Translate this page into:
Mesothelioma

-
Received: ,
Accepted: ,
How to cite this article: Rao N, Wei S. Mesothelioma. CytoJournal 2022;19:10.
Abstract
Mesothelioma arises from the surface serosal cells lining the pleural, peritoneal, and pericardial cavities. It has three variants including: epithelioid, sarcomatous/desmoplastic, and biphasic types. Mesothelioma cells, predominantly of the epithelioid type, can shed into effusions as sheets, clusters/ morulae, papillae, or single cells. The challenges to cytologic diagnosis of mesothelioma are two-fold: 1. distinguishing mesothelial cells from metastatic malignant (most commonly carcinoma) cells; 2. distinguishing reactive mesothelial from mesothelioma cells. Immunocytochemistry is a helpful aid to cytologic evaluation for the former. The distinction of reactive mesothelial cells from mesothelioma can be more difficult, as there is considerable overlap in their appearances in effusion specimens. Recently developed ancillary molecular and genetic tests are proving to be useful in confirming the diagnosis of malignant mesothelioma in cytology specimens.
Keywords
Mesothelioma
Cytomorphology
Cell-block
Immunocytochemistry
Molecular and genetic tests

INTRODUCTION
Mesothelioma is a tumor that arises from the surface serosal cells lining the pleural, peritoneal, and pericardial cavities. Pleural mesotheliomas are the commonest (~90%), followed by peritoneal and pericardial mesotheliomas (6–10%).1 Other locations are vanishingly rare. A strong link exists between mesothelioma and asbestos exposure, first reported in 1960.2 Recently, genomic analyses have revealed several genetic mutations in mesothelioma. The commonly mutated genes include BAP1, NF2, TP53, SETD2, DDX3X, ULK2, RYR2, CFAP45 and SETD1.[3]
Ninety percent of patients with pleural mesothelioma present with pleuritic pain associated with recurrent, unilateral, bloody pleural effusions which usually contain malignant mesothelial cells.[4] Therefore, effusion cytology assumes significant clinical importance in the diagnosis of mesotheliomas. Nevertheless, cytologic diagnosis of effusions of unknown etiology is difficult. Diagnostic accuracy of effusion cytology is variable, with a particular problem being relatively high false-negative rates, owing to sampling and screening errors. [5] In addition, there is the well-known cytologic problem of distinguishing between reactive inflammatory/hyperplastic and neoplastic mesothelial cells, and in distinguishing between mesothelioma and adenocarcinoma cells.[5] The use of adjuvant methods is therefore highly recommended and performed as a routine in most centers.[5]
CYTOLOGIC FEATURES OF MESOTHELIOMA
Cytologic diagnosis of mesothelioma is a difficult proposition, as mentioned, mainly because of the significant overlap that exists between benign and malignant mesothelial cells, and between mesothelioma and adenocarcinoma cells. The basic diagnostic feature of mesothelioma is the resemblance to ‘normal’ or reactive mesothelial cells. On the other hand, this resemblance is at the root of a major diagnostic conundrum; i.e. is this reactive or a neoplastic mesothelial cell population? Diagnostic cytologic criteria have been outlined by various authors.[6-11]
Relatively constant cytologic features seen in retrospective studies, which are useful for a diagnosis of mesothelioma (expanded below), include the presence of a single malignant mesothelial cell population, multinucleation, articulation between mesothelial cells (intercellular windows), cell-in-cell arrangements, cytoplasmic vacuoles, peripheral blebs, cluster formation with knobby outlines (scalloped borders), variable nuclear enlargement, prominent nucleoli, and cytoplasmic metachromasia. Based on morphologic cell types, mesotheliomas have been divided into epithelioid, sarcomatoid (including desmoplastic mesothelioma), and biphasic varieties. Effusion specimens from epithelioid mesotheliomas are generally hypercellular, whereas the spindle cell components in biphasic and sarcomatoid mesotheliomas do not usually exfoliate into effusion fluids.[6]
A laboratory can decide on the optimal preparation methods for handling an effusion fluid specimen for cytologic examination. Regardless of the preparation method used, the Papanicolaou (PAP) and a Romanowsky stain are almost always used. Splitting the sample facilitates evaluation of both nuclear (PAP) and cytoplasmic (Romanowsky) characteristics and is optimal for identifying the various discriminatory features of mesothelial cells and adenocarcinoma cells. In our laboratory a Cytospin preparation stained with Diff-Quik (DQ) (Romanowsky), and a liquid-based cytology preparation stained using the PAP stain are used. A cell block [Figures 7 and 8] is also mandatorily performed whenever a diagnosis of malignancy is clinically entertained and/or cytologically suspected. Apart from providing an additional perspective to the cytologic assessment, the cell block facilitates performance of immunocytochemistry epithelioid mesotheliomas exfoliate richly into effusion fluids, producing hypercellular specimens with monolayered sheets as well as three-dimensional cell groups [Figure 1]. These groups can take various forms, but characteristic of mesothelioma are clusters of cells with irregular, knobby outlines [Figure 3]. Sometimes they take on a papillary architecture, reproducing a common pattern seen histologically [see Figure 3]. Acinar formation (a feature of adenocarcinoma) is not seen in mesotheliomas, although intercellular windows between mesothelial cells may appear like acini on occasion [Figure 5]. Cell engulfment is a common feature. Benign effusions are relatively less cellular, with smaller cell groups that are largely two-dimensional [Figure 2]. This is an important feature, because even though the individual cells may show considerable atypia, the two-dimensional nature of the cell groups is retained.
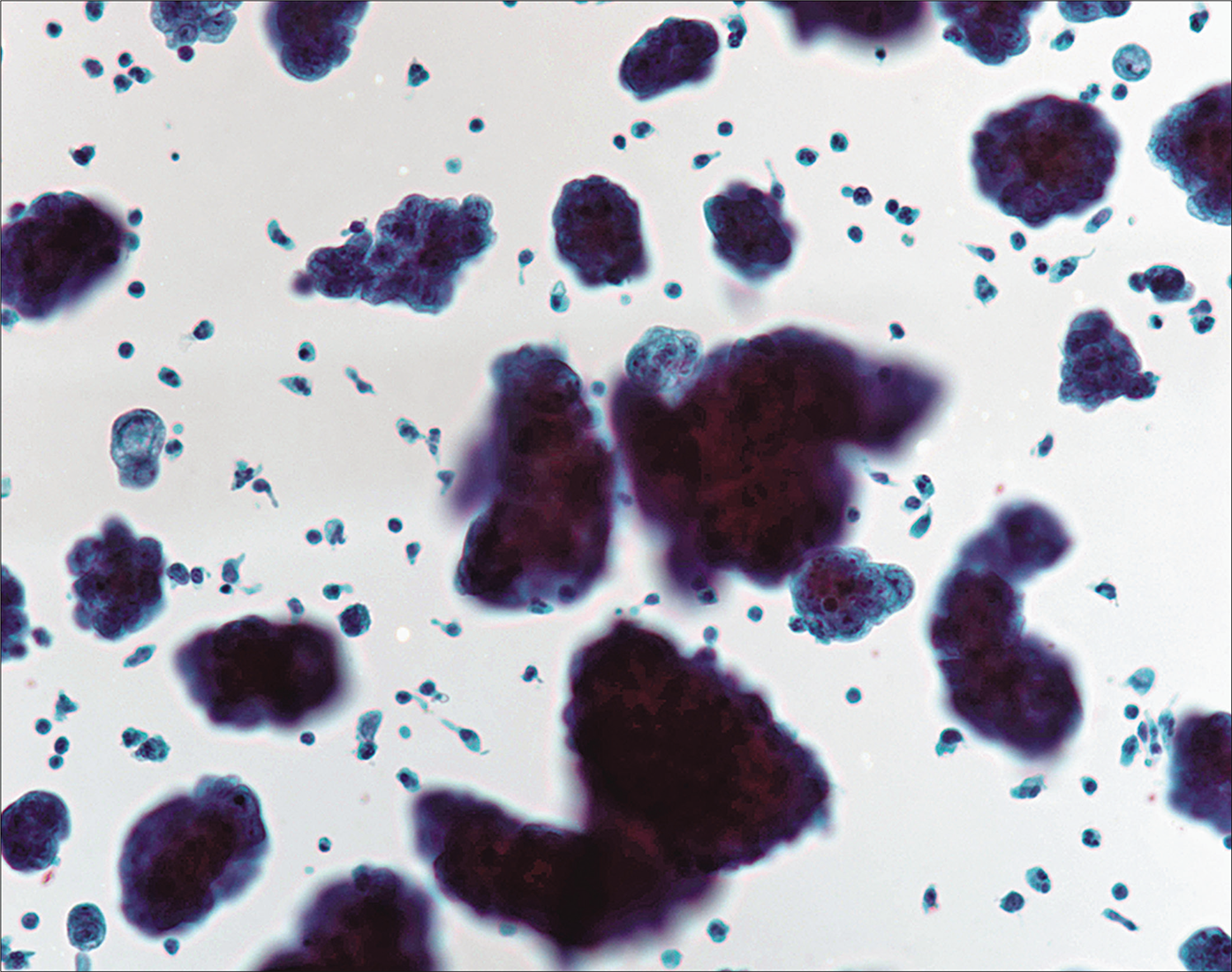
- Hypercellular specimen in a case of mesothelioma with three-dimensional clusters and two-dimensional sheets. [Autocyte Prep, PAP stain, 10X.]
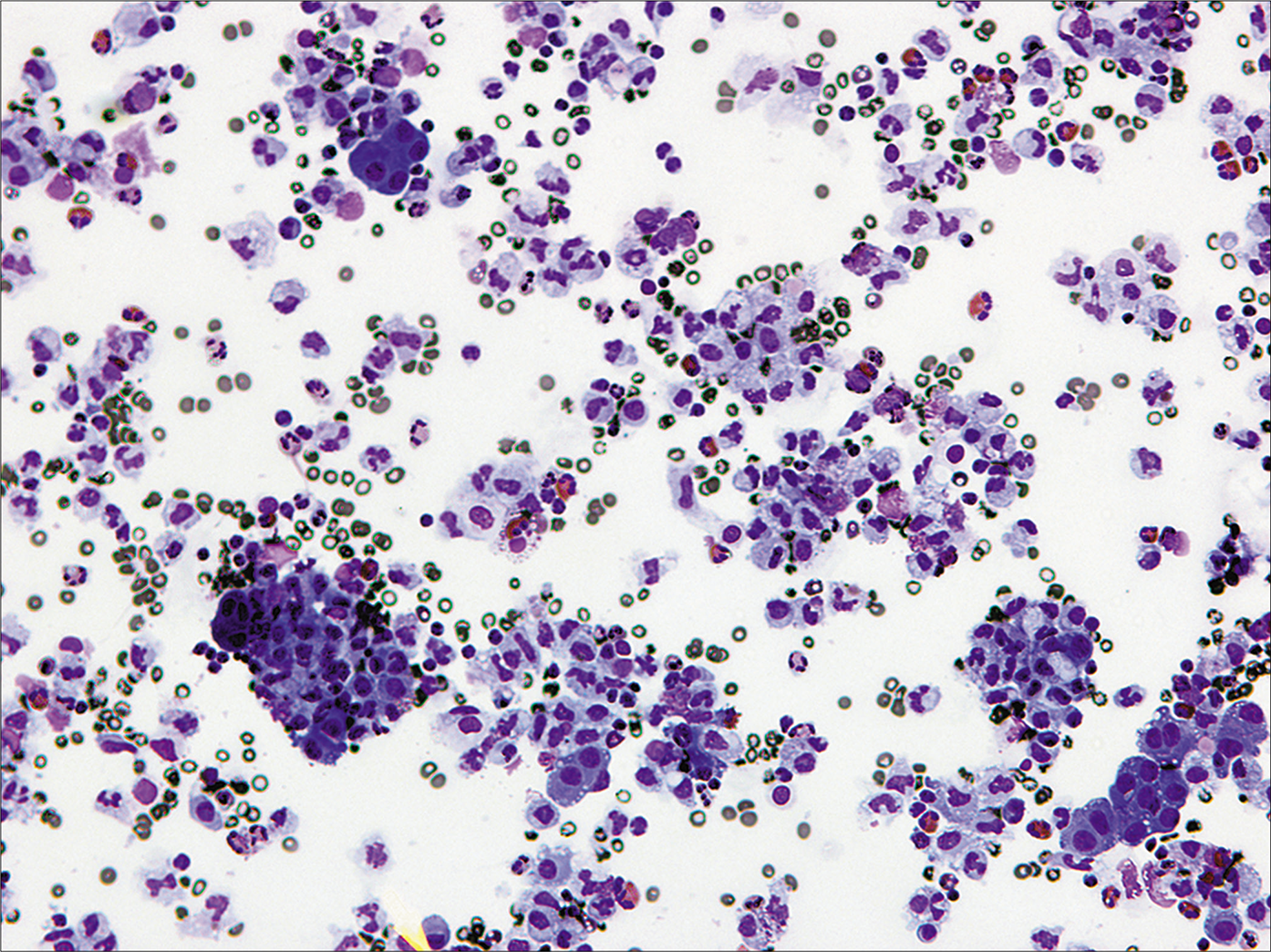
- Moderately cellular specimen of pleural fluid with reactive mesothelial cells with dense cytoplasm arranged in two dimensional groups. Admixed macrophages with feathery cytoplasm and inflammatory cells are present in the background. [DQ, 20X.]
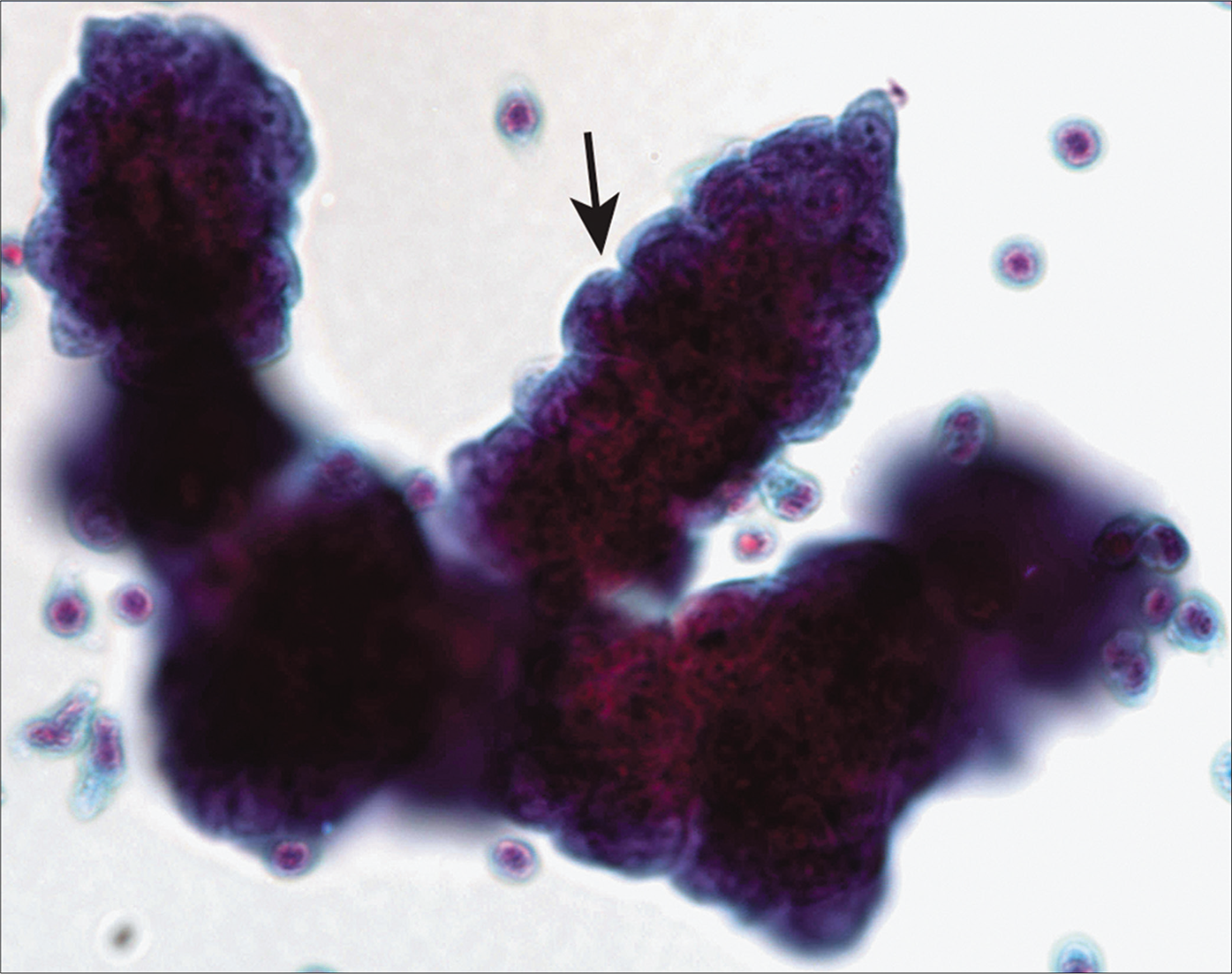
- Three-dimensional papillary groups with knobby outlines (arrow) in a case of mesothelioma. [Autocyte Prep, PAP stain, 20X.]
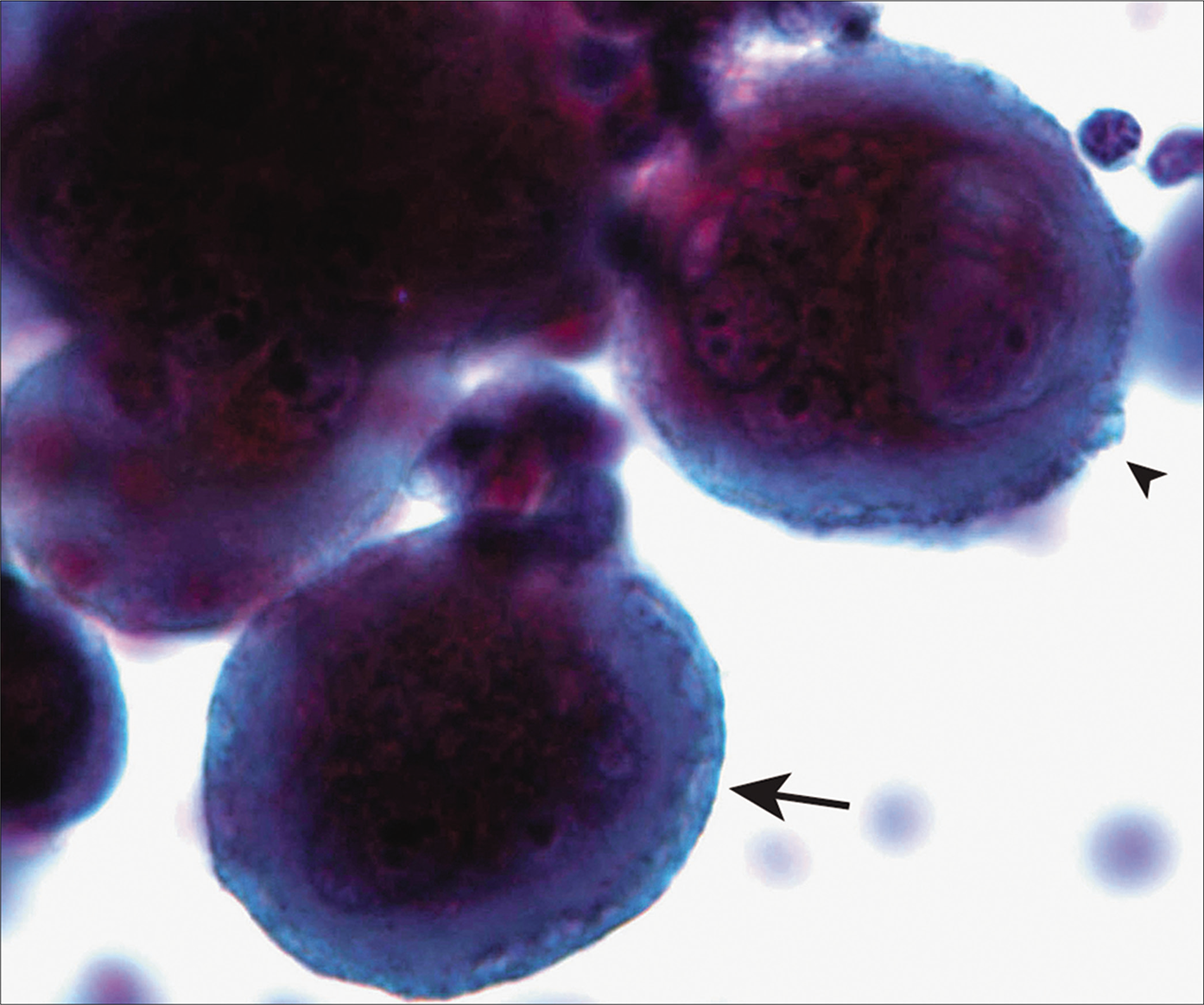
- Mesothelial cells with two-tone cytoplasm (arrow) and peripheral cytoplasmic blebs (arrowhead). [Autocyte Prep, PAP stain 40X.]
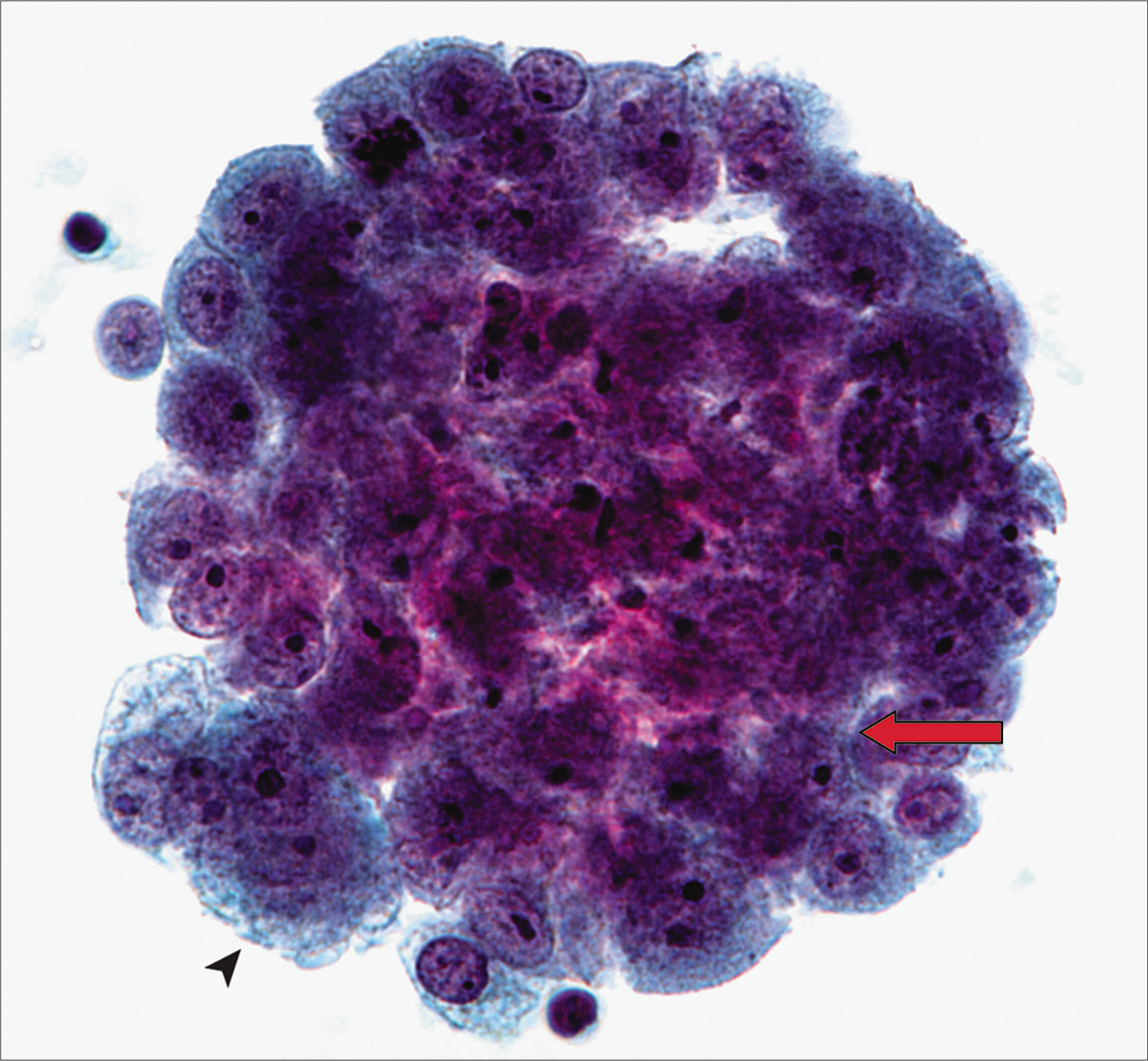
- Two-dimensional mesothelial cell groups in a case of mesothelioma with intercellular windows (arrow). Also note a multinucleate atypical mesothelial cell at the periphery of the group (arrowhead). [Thinprep, PAP stain, 40X.]
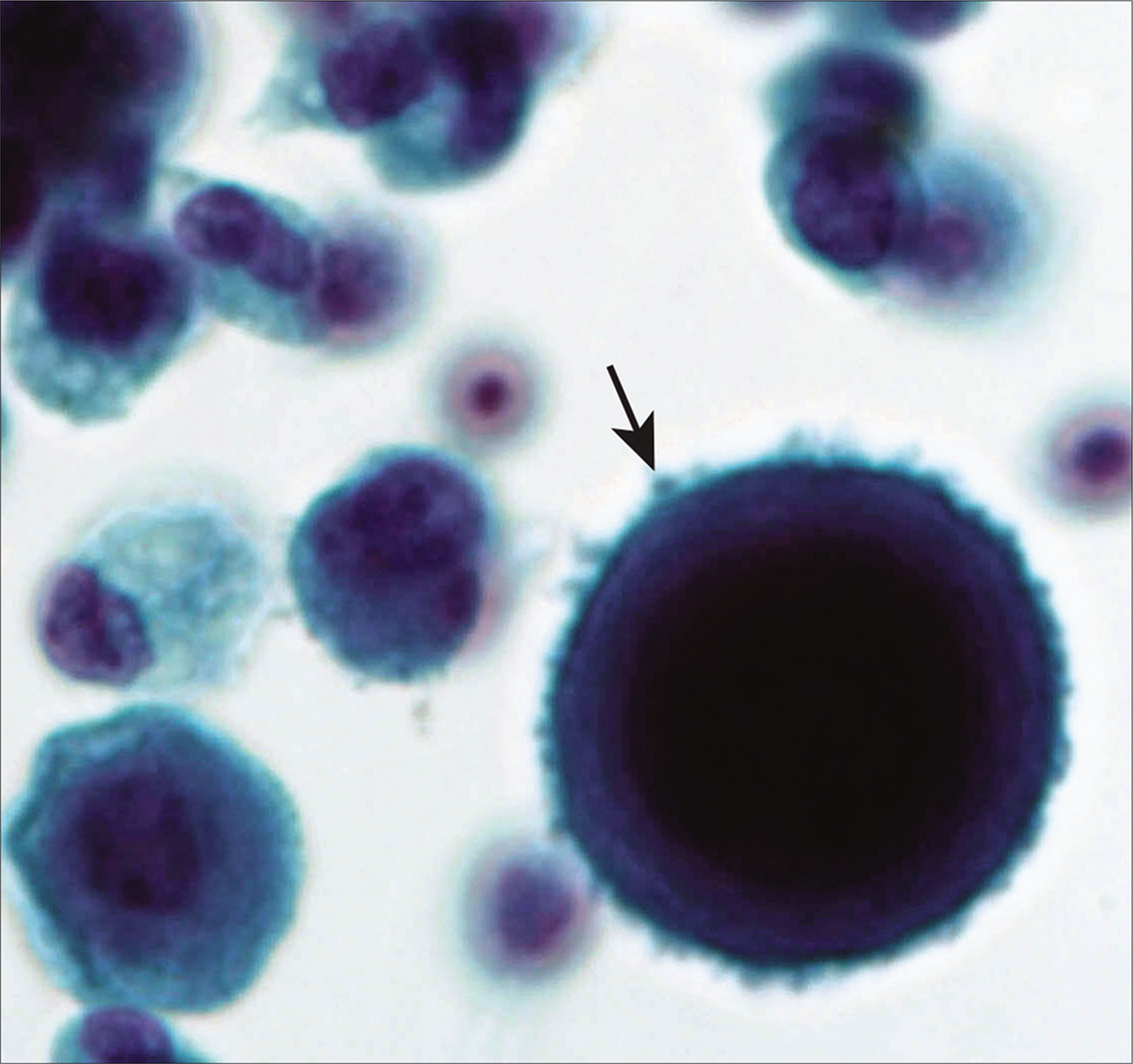
- Mesothelial cell with prominent microvilli (arrow). [Autocyte Prep, PAP stain, 60X.]
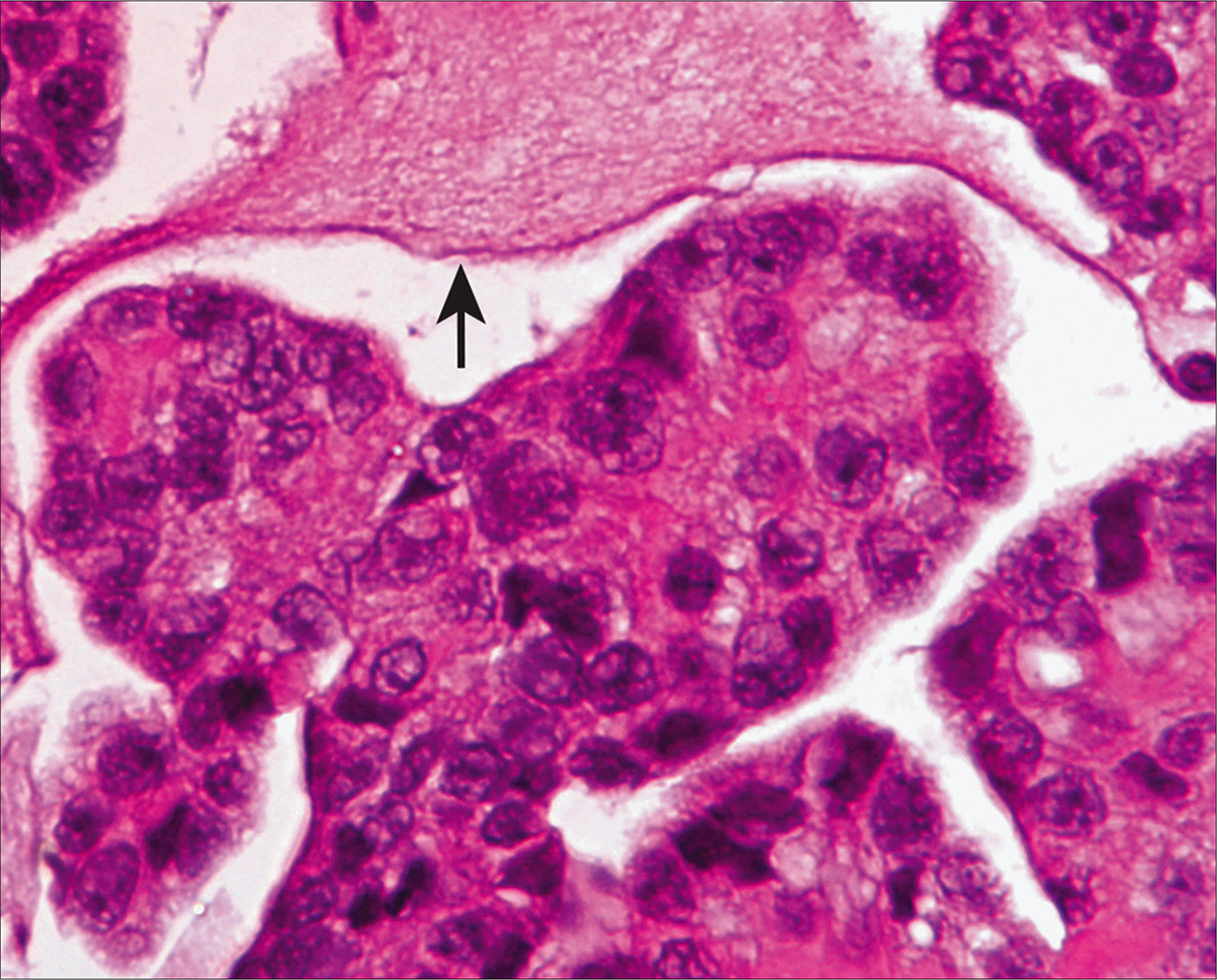
- Cell block from a case of mesothelioma showing hypercellular atypical mesothelial cell groups. Extracellular mucinous material is present in the background (arrow). [Hematoxylin and eosin (HE), 40X.]
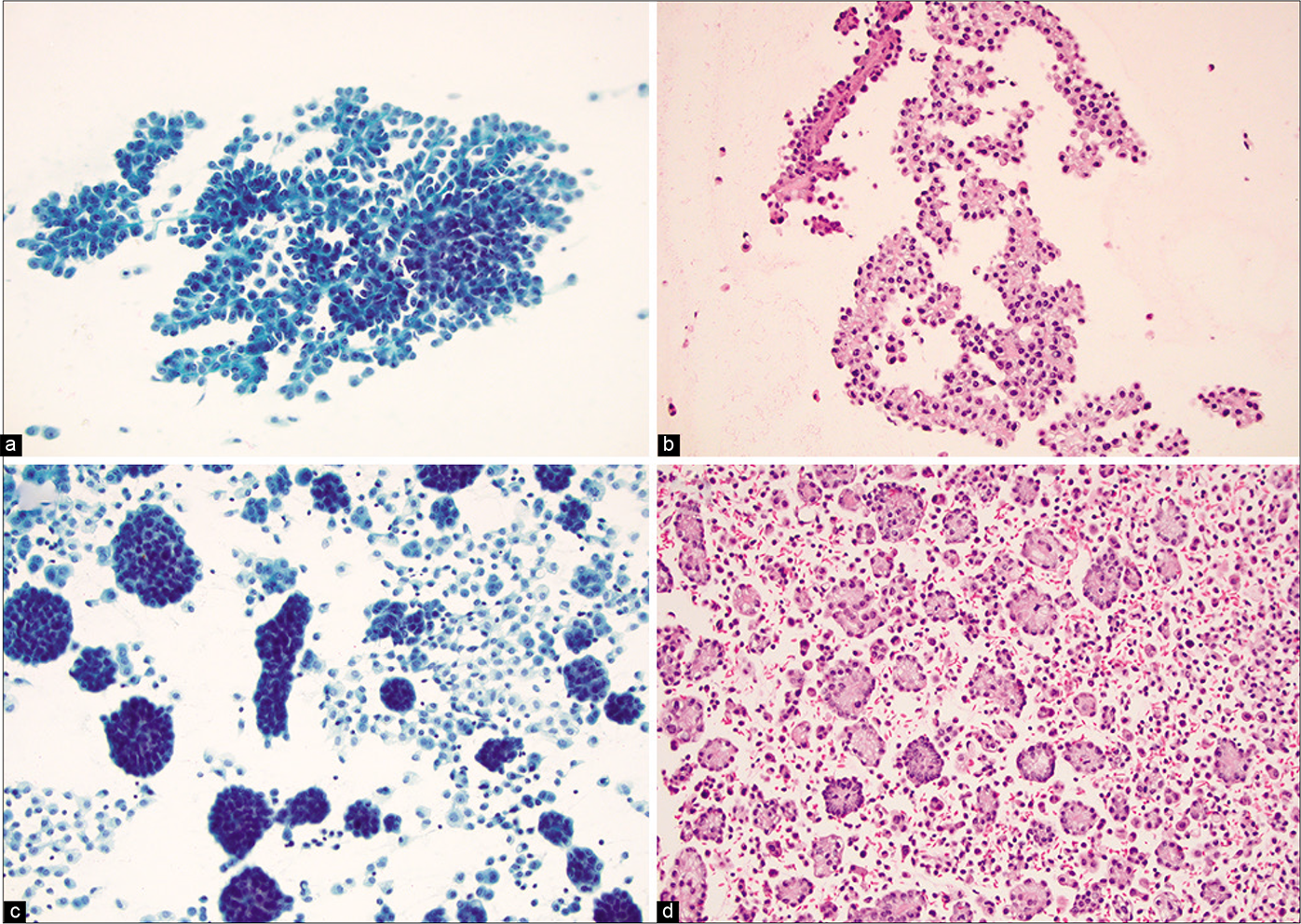
- (a) Mesothelioma presents with papillary architecture (Pap stained smear). (b) The corresponding cell block from Fig 8a (Hematoxylin and eosin). (c) Clusters of morulae comprised of mesothelioma cells (Pap stained smear). (d) The corresponding cell block of Fig 8c (Hematoxylin and eosin).
In adenocarcinoma, although the cell groups are three-dimensional and complex, they generally retain a smooth contour (the so-called ‘community border’) [Figure 11] as opposed to the knobby outlines of mesothelioma cell groups [Figures 3, 5].
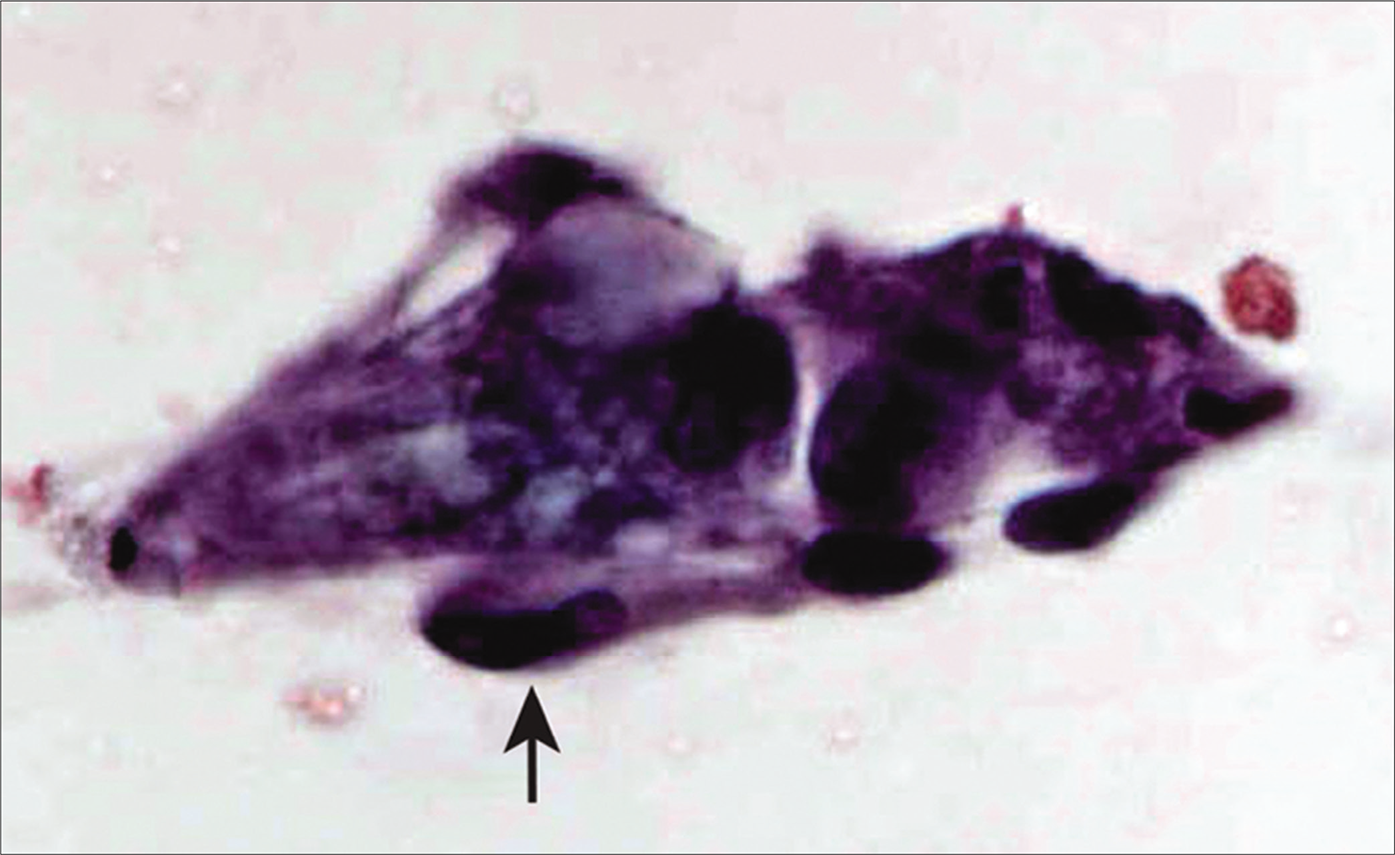
- Loosely cohesive small group of spindled mesothelial cells (arrow) in a case of biphasic mesotheliom a [Autocyte Prep, PAP stain, 40X.].
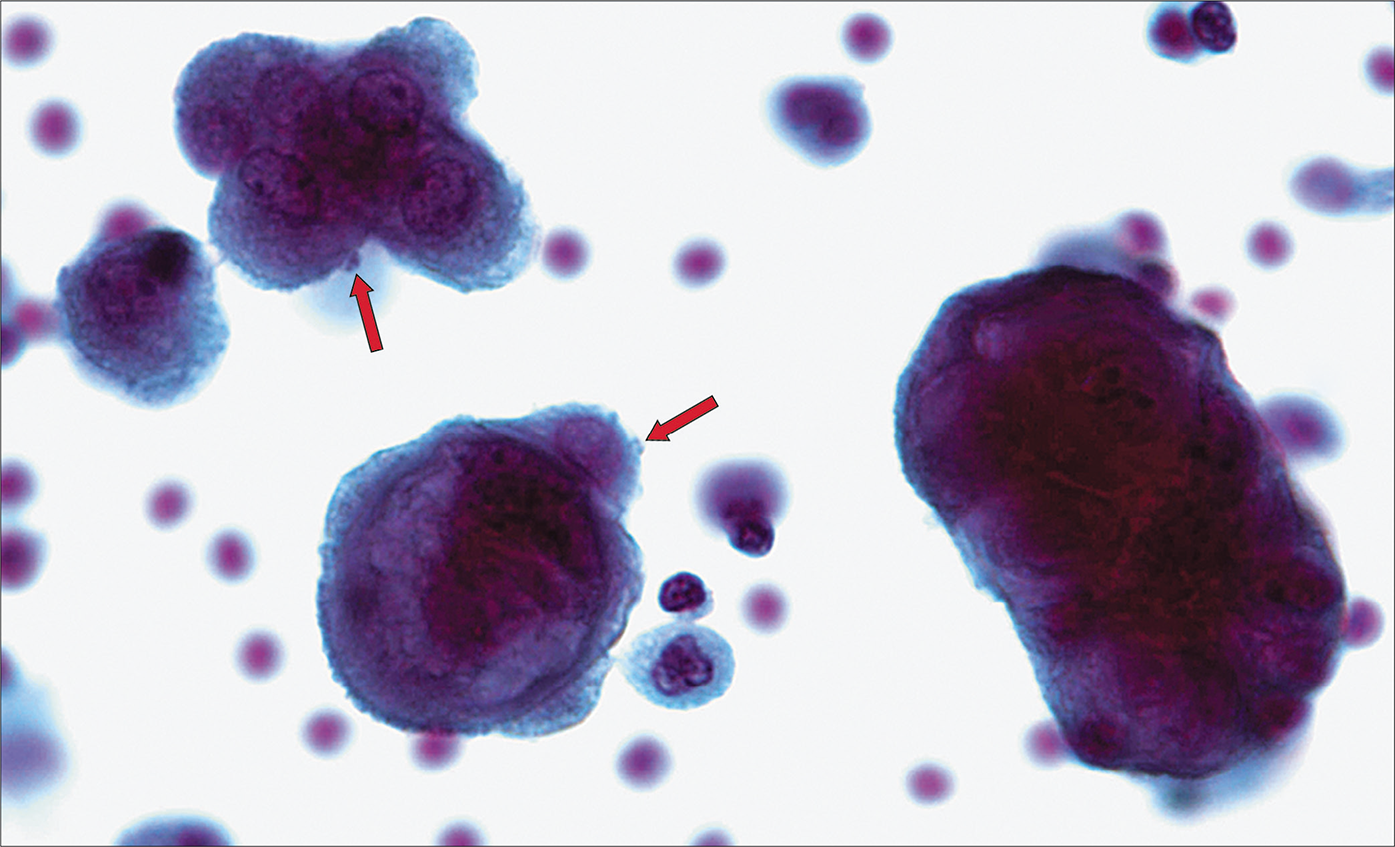
- Mesothelial cell with two-zone cytoplasm and peripheral blebbing (arrow). [Autocyte Prep, PAP stain, 40X.]
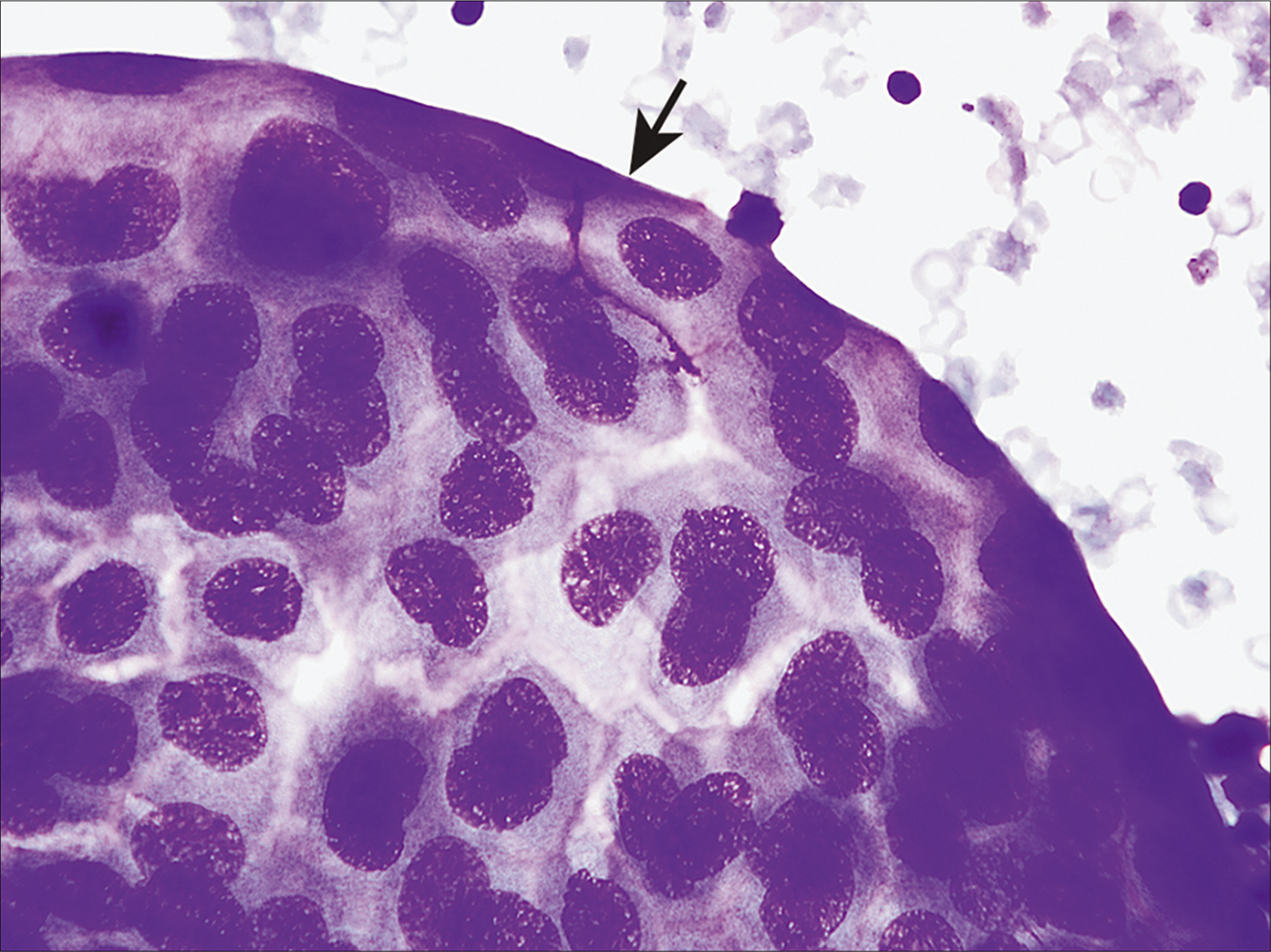
- A case of metastatic pancreatic carcinoma in peritoneal fluid, highlighting a cohesive group of neoplastic cells with smooth community borders (arrow). Compare with knobby outlines in mesothelioma [Figure 3]. [DQ stain, 20X.]
Individual neoplastic mesothelial cells are larger and more variable in size, while retaining a basic resemblance to benign/reactive mesothelial cells. Giant mesothelial cells can be present [see Figure 5]; they are usually lacking in benign effusions and adenocarcinoma. However, bizarre malignant cells are a feature of adenocarcinoma.
• Cytoplasm of reactive as well as malignant mesothelial cells has a distinct two-zone appearance with an inner dense ring fading into an outer delicate, lacy area [Figure 4]. This feature is useful in distinguishing mesothelial from adenocarcinoma cells, where the cytoplasm has a diffuse pale appearance.
Mesothelial cells, benign or malignant, tend to articulate with one another in a particular fashion, leading to the formation of ‘intercellular windows’; these are not a characteristic feature of adenocarcinoma. Peripheral cytoplasmic blebs [Figures 4, 10] and slender microvilli [Figure 6] are seen in both benign and malignant mesothelial cells, but are more prominent in the latter. The ultrafast PAP stain is reported to enhance the direct visualization of the microvilli, facilitating the distinction between mesothelial and carcinoma cells.[12]
Cytoplasmic vacuoles are a non-specific finding. Nevertheless, glycogenor lipid-containing vacuoles may be seen in mesothelial cells. While lipid vacuoles are located centrally, glycogen vacuoles, which stain a golden yellow color on the PAP stain and are periodic–acid Schiff (PAS)-stain positive, have a peripheral localization. Mucin-containing vacuoles in adenocarcinoma are usually irregular, pushing the nucleus into an eccentric position, but degenerative vacuoles can appear similar. A mucin stain can be useful in this regard.
• General cytologic nuclear features of malignancy apply to mesothelioma also. Irregular, pleomorphic, and enlarged nuclei, prominent nucleoli (including macronucleoli), binucleation, and multinucleation are characteristics of malignancy. However, the features may be somewhat subtle and need to be carefully searched. Atypical mitoses may be seen rarely.
Spindle cells rarely exfoliate in effusion fluids, and when they do, they may appear polyhedral owing to the surface tension phenomenon. The presence of scattered atypical spindle cells is suspicious for mesothelioma [Figure 9]. Other features include the presence of background extracellular/stromal hyaluronic acid [see Figure 7]. This takes on a metachromatic appearance on Romanowsky stains and a fluffy pale green/ blue appearance on the PAP stain.
RARE VARIANTS
These are uncommonly seen and infrequently reported. Consequently, most cytopathologists are unfamiliar with their cytologic appearances and a prospective cytodiagnosis is often quite difficult. Occasional case reports of some of these variants[13] are retrospective in nature where the cytologic features have been recognized in the light of a subsequent histologic diagnosis. Nevertheless, it is important to be aware of their existence and to raise the suspicion of a possible rare variant in problematic cases. Again, it cannot be overstated that the clinical history, radiologic findings, and ancillary studies all have an important role to play in differentiating mesothelioma from other conditions. Some of the rare variants and their cytologic appearances are described below.
Clear cell pattern
This pattern has mesothelial cells with predominantly clear cytoplasm. Epithelioid mesothelial cells of conventional type may have an admixture of varying amounts of clear cells. Diagnostic clear cells may not exfoliate in sufficient amounts, thus precluding definitive cytologic diagnosis or suspicion.[14] The cytologic differential diagnosis with this pattern includes other tumors with clear cell features including clear cell renal cell carcinoma metastasizing to the pleura, and clear cell carcinoma of the lung. Positivity for mesothelial immunohistochemical markers in neoplastic cells is useful in differentiation from these tumors.
Deciduoid pattern[13]
This pattern is characterized by large, round to polygonal cells with regular cell borders and abundant glassy eosinophilic cytoplasm [Figures 12, 13]. Sometimes a two-zone appearance may be seen with an outer paler zone present, along with an inner glassy zone. The nuclei are vesicular with prominent nucleoli [see Figure 13]. Although cellular pleomorphism, binucleation, and multinucleation may be prominent, infrequent mitotic figures are present.

- Peritoneal fluid with deciduoid mesothelioma. Dyscohesive scattered single atypical enlarged cells. [PAP stain, 10X.] (Courtesy of Dr Bernard Naylor.)

- Peritoneal fluid with deciduoid mesothelioma. Enlarged malignant cells with a binucleate form, and glassy cytoplasm. Scattered smaller atypical mesothelial cells are present in the background. [PAP stain, 40X.] (Courtesy of Dr Bernard Naylor.)
Rhabdoid pattern
The tumor cells have cytoplasmic eosinophilic globules which impart a rhabdoid appearance, express pancytokeratin, and are negative for muscle markers such as desmin and muscle specific actin. It is associated with aggressive behavior.[15]
Pleomorphic pattern
This variant often has multinucleated tumor giant cells with bizarre anaplastic nuclei.[16,17] This pattern is also associated with aggressive behavior.
Lymphohistiocytoid pattern
The mesothelial cells of this variant have a histiocytoid appearance and are admixed with inflammatory lymphomononuclear cells. Although histologically they also possess an unequivocal sarcomatous component, the spindle cells, as mentioned before, may not exfoliate readily into effusion fluids. Therefore, the cytodiagnosis of this variant would be difficult. Atypical mesothelial cells with a histiocytoid morphology admixed with a rich inflammatory infiltrate might raise suspicion. Use of ancillary immunohistochemical studies would decidedly be of importance in these cases.[18]
Signet-ring cell pattern
The tumor cells in the signet-ring cell variety contain cytoplasmic vacuoles that do not contain mucin.[19] They are, instead, said to be lipid-rich.
Small cell pattern
This extremely rare variant shows small, uniform round cells with bland nuclei and high nuclear-cytoplasmic ratio.[20] This should be differentiated from other small round cell tumors that can be metastatic to the pleura. The tumor cells are non-immunoreactive for the usual neuroendocrine immunomarkers.
DIAGNOSTIC PROBLEMS
False-negative diagnoses of mesothelioma occur with inadequate samples (quantititative), presence of excess blood, and conditions that prevent exfoliation of diagnostic cells into the effusion fluid such as a coexistent fibrinous pleuritis. Cytologically bland-appearing mesotheliomas can also be a source of false-negative diagnosis. For all of the above reasons, the sensitivity of conventional cytology for malignant cell detection in effusion fluids is reportedly around 58% (average from 6001 cases reported in six studies), with a specificity of 97%.[21] Using ancillary methods and relying on radiology and clinical data, the sensitivity can be improved significantly.[21] False-positive diagnoses may occur rarely when large aggregates of reactive mesothelial cells are misinterpreted.
• In resolving the differential diagnosis between reactive mesothelial cells and mesothelioma, important clues are quantity and quality. Quantity refers to hypercellular smears with the presence of numerous single mesothelial cells and quality refers to the presence of large groups of three-dimensional mesothelial cells in mesothelioma. These features are evaluated better on Romanowsky-stained smears.
Comparative cytologic features of reactive mesothelial cells, malignant mesothelial cells, and adenocarcinoma cells are provided in Tables 1 and 2.
| Reactive mesothelial cells | Mesothelioma |
|---|---|
| Slightly to moderately cellular specimens | Hypercellular specimens |
| Mainly mono-layered sheets | Two-dimensional sheets and three-dimensional cell groups |
| Cell groups (relatively smaller) with knobbly outlines | Cell groups (relatively larger) with knobbly outlines |
| Intercellular windows present | Intercellular windows present |
| No acinus formation | No acinus formation |
| Mild size variability | Greater variation in size |
| Giant mesothelial cells and multinucleate cells usually absent | May be present |
| Peripheral cytoplasmic blebs and microvilli may be present, but not very prominent | Usually prominent |
| Nuclear features of malignancy—pleomorphic and enlarged nuclei, prominent nucleoli, and atypical mitoses—are not prominent | May be present |
| Mesothelioma | Adenocarcinoma |
|---|---|
| Hypercellular specimens | Hypercellular specimens |
| Two- and three-dimensional cell groups | Two- and three-dimensional cell groups |
| Knobbly outlines to cell groups | Smooth contours (‘community borders’) |
| Acinus formation usually not present | Usually present |
| Cellular variability present | Cellular variability present |
| Giant mesothelial cells present | Bizarre malignant cells present |
| Nuclear features of malignancy—pleomorphic and enlarged nuclei, prominent nucleoli, and atypical mitoses—may be subtle but usually present | Nuclear features of malignancy—pleomorphic and enlarged nuclei, prominent nucleoli, and atypical mitoses—are present |
| Two-tone cytoplasmic appearance present | Absent |
| Intercellular windows present | Absent |
| Peripheral cytoplasmic blebs with microvilli present | Absent |
| Spectrum of changes without a distinct ‘foreign’ population | Usually identifi able as a ‘foreign’ cell population |
SPECIAL STAINS IN MESOTHELIOMA
The use of mucin stains for the differential diagnosis of mesothelioma and adenocarcinoma is based on differences in the quality of mucin. Mesothelial cells produce hyaluronic acid, an acidic mucin, which is also seen in the background [see Figure 7] in effusion fluids. Adenocarcinoma cells produce neutral mucin. Accordingly, mesotheliomas can be positive for acidic mucin stains such as Alcian blue. The staining disappears with hyaluronidase predigestion. Epithelial (neutral) mucin in adenocarcinomas is positive with mucicarmine, and is PAS-positive and diastase-resistant. Glycogen, which is present in mesothelial cells, disappears with diastase-digestion. • It needs to be remembered that a positive stain for an epithelial mucin is useful for a diagnosis of adenocarcinoma, but a negative result does not exclude it.
IMMUNOCYTOCHEMISTRY
As stressed before, based on morphology alone, it may be difficult to distinguish between reactive mesothelial cells, mesothelioma, and adenocarcinoma in effusion fluids. Immunocytochemistry is now the predominant method of elucidating problematic cases. But there are a number of problems associated with the performance and interpretation of immunocytochemistry in effusion fluids.
A variety of antibodies of variable efficacy have been employed in the diagnosis and differential diagnosis of mesotheliomas.[22-25] They can broadly be divided into those that mark mesothelial cells, those that are negative for mesothelial cells and serve as a negative marker, and other antibodies that are non-specific for mesothelial cells. The immunocytochemical diagnosis of mesothelioma has traditionally rested on demonstration of negative markers. Markers that are immunoreactive for mesothelial cells and mesothelioma and non-immunoreactive for adenocarcinoma are recognized and employed in routine diagnostic practice with greater regularity.[22-25] Most centers use a panel, comprising a combination of the above three classes of antibodies, to assist in the differential diagnosis. However, there is no agreement on the ‘optimal’ diagnostic panel. An approach emphasized by some authors is to use three adenocarcinoma markers and two mesothelioma markers.[26]
• Positivity for at least one mesothelial marker and negativity for all adenocarcinoma markers has been suggested to be optimal for a diagnosis of mesothelioma. Conversely, two positive adenocarcinoma markers with a negative mesothelioma marker may be considered optimal for a diagnosis of adenocarcinoma.
Problems exist in interpretation of data on immunocytochemistry in effusion fluids, due to differences in the specimen types, different vendors of the primary antibody, different dilutions, incubations, epitope retrieval techniques, etc. The inability to evaluate immunoreactivity for different immunomarkers in the same cells limits application of cytology preparations. Limited literature is available on this aspect.
Recently, BAP1 and MTAP immunocytochemistry has come into diagnostic usage in many laboratories to confirm the malignant nature of mesothelioma in effusion specimens.[3,27,28] Loss of nuclear staining for BAP1 [Figure 15a] can be found in 60% to 70% epithelioid mesotheliomas; and is highly specific. Contrarily, reactive mesothelial cells typically retain nuclear staining [Figure 15b]. The gene for MTAP is located close to CDKN2A/p16/p14 at the 9p21.3 locus and has been reported to be deleted in tandem with CDKN2A in the vast majority of pleural and peritoneal mesotheliomas. Loss of cytoplasmic staining of MTAP is very specific for mesothelioma.
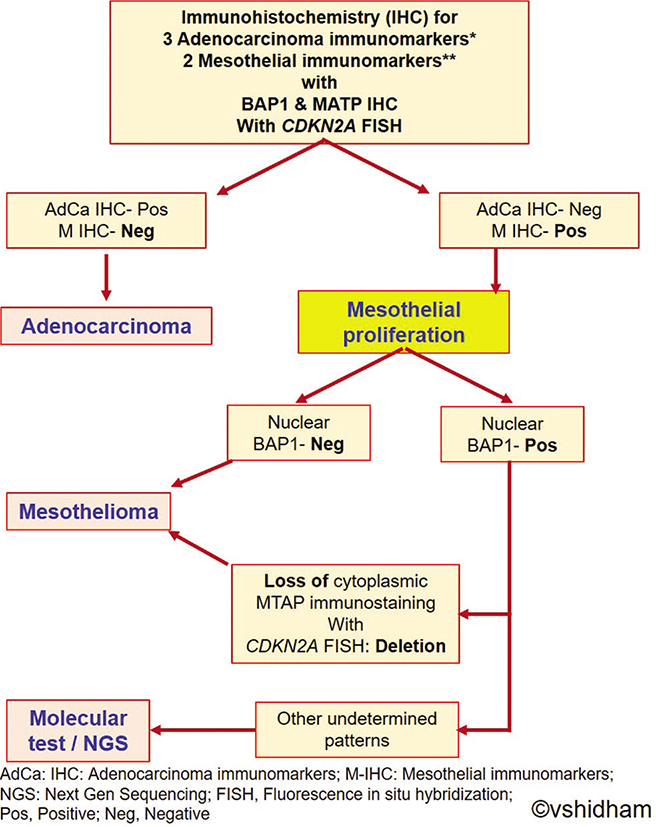
- Algorithmic approach for diagnostic work-up for evaluation of mesothelial proliferations. (Reproduced from Open Access publication: CytoJournal 2022;19:3).[30]
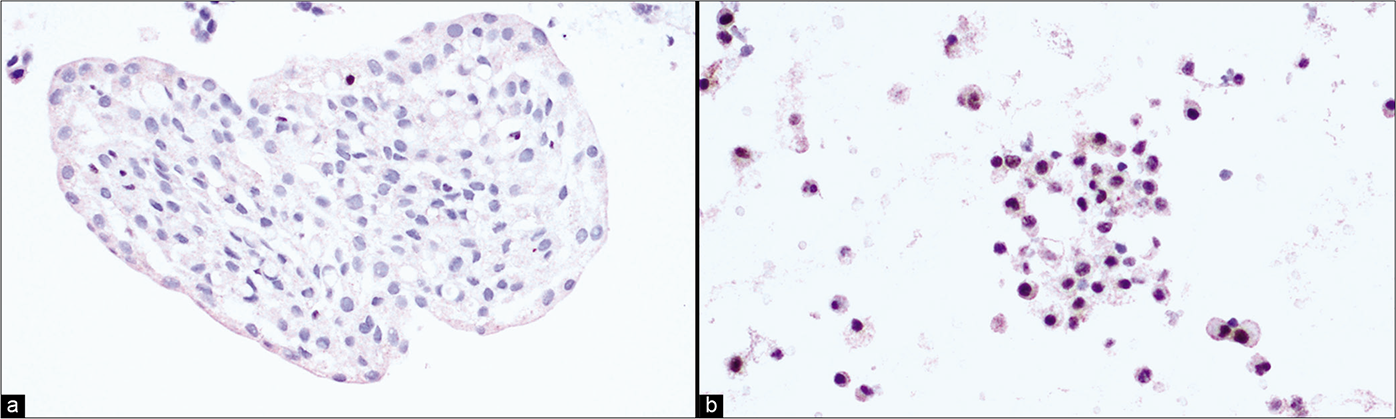
- (a) Pleural fluid cell-block sections: Loss of nuclear BAP1 staining in mesothelial cells is suggestive of mesothelioma. (b) Pleural fluid cell-block sections: Effusion cytology. Retention of BAP1 nuclear staining in mesothelial cells is suggestive of reactive mesothelial proliferation.
An algorithmic approach for diagnostic work-up of effusion cytology or small biopsy specimens with mesothelial proliferations is suggested below [Figure 14]:[29]
Perform a panel of three carcinoma and two mesothelial IHC markers along with BAP1.
If mesothelial IHC markers are positive with loss of BAP1 staining, the findings would be consistent with mesothelioma.
If BAP1 is intact, MTAP IHC or CDKN2A FISH (see below) could be attempted. Loss of MTAP IHC staining or CDKN2A deletion, would again be consistent with mesothelioma.
Retention of MTAP staining and/or intact CDKN2A are suggestive of a reactive mesothelial proliferation; however, a mesothelioma cannot be excluded since some cases can have intact both BAP1 and CDKN2A.
The use of cell-blocks can overcome many of these problems, as the results are somewhat more standardized. As opposed to tissue biopsies, coordinate immunoreactivity, where the same cells are evaluated on different slides for different immunomarkers, is challenging on cell-blocks, due to the lack of proper reference points. Subtractive coordinate immunoreactivity pattern’ (SCIP), applying a combination of various immunomarkers establish the basic map of different components in the cell-block sections.
OTHER ANCILLARY METHODS
Recent developments in molecular and genetic tests have considerably advanced the ability to reliably recognize neoplastic mesothelial proliferations in cytology specimens.[3,27,28] Homozygous deletion of CDKN2A is one of the most frequent genetic alterations in malignant mesothelioma. Fluorescence in situ hybridization (FISH) for homozygous deletion of CDKN2A/p16 (9q21) can be used reliably to distinguish mesothelioma from benign mesothelial proliferations, especially when used together with immunocytochemistry of BAP1 and MTAP. Next-generation sequencing can also help confirm the diagnosis of mesothelioma when there are mutations of BAP1, CDKN2A, NF2, SETD2 and p53.
Electron microscopy can be performed on cells derived from effusion fluids, and has been applied to distinguish adenocarcinoma cells from mesothelial cells.[31,32] However, it does not help to distinguish reactive from malignant mesothelial cells; nor does it help, in contextual situations, to distinguish benign from malignant neoplastic cells. Careful correlation with light microscopic appearances is essential when interpreting ultrastructural data. With the availability of a variety of immunomarkers, the role of electron microscopy has regressed considerably.
• A distinctive feature of mesothelial cells is the presence of long, slender microvilli, which tend to branch, along with welldeveloped desmosomes and tonofilament bundles.[32] In adenocarcinoma, the villi tend to be short and blunt with denser filamentous cores and less-numerous intermediate filaments. Prominent glycogen accumulation is seen in mesothelial cells. Mucin droplets are a feature of adenocarcinoma. There may be an overlap, or poor expression of many of these features, particularly in poorly differentiated tumors, rendering an ultrastructural diagnosis difficult in those cases.
Malignant mesothelioma in situ
Malignant mesothelioma in situ (MMIS) has been included in the current WHO classification of thoracic tumors (2021) as a diagnostic category. Although there are published criteria for diagnosis, the clinical significance is incompletely understood. [33,34] The cytologic diagnosis, as expected, is fraught with difficulty. In general, a suggested approach is to label those effusion cytology cases that fulfill the diagnostic criteria (as discussed above), but lack clinical-radiological correlation, as “atypical mesothelial proliferation, cannot exclude malignant mesothelioma in situ”. It is still evolving as a diagnostic entity, with foreseeable changes in the near future.[33]
Acknowledgment
Authors thank Janavi Kolpekwar for copy-editing assistance.
ABBREVIATIONS (IN ALPHABETIC ORDER)
DQ - Diff-quik
FISH - Fluorescence in situ hybridization
IHC – Immunohistochemistry
MMIS - Malignant mesothelioma in situ
PAP – Papanicolaou
PAS - Periodic-acid Schiff
SCIP - Subtractive coordinate immunoreactivity pattern
WHO - World Health Organization.
References
- Clinical presentation and natural history of benign and malignant mesothelioma. Semin Oncol. 1981;8:313-320.
- [Google Scholar]
- Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260-271.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407-16.
- [CrossRef] [PubMed] [Google Scholar]
- Diffuse malignant mesothelioma. Prospective evaluation of 69 patients. Ann Intern Med. 1982;96(6 Pt 1):746-55.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20:350-7.
- [CrossRef] [Google Scholar]
- Diagnosis of pleural malignant mesothelioma in life: A practical approach. J Pathol. 1984;143:147-75.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic presentation of malignant mesothelioma in pleural effusion. Exp Cell Biol. 1988;56:211-216.
- [CrossRef] [PubMed] [Google Scholar]
- Effusion cytology in the diagnosis of malignant epithelioid and biphasic mesothelioma. Arch Pathol Lab Med. 1990;114:845-851.
- [Google Scholar]
- The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest. 1997;111:106-109.
- [CrossRef] [PubMed] [Google Scholar]
- The cytology of malignant mesothelioma. Invited Review. Cytopathology. 2000;11:139-151.
- [CrossRef] [PubMed] [Google Scholar]
- Long microvilli of mesothelioma are conspicuous in pleural effusions processed by Ultrafast Papanicolaou stain. Cancer. 2003;99:17-22.
- [CrossRef] [PubMed] [Google Scholar]
- Deciduoid peritoneal mesothelioma. A report of the cytological appearances. Cytopathology. 2001;12:57-61.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual clear cell variant of epithelioid mesothelioma. Arch Pathol Lab Med. 2001;125:1588-1590.
- [CrossRef] [PubMed] [Google Scholar]
- Mesothelioma with rhabdoid features: An ultrastructural and immunohistochemical study of 10 cases. Mod Pathol. 2006;19:373-83.
- [CrossRef] [PubMed] [Google Scholar]
- Pleomorphic epithelioid diffuse malignant pleural mesothelioma: A clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol. 2011;6:896-904.
- [CrossRef] [PubMed] [Google Scholar]
- Pleomorphic mesothelioma: Report of 10 cases. Mod Pathol. 2012;25:1011-22.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphohistiocytic mesothelioma: an often misdiagnosed variant of sarcomatous malignant mesothelioma. Am J Clin Pathol. 2000;113:649-654.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20:350-357.
- [CrossRef] [Google Scholar]
- Immunohistochemistry in the distinction between malignant mesothelioma and pulmonary adenocarcinoma: a critical evaluation of new antibodies. J Clin Pathol. 2002;55:662-668.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol. 2002;26:61-66.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant mesothelioma: immunohistochemistry and DNA ploidy analysis as methods to differentiate mesothelioma from benign reactive mesothelial cell proliferation and adenocarcinoma in pleural and peritoneal effusions. Arch Pathol Lab Med. 1996;120:959-966.
- [Google Scholar]
- Role of immunohistochemistry in differentiating epithelial mesothelioma from adenocarcinoma. Review and update. Am J Clin. 1999;112:75-89.
- [CrossRef] [PubMed] [Google Scholar]
- The value of ThinPrep and cytospin preparation in pleural effusion cytological diagnosis of mesothelioma and adenocarcinoma. Diagn Cytopathol. 2005;32:137-144.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of methylthioadenosine phosphorylase compared with BAP1 Immunohistochemistry, and CDKN2A and NF2 fluorescence in situ hybridization in separating reactive mesothelial proliferations from epithelioid malignant mesotheliomas. Arch Pathol Lab Med. 2018;142:1549-53.
- [CrossRef] [PubMed] [Google Scholar]
- Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108-13.
- [Google Scholar]
- The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668-72.
- [CrossRef] [PubMed] [Google Scholar]
- Immunocytochemistry of effusion fluids: Introduction to SCIP approach. Cytojournal. 2022;19:3.
- [CrossRef] [Google Scholar]
- Malignant mesothelioma: current conundrums and whither electron microscopy for diagnosis? Ultrastruct Pathol. 1997;21:315-320.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrastructural features of diffuse malignant mesothelioma. Hum Pathol. 1998;29:13821392.
- [CrossRef] [Google Scholar]
- Guidelines for the cytopathologic diagnosis of epithelioid and mixed-type malignant mesothelioma: Complementary statement from the international mesothelioma interest group, also endorsed by the international academy of cytology and the papanicolaou society of cytopathology. A proposal to be applauded and promoted but which requires updating. Diagn Cytopathol. 2020;48:877-9.
- [CrossRef] [PubMed] [Google Scholar]
- The concept of mesothelioma in situ, with consideration of its potential impact on cytology diagnosis. Pathology. 2021;53:446-53.
- [CrossRef] [PubMed] [Google Scholar]








