Translate this page into:
Metastatic prostatic stromal sarcoma: A challenging diagnosis on fine-needle aspiration with broad differential diagnosis
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Prostatic stromal sarcomas (PSS) are rare solid organ mesenchymal sarcomas. PSS may pose difficult diagnostic challenges on fine needle aspiration biopsy. We report a 48-year-old man diagnosed with metastatic high grade prostatic stromal sarcoma by a CT-scan guided fine needle aspiration (FNA) biopsy of a right lower lung lobe nodule. We reviewed the literature on the epidemiologic, cyto-histological, and immunophenotypic findings and discussed the differential diagnosis for this rare entity.
Keywords
Fine-needle aspiration
high-grade
metastatic
prostate
stromal sarcoma
INTRODUCTION
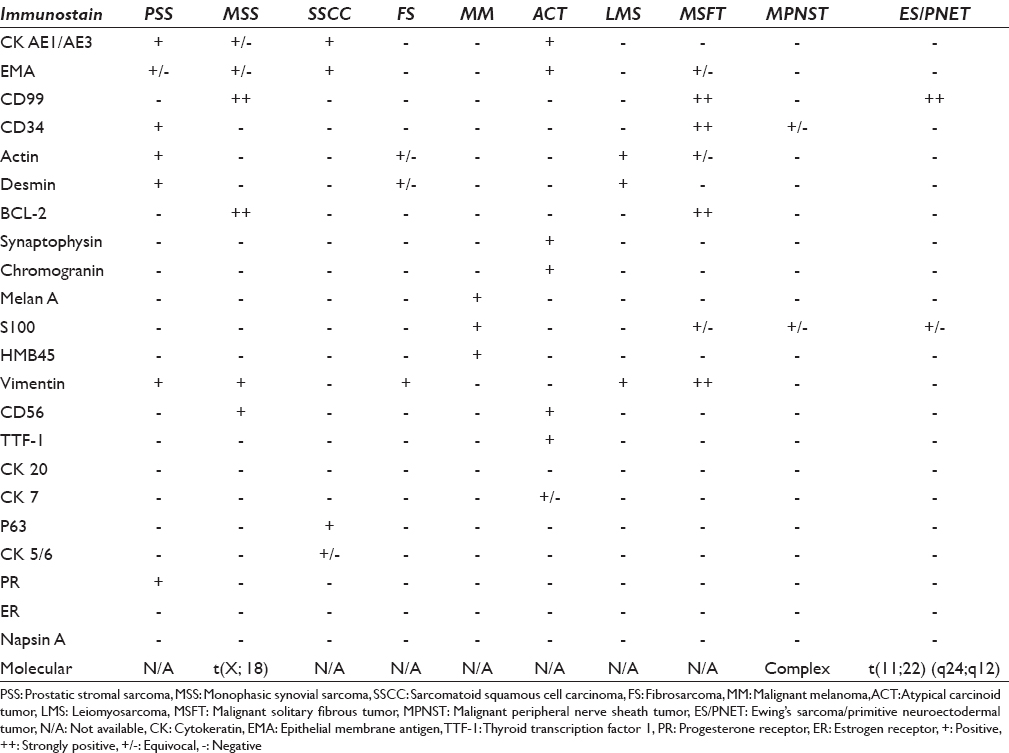
Prostatic stromal sarcomas (PSSs) are malignant cellular spindle cell tumors of specialized prostatic stromal cells. PSS include pure mesenchymal and mixed epithelial stromal (phyllodes) tumors.[1] More than half of patients with PSS are younger than 50 years old. Majority of the patients present with lower urinary tract symptoms and urinary retention.[2] PSS may form solid or partially cystic masses which may contain necrosis.[3] PSS have broad differential diagnosis includes sarcomatoid squamous cell carcinoma (SSCC), malignant spindle cell melanoma, malignant solitary fibrous tumor (MSFT), atypical carcinoid tumor (ACT), metastatic fibrosarcoma (FS), malignant peripheral nerve sheath tumor (MPNST), primitive neuroendocrine tumor/Ewing sarcoma (PNET/ES), and leiomyosarcoma (LMS). The main modality for treatment is radical prostatectomy with chemotherapy. Recent literature showed that PSS have high rate of local recurrence and distant metastasis.[4]
MATERIALS AND METHODS
Computed tomography (CT)-guided fine -needle aspiration (FNA) biopsy was performed using a 21-gauge needle on a lung nodule. Material from the FNA was expelled onto glass slides and smeared. Some of the smears were air-dried and stained with Diff-Quik stain. The remaining smears were immediately wet fixed with 95% ethyl alcohol and stained with Papanicolaou (PAP) stain. Material for cell block was rinsed from the needle in 10% neutral buffered formalin.[5] Paraffin-embedded sections from the cell block were stained with hematoxylin and eosin. Immunohistochemistry stains were performed on unstained sections of formalin fixed, paraffin embedded cell block by the standard avidin–biotin technique. The panel of antibodies used included pancytokeratin (cytokeratin [CK] adverse event 1/3 [AE1/AE3]), CK 7, epithelial membrane antigen (EMA), progesterone receptor (PR), estrogen receptor (ER), thyroid transcription factor 1 (TTF-1), prostatic specific antigen (PSA), CD117, S100, CD34, actin, napsin A, desmin, melan A, and vimentin.
CASE REPORT
Subject
A 48-year-old Puerto Rican male patient with a history of recurrent lower urinary tract infections and urine retention. The medical history of the patient is remarkable for long-standing ulcerative colitis led to a total colectomy and ileocolic anastomosis 8 years ago. Physical examination revealed enlarged right and left prostatic lobes with no apparent problem in the testis or phallus. PSA was 9.4 ng/ml. The patient underwent prostatic biopsies which showed prostatic adenocarcinoma, acinar type, Gleason score of 3 + 3 = 6, affecting 2% of the right lobe of the prostate, as well as high-grade PSS occupying 50% of the left lobe of the prostate. Chest CT-scan showed a 1.8 cm × 0.9 cm nodule in the right lower lung lobe [Figure 1]. Infectious or inflammatory etiologies were considered. A CT-scan guided FNA biopsy on the lung nodule to exclude any lung primary tumors. The biopsy showed malignant neoplasm with mixed spindle and epithelial cell features confirmed to be metastatic PSS with proper immunohistochemical stains [Figure 2a–d]. Shortly after the lung biopsy, the patient had radical prostatectomy, which showed prostatic adenocarcinoma, acinar type, Gleason score 3 + 3 = 6, occupying 5% of the right prostatic lobe, as well as a 4.0 cm × 3.5 cm × 3.0 cm cystic mass occupying 60% of the left prostatic lobe and all left seminal vesicles, which diagnosed as high grade PSS. The morphologic picture and the immunohistochemical profile of the PSS are identical to the lung nodular lesion [Figure 2e and f].
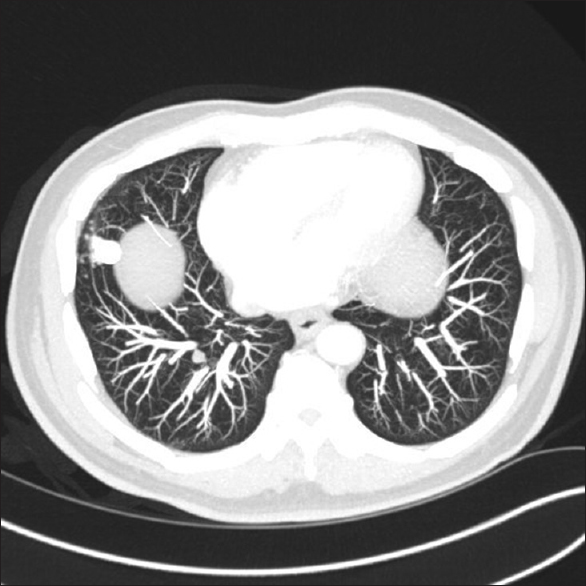
- Chest computed tomography scan image show the right lower lobe nodule of the lung
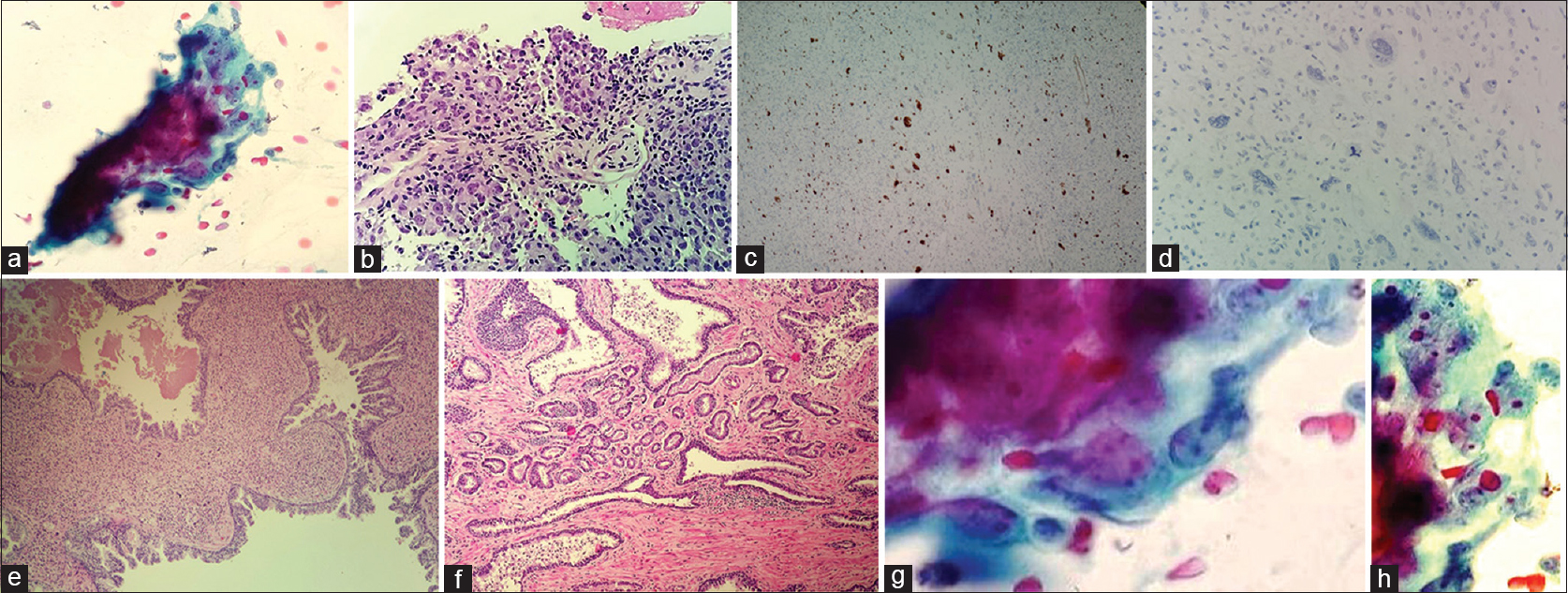
- (a) Papanicolaou stain smears show high grade spindle cell neoplasm. (b) H and E stained section from cell block shows sweeping fascicles of spindle cell lesion with nuclear atypia. (c) Cell block; prostatic specific antigen immunostains highlighted the large atypical epithelial cells. (d) Cell block; cytokeratin adverse event 1/3 immunostains is negative in the large atypical stromal cells. (e) Prostatectomy resection; left lobe shows prostatic stromal sarcoma. (f) Prostatectomy specimen; right lobe, prostatic adenocarcinoma, acinar, type, Gleason score 3 + 3 = 6. (g) Neoplastic spindle cells on cytology. (h) Cytomorphology showing high grade spindle cells
Radiology
The initial pelvic CT-scan showed a 4.0 cm × 3.5 cm × 3.0 cm cystic mass occupying the left prostatic lobe and involving the left seminal vesicle. No pelvic lymphadenopathy identified. The staging abdominal CT scan showed areas of fibrosis and the ileocolic anastomosis with no edema or effusions. No abdominal lymphadenopathy identified. The chest CT scan showed a 1.8 cm × 0.9 cm nodule in the right lower lung lobe [Figure 1]. No pleural or pericardial effusion or lymphadenopathy were identified.
Cytology
Moderate to high cellular smears composed of pleomorphic large spindle cells with prominent nuclear atypia. Multiple atypical mitotic figures as well as focal areas of necrosis admixed with red blood cells (RBCs) extravasations are identified. The cells had a fascicular arrangement with focal storiform pattern with no glandular architectures were identified [Figure 2a, b, g and h].
Immunohistochemical findings
A properly controlled immunostain panel was performed on the cell block to determine the nature of the tumor. Differential diagnosis of this spindle cell tumor includes SSCC, malignant spindle cell melanoma, MSFT, ACT, metastatic FS, MPNST, PNET/ES, and LMS. The current case tumor cells were positive for CK AE1/AE3, PSA, PR, CD34, actin, desmin and vimentin, and negative for S100, TTF-1, napsin A, CK 7, CD117, ER, and melan A [Figure 2c and d].
DISCUSSION
Prostatic stromal tumors (PST) are putatively derived from specialized stromal cells of prostate and include pure mesenchymal and mixed epithelial stromal (phyllodes) tumors. PST are classified into stromal tumor of uncertain malignant potential (STUMP) and PSS in 2004 WHO classification of prostate tumors.[6] STUMP has been previously classified under a variety of names including phyllodes tumor of the prostate, atypical stromal hyperplasia, cystosarcoma phyllodes, and cystic epithelial-stromal tumor. Gaudin et al.,[7] classified these lesions in 1998 as prostatic stromal lesions that were not obvious sarcomas, designated as STUMP,[8] reflecting the uncertain nature of the clinical course in affected patients. STUMP and PSS are often difficult to distinguish morphologically. Different parameters used to differentiate these two processes include cellularity, mitotic activity, necrosis, and extension into surrounding organs.[9] STUMP encompasses cellular spindle cell lesions of specialized prostatic stroma with or without epithelial component. STUMP lacks significant cellular atypia, mitotic activity, necrosis, or extraprostatic growth.
PSS are rare solid organs mesenchymal sarcomas that originate in the cells of the stromal prostate. PSS represents < 0.1% of primary prostate malignancies in adults.[9] PSS affect male adults with age predilection between 25 and 86 years, approximately half of all reported cases of stromal sarcoma occur before the age of 50 years. PSS may pose difficult diagnostic challenges on cytology. The common clinical presentation of adult prostate sarcoma is urinary tract infections and urinary retention.[10] PSA levels are usually normal.[11] No known risk factors are described in literature for PSS.[12] Stromal sarcomas may arise de novo or may exist in association with either a preexistent or concurrent STUMP.[13] It is suggested that some STUMP cases to dedifferentiate to sarcomas in rare occasions.[14]
Gross examination of PSS demonstrates predominantly tan-white, solid, fleshy lesions ranging in size from 2 to 18 cm.[15] Occasionally, areas of edema, hemorrhage, or small cysts may be identified. Cytologically, PSS show moderate to high cellular smears composed of pleomorphic large spindle cells with prominent nuclear atypia. Multiple atypical mitotic figures as well as focal areas of necrosis admixed with RBCs extravasations are identified.[13] The cells had a fascicular arrangement with focal storiform pattern with no glandular architectures were identified. Grading of PSS as high grade or low grade was proposed in the AFIP fascicle,[14] which is preferred by authors. High-grade tumors based on increased cellularity, frequent mitoses, cytologic atypia, necrosis, and stromal overgrowth. Low-grade tumors are less atypical; prominent cellularity, appreciable mitoses, nuclear atypia, necrosis, and extraprostatic spread indicate malignant nature.[16] A 3-tier grading system for PSS has been proposed by some authors [Table 1].[17] Recurrent tumor of PSS may show higher grade features than an initial tumor.[18]

Proper clinical history and radiological correlation as well as a wise use of immunocytochemistry stains are vital for diagnosing metastatic PSS, as the lung is the primary site for secondaries. PSS cells are positive for vimentin, CD34, PR, with variable reactivity to PSA, desmin, SMA, CK (AE1/AE3), and PAP, whereas they are negative for S100, CD117, TTF-1, and ER.[19] Spindle cell squamous cell carcinoma is one of the main differential diagnosis, tumor cells are positive for CK AE1/AE3, p63, or p40 and negative for PSA and PR. The other differential diagnosis is LMS which have cigar-shaped nuclei with perinuclear vacuoles; however, they are almost always CK AE1/AE3, PSA, PR, and EMA negative.[20] MSFT shows stag-horn shaped vasculature with ropy collage, and usually positive for CD34 and BCL-2;[21] however, it is usually negative for CD99, PSA, PR and CK AE1/AE3. ACT could pose similar tumor morphology with more spindle features; however, they are CK AE1/AE3, synaptophysin, and chromogranin positive and both CD99 and CD34 negative. Malignant melanoma (MM) could mimic the cytologic features of PSS; however, MM is positive for S100, melan A, HMB45, and negative for CK AE1/AE3, PSA, and PR.[22] Table 2 shows the differential diagnosis with related the immunohistochemical stains as well as the molecular findings in each entity.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors have contributed significantly and agree with the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from the Institutional Review Board (IRB). documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
ACT - Atypical carcinoid tumor
CK – Cytokeratin
CT - Computed tomography
EMA - Epithelial membrane antigen
ER - Estrogen receptor
FNA - Fine needle aspiration
FS - Fibrosarcoma
LMS - Leiomyosarcoma
MPNST - Malignant peripheral nerve sheath tumor
MSFT - Malignant solitary fibrous tumor
PAP - Papanicolaou
PNET/ES - Primitive neuroendocrine tumor/Ewing sarcoma
PR - Progesterone receptor
PSA - Prostatic specific antigen
PSS - Prostatic stromal sarcomas
RBC - Red blood cells.
SSCC - Sarcomatoid squamous cell carcinoma
TTF-1 - Thyroid transcription factor 1
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Specialized stromal tumors of the prostate: A clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30:694-704.
- [Google Scholar]
- Phyllodes tumor of the prostate: Long-term followup study of 23 cases. J Urol. 2004;172:894-9.
- [Google Scholar]
- Chemotherapy induced complete remission in malignant phyllodes tumor of the prostate metastasizing to the lung. J Urol. 2002;168:1104-5.
- [Google Scholar]
- Metastatic Chordoma: A Diagnostic Challenge on Fine Needle Aspiration. Case Reports in Pathology, 2016 2016
- [Google Scholar]
- Molecular genetic evidence for different clonal origins of epithelial and stromal components of phyllodes tumor of the prostate. Am J Pathol. 2004;165:1395-400.
- [Google Scholar]
- Prostatic stromal hyperplasia with atypia: Follow-up study of 18 cases. Arch Pathol Lab Med. 2008;132:1729-33.
- [Google Scholar]
- Malignant phyllodes tumor of the prostate: Retrospective review of specimens obtained by sequential transurethral resection. Pathol Int. 2002;52:777-83.
- [Google Scholar]
- Phyllodes tumor of the prostate. A case report and review of the literature. Eur Urol. 2000;38:649-53.
- [Google Scholar]
- Sarcomas and related proliferative lesions of specialized prostatic stroma: A clinicopathologic study of 22 cases. Am J Surg Pathol. 1998;22:148-62.
- [Google Scholar]
- Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS) and the Granulomatous Lung Diseases, Should We Mention The S Word!? J Am Soc Cytopathol. 2015;4:S45.
- [Google Scholar]
- Prostatic cystic epithelial-stromal tumors: A report of 2 new cases. J Urol. 1993;149:860-4.
- [Google Scholar]
- Giant cystosarcoma phyllodes of the prostate associated with adenocarcinoma. Arch Pathol Lab Med. 1992;116:195-7.
- [Google Scholar]
- Malignant phyllodes tumor of the prostate. A case report with immunohistochemical and ultrastructural studies. Arch Pathol Lab Med. 1992;116:296-9.
- [Google Scholar]
- Clear cell cribriform hyperplasia of prostate. Report of 10 cases. Am J Surg Pathol. 1986;10:665-71.
- [Google Scholar]
- Atypical stromal hyperplasia of the prostate gland. Am J Clin Pathol. 1977;67:324-7.
- [Google Scholar]
- EUS-guided fine-needle biopsy using a novel fork-tip needle: A case control study. Gastrointestinal Endoscopy 2016
- [Google Scholar]
- Leiomyosarcoma arising in atypical fibromuscular hyperplasia (phyllodes tumor) of the prostate with distant metastasis. Cancer. 1991;68:910-5.
- [Google Scholar]
- High diagnostic accuracy of core needle biopsy of soft tissue tumors: An institutional experience. Diagnostic Cytopathology 2016
- [Google Scholar]








