immunohistochemical panel (TLE1, STAT6) and evaluation of SYT/SSX translocation are useful.
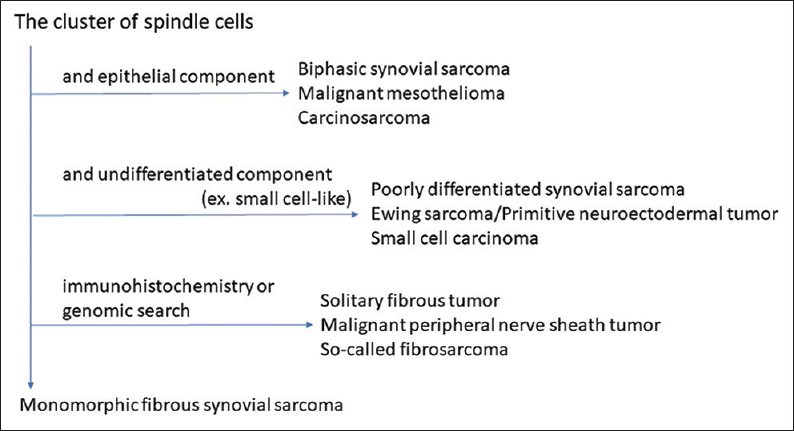
- The algorithmic approach of cytological feature
SUMMARY
In common with SS of the soft tissue, the cytologic features of pulmonary SS are characterized by hypercellular smears composed of small, oval to short-spindled single cells, and branching or irregular-shaped clusters with frayed edges and thin-walled capillaries. Since the tumor cells of SS are frequently small sized with scant cytoplasm, it is important not to confuse them with so-called small-round-cell tumors. For SS cases with metastasis, the cell block from cytology specimen can help with genetic research.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that we have no competing interests.
AUTHORSHIP STATEMENTS BY ALL AUTHORS
All authors have participated sufficiently and took responsibility for the appropriateness of content in this article.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board of all the institutions associated with this study as applicable.
LIST OF ABBREVIATIONS (In alphabetic order)
CD - Cluster of differentiation,
ETV6 - ETS variant 6 transcription factor,
EWSR - Ewing sarcoma,
FLI1 - Friend leukemia integration 1 transcription factor,
INI-1 - Integrase Interactor 1,
MIC2 - Microneme protein 2,
N/C ratio; nuclear-cytoplasmic ration,
NAB2 - NGFI-A-binding protein 2,
NKX3.1 - NK3 Homeobox 1,
NTRK - Neurotrophic tyrosine receptor kinase,
SMARCB1 - SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1,
SOX10 - SRY-related HMG-box 10,
SS - Synovial sarcoma,
SSX - Synovial sarcoma, X breakpoint 2,
STAT6 - Signal transducer and activator of transcription 6,
SYT - Synovial sarcoma translocation, chromosome 18,
TLE1 - Transducin-like enhancer of split 1
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Primary pulmonary synovial sarcoma: A clinicopathologic, immunohistochemical, and molecular study of 11 cases. Hum Pathol. 2004;35:850-6.
- [Google Scholar]
- Primary pulmonary synovial sarcoma. In: Hasleton P, Flieder DB, eds. Spencer's Pathology of the Lung (6th ed). New York: Cambridge University Press; 2013.
- [Google Scholar]
- Primary monophasic synovial sarcoma lung with brain metastasis diagnosed on transthoracic FNAC: Report of a case with literature review. Lung India. 2012;29:384-7.
- [Google Scholar]
- Fine-needle aspiration of synovial sarcoma: Criteria for diagnosis: Retrospective reexamination of 37 cases, including ancillary diagnostics. A Scandinavian sarcoma group study. Diagn Cytopathol. 2003;28:232-8.
- [Google Scholar]
- Monophasic synovial sarcoma: A cytologic spectrum. Diagn Cytopathol. 2004;30:19-23.
- [Google Scholar]
- PNET-like features of synovial sarcoma of the lung: A pitfall in the cytologic diagnosis of soft-tissue tumors. Diagn Cytopathol. 2001;24:283-8.
- [Google Scholar]
- Aspiration cytology of biphasic and monophasic synovial sarcoma: A report of two cases. Acta Cytol. 1993;37:413-7.
- [Google Scholar]
- Fine-needle aspiration biopsy of synovial sarcoma. A cytomorphologic analysis of primary, recurrent, and metastatic tumors. Am J Clin Pathol. 1996;106:769-75.
- [Google Scholar]
- Fine needle aspiration biopsy of monophasic spindle synovial sarcoma of lung with fluorescence in situ hybridization identification of t(x; 18) translocation: A case report. Acta Cytol. 2009;53:105-8.
- [Google Scholar]
- Primary pulmonary synovial sarcoma-transbronchial needle aspiration is a diagnostic approach: A case report with cytological features. Cytopathology. 2010;21:52-5.
- [Google Scholar]
- Synovial sarcoma presenting as a lung mass and diagnosed by cytology. Diagn Cytopathol. 2016;44:434-7.
- [Google Scholar]
- Diagnosis of a rare primary pulmonary synovial sarcoma with endobronchial ultrasound-guided transbronchial needle aspiration. Thorac Cancer. 2016;7:684-8.
- [Google Scholar]
- Reduced expression of SMARCB1/INI1 protein in synovial sarcoma. Mod Pathol. 2010;23:981-90.
- [Google Scholar]







