Translate this page into:
Metastatic hepatocellular carcinoma presenting as gynecomastia in male: A diagnostic dilema in fine needle aspiration cytology
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Hepatocellular carcinoma (HCC) is a common primary malignant tumor of the liver.[1] HCC is highly invasive and distant metastases are common at the time of diagnosis.[2] Among all the metastatic sites, the breast is an extremely rare site of metastasis from HCC.[2–6] Metastatic HCC has an aggressive course, with a poor outcome.[27]
A 56-year-old male developed right breast enlargement since four months. His past medical history was unremarkable. On examination, a well-circumscribed, non-tender, freely mobile, firm mass measured 6 cm ×5 cm in size. The overlying skin and axilla were unremarkable. The clinical impression was of gynecomastia. The routine laboratory findings were normal.
Ultrasound of the right breast showed a 5.8 cm ×4.6 cm-sized hypoechoic mass, with slightly irregular margins, suspicious for primary breast malignancy [Figure 1]. Right breast fine-needle aspiration cytology (FNAC) showed a cellular aspirate having numerous naked nuclei and large cohesive fragments of polygonal cells. The cells were enlarged, having pleomorphic nuclei, prominent nucleoli, and dense granular eosinophilic cytoplasm. Intranuclear inclusions and bile pigment were also evident. The cells were arranged in a trabecular pattern, with thick plates of atypical hepatocytes lined by elongated endothelial cells [Figure 2]. The possibility of metastatic HCC was suggested. However, hepatoid adenocarcinoma could not be ruled out. Tru-cut biopsy showed a trabecular arrangement of polygonal cells having pleomorphic nuclei, intranuclear inclusions, and clear-to-granular amphophilic cytoplasm [Figure 3]. On immunohistochemistry, the tumor cells were positive for alpha-fetoprotein (AFP) (BioGenex, C3). The cells were negative for cytokeratin 7, cytokeratin 20, gross cystic disease fluid protein-15 (GCDFP-15), estrogen receptors, and progesterone receptors. A final diagnosis of metastatic HCC in the breast was made.
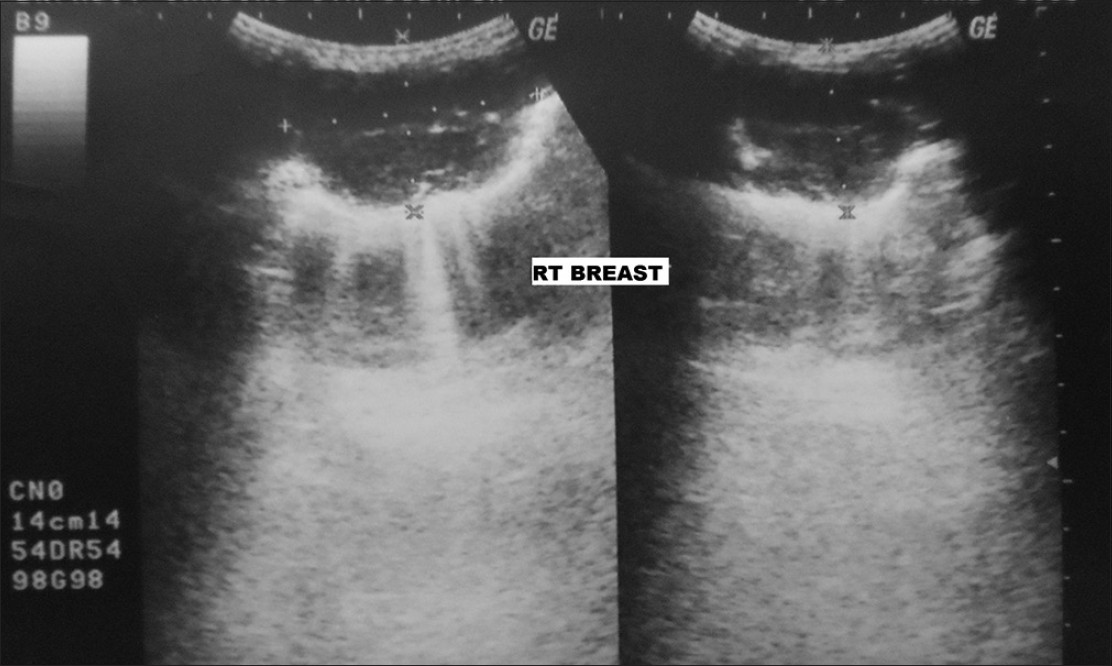
- Ultrasound of right breast shows a 5.8 cm × 4.6 cm sized hypoechoic mass with irregular margins
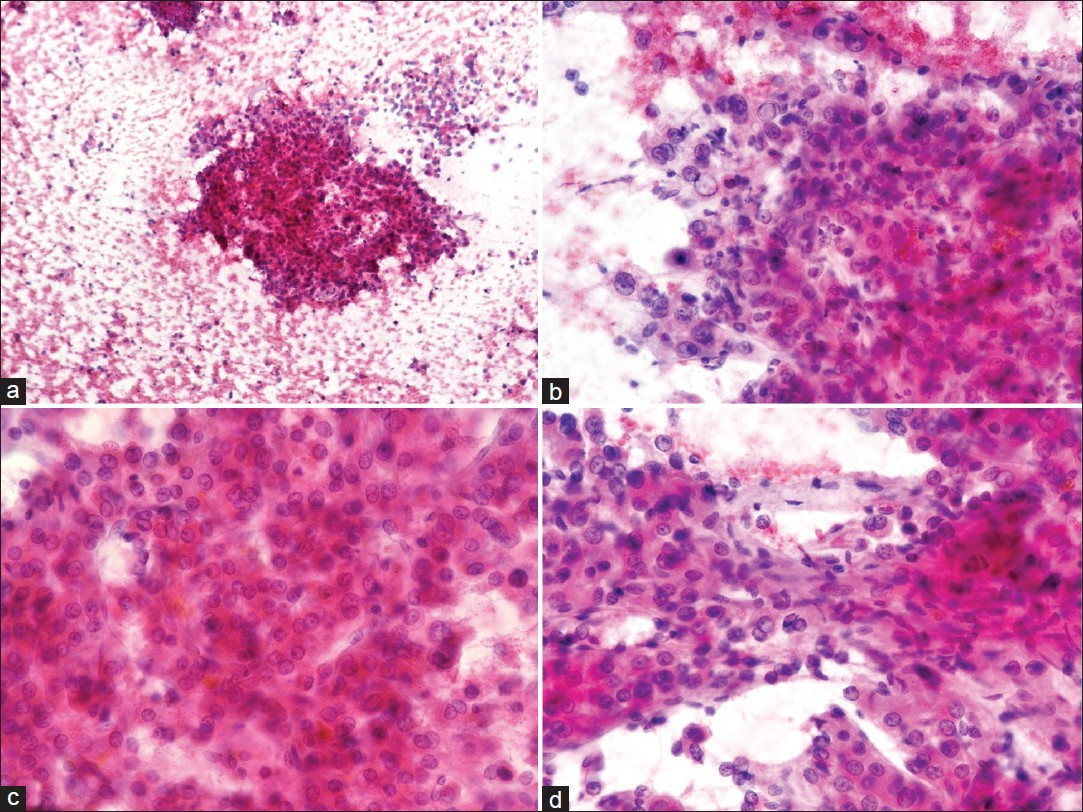
- Right breast fine-needle aspiration cytology (FNAC): PAP stain: (a) Cellular aspirate shows numerous naked nuclei and large cohesive fragments of polygonal cells (×100). (b) Cells have enlarged pleomorphic nuclei, prominent nucleoli and dense granular eosinophilic cytoplasm. Tumor cells show intranuclear inclusions (×400). (c) Cells are arranged in trabecular pattern with thick plates of atypical hepatocytes showing bile pigmentation (×400). (d) Cluster of atypical hepatocytes is lined by elongated endothelial cells (×400)
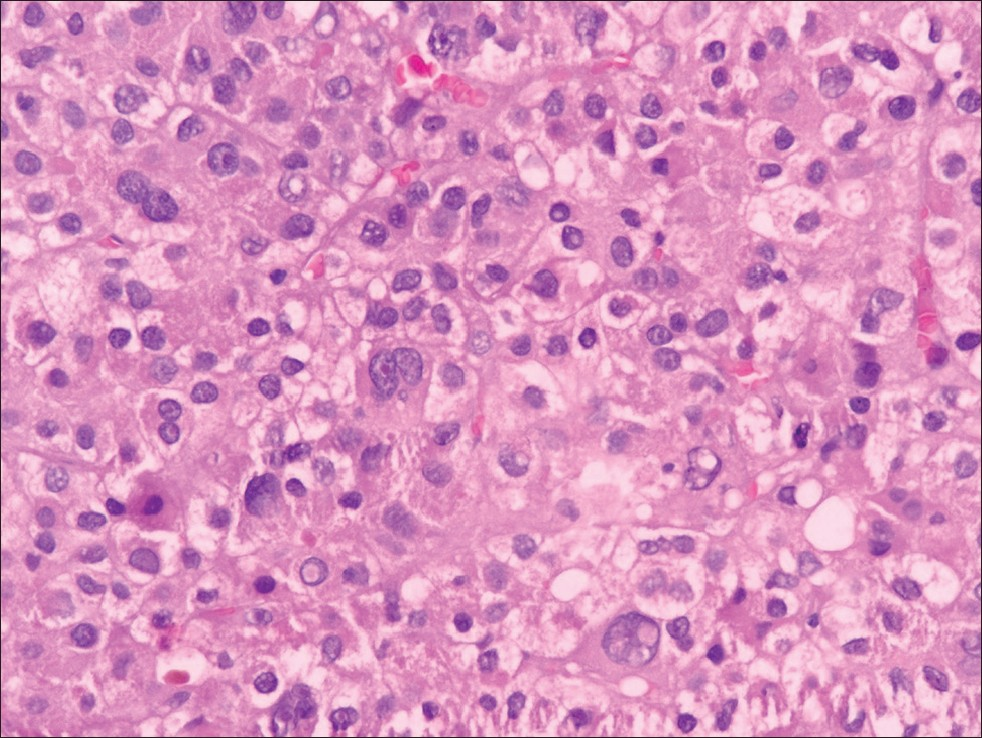
- Right breast tru-cut biopsy: Tumor shows trabecular arrangement of polygonal cells having pleomorphic nuclei, occasional prominent nucleoli, intranuclear inclusions and clear to granular amphophilic cytoplasm (Hematoxylin-eosin stain ×400)
Subsequent investigation showed raised serum AFP (18302.8 ng / ml, normal range, 0.5 – 35 ng / ml) and a positive hepatitis B surface antigen marker. Computerized tomography (CT) of the abdomen showed a hepatic mass. CT-guided FNAC and biopsy of the hepatic mass confirmed the diagnosis of primary HCC. Cirrhotic changes were not evident. On the whole body scan, HCC metastasis to other sites was not found. In view of the good general condition and metastatic breast disease, chemotherapy was given. He remained in good general health for four months, and then he developed breathlessness. A chest radiograph revealed extensive bilateral pulmonary infiltrates. The patient died after five months with progressive metastatic disease being the most likely cause.
Chronic hepatitis B or C infection or cirrhosis secondary to other chronic liver diseases are predisposing factors for HCC.[13] The most common site of HCC metastasis is the lung, followed by lymph nodes, bone, and the adrenal gland.[1–47–9] Other unusual metastatic sites like the brain, bladder, gastrointestinal tract, diaphragm, seminal vesicles, pancreas, kidney, and spleen are almost never represented as the initial manifestation of HCC, but occur only after metastasis at more common sites.[9] To the best of our knowledge, only six cases of breast metastasis from HCC have been reported in the literature.[2–46]
Breast metastasis from extramammary malignancy is rare, with an incidence of 0.4 – 1.3%.[4] Male patients are affected five to six times less frequently than female patients.[5] The common sources of primary tumors that metastasize to the breast are hematological malignancies, malignant melanoma, lung tumors, renal cell carcinoma, ovarian tumors, and small bowel carcinoid. Other tumors metastasized to breast are prostate, stomach, malignant mesothelioma, rhabdomyosarcoma, osteosarcoma, thyroid neoplasm, and cervical, vaginal, and endometrial carcinoma.[235]
Metastatic carcinoma to the breast usually presents as a rapidly growing, well-circumscribed, painless, freely mobile, firm mass, in the upper outer quadrant of the breast.[210] Skin and underlying muscle are free from the tumor.[11] Mammography shows a well-circumscribed, round nodule, with slightly irregular margins, without desmoplasia, speculation, or microcalcification. Rarely diffuse parenchymal and skin involvement is seen.[10] However, to distinguish primary breast cancer from metastatic disease, FNAC and core biopsy are usually needed for the final diagnosis.
In a majority of the cases, a history of extramammary malignancy or widespread metastases are present.[210] Like in our case, where metastatic HCC exists without a known primary within the liver, one has to consider the possibility of hepatoid adenocarcinoma. Hepatoid adenocarcinoma is a rare variant of adenocarcinoma, characterized by adenomatous and hepatocellular differentiation. It produces AFP, having the same function and form as HCC. It is most common in the stomach, followed by esophagus, gall bladder, ovaries, pancreas, lungs, kidneys, uterus, adrenal gland, and urinary bladder.[8] A majority of the gastric hepatoid adenocarcinomas would be associated with the foci of conventional intestinal adenocarcinoma (in situ or invasive) that merge with the hepatoid areas.[12] Hepatoid adenocarcinoma shows positivity for AFP and hepatocyte paraffin 1 (Hep Par-1) Therefore, presence of AFP and Hep Par-1 reactivity is not unique to HCC, and therefore, hepatoid adenocarcinomas may be considered in the differential diagnosis.[11] In such cases, the clinical and morphological findings may serve as useful markers for the diagnosis of HCC. Our case was composed solely of HCC with AFP positivity, without any evidence of adenocarcinoma elsewhere.
Treatment of breast metastasis from HCC is not well known due to its low incidence.[2] Surgical excision and infusion chemotherapy in localized metastases have been shown to provide reasonable control.[7] Breast metastasis usually indicates disseminated disease and most of the patients die within a year of diagnosis.[2]
To conclude, breast metastasis from HCC is very rare and represents an advanced stage of HCC, with poor prognosis. When HCC is found outside the liver, without a known primary within the liver, a tissue biopsy must be obtained, to confirm the presence of HCC and rule out hepatoid adenocarcinoma. The immunohistochemical marker must be interpreted in the context of the morphological and clinical findings.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
We have no financial or non-financial competing interests that may cause them embarrassment were they to become public after the publication of the manuscript.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Authorship credit should be based on (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from the Institutional Review Board (IRB) (or its equivalent).
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of the CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/21/102863
REFERENCES
- Atypical presentation of hepatocellular carcinoma: A mass on the left thoracic wall. BMC Cancer. 2004;4:89.
- [Google Scholar]
- Positive positron emission tomography scan in sarcoidosis and two challenging cases of metastatic cancer. CASE 2. Hepatocellular carcinoma with metastasis to the breast. J Clin Oncol. 2005;23:8908-9.
- [Google Scholar]
- Breast metastasis from hepatocellular carcinoma. Hepatogastroenterology. 2004;51:387-90.
- [Google Scholar]
- Breast metastasis from carcinoma of the posterior pharyngeal wall. JK Science: J Med Educ Res. 2010;12:149-50.
- [Google Scholar]
- Metastatic hepatocellular carcinoma of the breast, simulating gynecomastia: Diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 1992;8:588-92.
- [Google Scholar]
- Chest wall metastasis from hepatocellular carcinoma in the absence of a primary: An unusual presentation. J Cancer Res Ther. 2008;4:42-3.
- [Google Scholar]
- Chest wall metastasis from unknown primary site of hepatocellular carcinoma. World J Gastroenterol. 2006;12:2139-42.
- [Google Scholar]
- Fine-needle aspiration cytology of extra mammary metastatic lesions in the breast: A retrospective study of 36 cases diagnosed during 18 years. Cytojournal. 2010;7:10.
- [Google Scholar]
- Hepatoid adenocarcinoma of the stomach with liver metastasis mimicking hepatocellular carcinoma: A case report. Cases J. 2009;2:6317.
- [Google Scholar]
- Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol. 2001;115:689-94.
- [Google Scholar]







