Translate this page into:
Microcystic variant malignant mesothelioma presenting as a localized paraspinal mass
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 58-year-old man presented with productive cough and fever. Computed tomography (CT) scan of the chest showed an upper right paraspinal mass. CT-guided fine-needle aspiration biopsy showed lobules of vacuolated cells against a background of myxoid material. The cells demonstrated moderate to severe nuclear atypia and occasional mitoses. Immunohistochemistry revealed tumor cells to be immunoreactive for calretinin, WT-1, D2-40, cytokeratin (CK) 7, AE1/AE3, high molecular weight keratin, vimentin and epithelial membrane antigen, and negative for thyroid transcription factor-1, Ber-EP4, carcinoembryonic antigen, S100 protein, CK20, and CDX2. The combined morphologic and immunohistochemical findings confirmed the diagnosis of microcystic variant of localized malignant mesothelioma. The subsequent lung resection showed a pleural-based mass in the right upper lobe and confirmed the diagnosis. Awareness of the existence of unusual morphologic variants and localized forms of mesothelioma are necessary to avoid misdiagnosis of fine needle biopsy samples. Recognition of characteristic cytomorphologic features along with optimal use of panel of immunohistochemistry studies is crucial for making a specific diagnosis.
Keywords
Fine-needle biopsy
lung
malignant localized mesothelioma
microcystic
INTRODUCTION
Localized malignant mesothelioma (MM) is classified as a distinct entity in a 2004 WHO classification[1] and has different clinical course and outcomes with better prognosis compared with diffuse forms.[23] Computed tomography (CT) usually provides precise localization and is useful in distinguishing pleural from peripheral pulmonary lesions. However, due to the rarity of this tumor and its lack of usual features of mesothelioma, it is rarely included in the differential diagnosis by radiologists. As radical local resection with or without additional treatment is considered the treatment of choice in localized MM, preoperative biopsy for confirmatory diagnosis is mandatory. When there is no effusion at the time of initial investigation, a fine-needle aspiration of the localized mass, with or without a core biopsy, may provide a diagnostic sample.
Histologically, localized MM is identical to the diffuse form and is classified into four major subtypes: Epithelioid, sarcomatoid, desmoplastic, and biphasic. The epithelioid type is the most frequent and can be further subdivided according to predominant growth patterns into tubulopapillary, adenomatoid (microglandular), small cell, clear cell, and deciduoid. Among these, the adenomatoid variant can present a wide spectrum of morphologic features and can potentially be misdiagnosed as a pulmonary/metastatic adenocarcinoma.[4] These difficulties may be amplified when the mesothelioma presents as a localized form.
We report the fine-needle aspirate biopsy features of a microcystic variant of localized MM of the pleura and discuss the differential diagnoses, including diagnostic caveats related to uncommon MM.
CASE REPORT
Clinical history
A 58-year-old man presented with productive cough and fever. His medical history was significant for hepatitis C and a maternal family history of colon cancer. The patient had no past history of cancer or surgery. He was an ex-smoker (40 pack/year), and worked as a screenwriter and photographer. He had no history of asbestos exposure. He was initially treated with antibiotics, and his symptoms were resolved. However, due to persistent abnormal chest X-ray findings, a CT scan of the chest was carried out revealing a 5 cm × 4 cm paraspinal mass in the upper right chest, which was also intensely hypermetabolic on a corresponding positron emission tomography scan without evidence of lymph node metastasis. No pleural effusion was detected [Figure 1]. A radiologic differential diagnosis included a posterior mediastinal neurogenic tumor and a metastatic carcinoma. Because the mass was located adjacent to the esophagus, an esophagogastroscopy was arranged to rule out esophageal cancer, and this was normal. A magnetic resonance imaging of the brain and a bone scan were negative. Laboratory studies were all within the normal range. The mass was aspirated under CT guidance using coaxial technique and a 22-gauge needle. Air-dried and alcohol fixed smears were stained with Romanowsky and Papanicolaou method. A cell block was prepared from sample rinsed in saline, using the histogel method. Rapid on-site assessment provided by a cytopathologist was recorded as an adequate sample showing an epithelioid neoplasm.
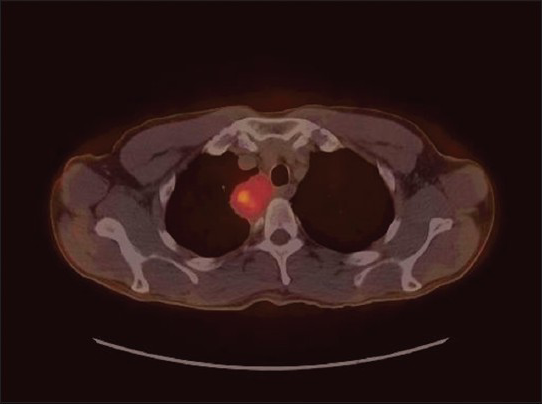
- Positron emission tomography-computed tomography chest image demonstrates a localized mass in right posterior mediastinal/ paraspinal region with maximum standardized uptake value of 6.6
Cytomorphological findings
The smears showed a hypercellular specimen consisting of loosely cohesive “lobules” of heavily vacuolated epithelioid cells displayed against a background of myxoid material, which was highlighted on Field's and Giemsa stained direct smears, suggesting the possibility of chordoma [Figure 2]. The vacuoles in the cells were filled with the same myxoid material seen in the background [Figure 3] and were negative for mucicarmine stain. Occasional cells with intracytoplasmic vacuoles displacing their nuclei to the periphery resembling signet-ring cells were also seen within the lobules, expanding the differential diagnosis to include the possibility of adenocarcinoma [Figure 4]. Individual microcysts were fused, resulting in secondary cystic dilatation. The epithelioid cells showed moderate to marked nuclear pleomorphism out of keeping with chordoma, hyperchromatic nuclei, prominent nucleoli, a dense chromatin pattern, and abundant cytoplasm. Mitotic activity was easily identified [Figure 3]. There was no evidence of necrosis. The working differential diagnoses included chordoma, benign adenomatoid tumor, epithelioid hemangioendothelioma, adenocarcinoma, and epithelioid MM.
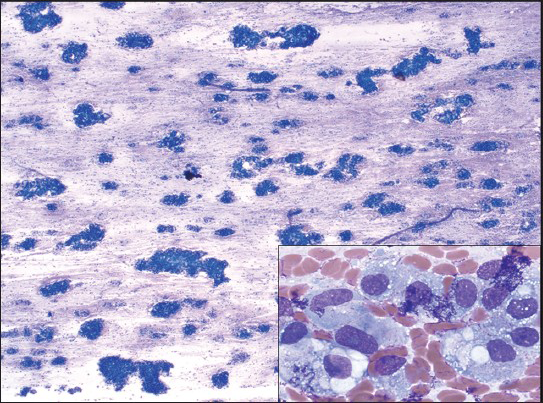
- Field's stained smear shows cohesive group of cells on a myxoid background (MGG, ×2.5). This smear pattern correlates with groups of tumor cells surrounded by fibrous septa on histology. High power view of vacuolated cells somewhat resembling physalipherous cells, but differentiated from them by pleomorphic large nuclei and prominent nucleoli (inset)
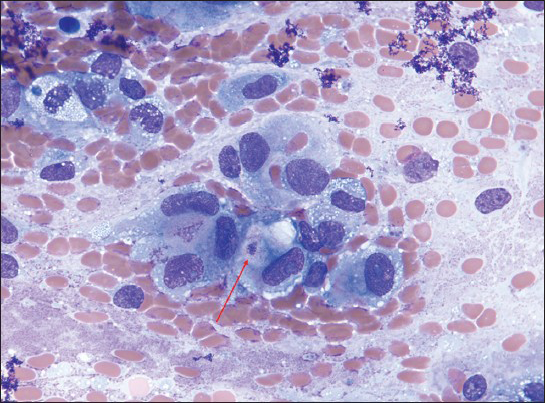
- Loosely cohesive epithelioid cells with eccentric nuclei, prominent nucleoli, and ample cytoplasm resembling adenocarcinoma. Note the cell showing vacuolated cytoplasm with magenta droplet (arrow) (myxoid background, ×63). Mucicarmine stains were negative to faintly positive
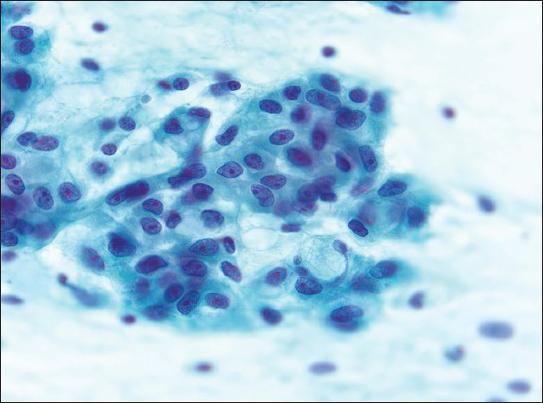
- Clusters of epithelioid cells with abundant, poorly defined cytoplasm, mildly pleomorphic nuclei with irregular nuclear membranes (grooves and notches), prominent eosinophilic nucleoli, and chromatin condensation. Rare signet-ring like cells are seen. The myxoid stroma is less obvious in the Papanicolaou (Pap)-stained smear (Pap, ×63)
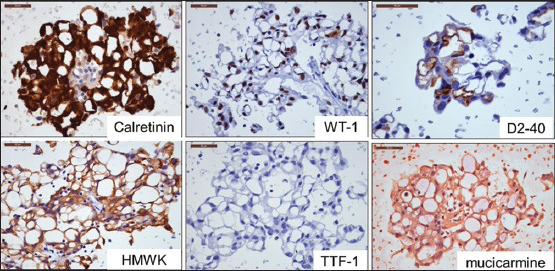
- Immunohistochemical results. Tumor cells are positive for calretinin, WT-1, and D2-40, and negative for high molecular weight keratin and thyroid transcription factor-1 supporting mesothelial origin. Mucicarmine stain is negative excluding adenocarcinoma
Immunohistochemical studies performed on cell block sections showed tumor cells were strongly immunoreactive for calretinin, WT-1, D2-40, cytokeratin (CK) 7, and AE1/AE3; and moderately positive for high molecular weight keratin (CK5/6), vimentin, and epithelial membrane antigen, which supported a mesothelial origin. Negative stains included thyroid transcription factor-1, Ber-EP4, carcinoembryonic antigen, S100 protein, CK20, and CDX-2, which excluded adenocarcinoma and chordoma and further supported the diagnosis of mesothelioma [Figure 5]. In the context of radiologic findings, a diagnosis of localized MM, microcystic (adenomatoid) variant, was made. The patient subsequently underwent right pneumonectomy. Examination of the lung found a localized, pleural-based 4.8 cm tumor located adjacent to the right upper lobe. The tumor involved parietal and visceral pleura, and focally invaded the underlying lung parenchyma and overlying chest wall soft tissue, confirming the diagnosis of MM. The patient remains disease free 29 months after the pneumonectomy.
DISCUSSION
Our study describes the FNA findings of a microcystic variant MM arising from the pleura, presented as a localized mass. Recognition of this uncommon type of MM requires recognition of characteristic cytomorphologic features as well as awareness of the unfamiliar clinical and radiologic settings that can be associated with the tumor. An FNA sample can be acceptable as an adequate specimen for definitive diagnosis of MM when immunohistochemistry studies can demonstrate mesothelial origin and exclude other differential diagnoses. The histomorphologic characteristic of the microcystic variant of pleural MM is well-described in a recent case report[5] but, to the best of our knowledge, this is the first case on which FNA was performed as the first-line investigation for primary diagnosis of a microcystic variant of localized MM.
Adenomatoid MM is a rare subtype of epithelioid mesothelioma and may be seen in about 6% of pleural MM.[6] Detailed clinicopathologic and immunohistochemical studies of adenomatoid variant pleural MM have been documented in a previous publication[4] in which all cases were diagnosed on surgical specimens. Most cases in this study were older males similar to our case. All cases presented with pleural effusions, and diffuse pleural thickening with multiple nodules, which are not seen in our case. The key features of these unusual cases were tubules and glandlike structures lined by plump or flattened epithelioid cells.[4] Cytologically, these lining cells showed nuclear atypia and marked cytoplasmic vacuolation, and occasional signet ring cell features, similar to those seen in our case. Another interesting observation in the present case was that the vacuoles of adjacent signet ring cells appeared to fuse to form large vacuoles, resulting in adenomatoid or multicystic features. Furthermore, some cells in the present case showed multiple intracytoplasmic microvacuoles, and progressively enlarged vacuoles were observed in adjacent cells making a single large lumen like signet ring cells. Similar morphologic findings were previously documented by Ordonez et al. Based on the 2004 WHO classification,[1] the adenomatoid form is defined as a microcystic structure, with a lace-like, adenoid cystic or signet ring appearance. Recent guidelines for the diagnosis of MM[7] separated adenoid cystic and signet ring cell forms from adenomatoid/microglandular forms independently. Our findings and a review of the limited literature for this unusual variant suggest a possibility of a progression from signet ring cells to microcyst formation. The characteristic features of a gland-like structure and cytoplasmic vacuoles in this variant can be potentially confused with more common mucin-producing cells in adenocarcinoma. In the current case, because of the localized growth, the lack of pleural effusion, and the resemblance to adenocarcinoma, it was difficult to diagnose microcystic MM by cytomorphology only. Histochemical stains with periodic acid–Schiff diastase (PAS-D) and mucicarmine were negative in cytoplasmic vacuoles and microcysts, which support a diagnosis of mesothelioma but do not exclude the possibility of adenocarcinoma. Also, because rare mesotheliomas have been reported containing PAS-D-positive and mucicarmine-positive droplets[8910] mucin stains must be interpreted in combination with immunohistochemical results. Our case maintained the typical immunophenotype of epithelioid mesothelioma and the mucicarmine stain was negative excluding adenocarcinoma.
Given the growth pattern and localized lesion of the present case, benign adenomatoid tumor of the pleura had to be ruled out, even though it is extremely rare with only three cases reported in the English literature.[1112] It has been found incidentally as discrete nodules in the pleura during unrelated surgical procedures. In contrast to adenomatoid MM, the cytologic features are bland with minimal nuclear pleomorphism, no mitotic activity, and no invasive growth, all of which our case showed. Immunohistochemistry is not helpful for a differential diagnosis since both entities share an identical immunophenotype.
Giemsa stained smears showed a prominent myxoid background in our case. At the time of rapid on-site assessment, this metachromatic myxoid material opened the differential diagnosis to tumors characterized by a myxoid stroma, which is typically due to glycosaminoglycans such as chondroitin sulfate and hyaluronic acid. Some mesotheliomas have secretion rich in hyaluronic acid which can be stained with Alcian blue or Hales colloidal iron and the staining is removed by a pretreatment of a section with hyaluronidase.[13] Since this myxoid material was not apparent in cell block sections, and the surgical resection specimens showed inconspicuous stroma, we did not attempt Alcian blue staining with and without hyaluronidase. We believe the background myxoid material in the current case originated from the content of microcysts ruptured in the smearing process. The findings of vacuolated epithelioid cells displaying against a myxoid background may raise the possibility of epithelioid hemangioendothelioma of the pleura. This tends to appear in different demographics from MM with an average age of 36 years, female sex predilection, and a multifocal process involving more than one organ.[141516] It shows bland cytologic features with no or rare mitotic activity, and tumor cells display a vascular immunophenotype with positivity for factor VIII-related antigen, CD31, and CD34.[416] However, in the case presented here, tumor cells had marked cellular atypia and were negative for CD34. A prominent myxoid background and epithelioid cells with multi-vacuolated cytoplasm also suggested a chordoma arising from the posterior mediastinum. Although it rarely presents as a discrete posterior mediastinal tumor,[17] the marked nuclear atypia observed in our sample is exceedingly rare in chordoma, and S-100 protein was negative further excluding the diagnosis.
Localized MM commonly presents in the pleura, in males, and as the epithelioid type.[2] Localized pleural MM is a solitary, circumscribed nodular tumor attached to either the visceral pleura or the parietal pleura and most cases present as incidental findings or with nonspecific symptoms.[2] Our patient presented pneumonia-like symptoms, which had been treated with antibiotics. Nonspecific clinical symptoms, an unusual growth pattern, and lack of pleural effusion made it difficult for the cytopathologist to recognize this rare entity. In diffuse MM, the epithelioid type has a somewhat better prognosis than other types, but the histologic subtype does not correlate with survival or clinical course in the localized form.[2] However, because of its localized nature, it can be cured by surgical excision with longer survival rate than diffuse MM.[3] Weissferdt et al. have reported 10 cases of adenomatoid variant diffuse MM that showed a mean survival of only 10 months after diagnosis. A recent case report of the localized form of this unusual type documented a patient alive 2 years after the operation with recurrence.[5] Our patient is disease free 29 months after pneumonectomy.
CONCLUSION
In summary, MM, including the microcystic variant, can present as a localized mass, and awareness of the existence of unusual morphologic variants and localized forms of mesothelioma is necessary because they can be potentially confused with other tumors exhibiting similar morphology. A broad differential diagnosis, recognition of characteristic cytomorphologic features, and optimal use of immunohistochemistry is crucial in arriving at a specific diagnosis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version was read and approved. All authors qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from Review Ethics Board (REB).
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004.
- [Google Scholar]
- What is the survival after surgery for localized malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2013;16:533-7.
- [Google Scholar]
- Malignant mesothelioma with prominent adenomatoid features: A clinicopathologic and immunohistochemical study of 10 cases. Ann Diagn Pathol. 2011;15:25-9.
- [Google Scholar]
- Microcystic variant of localized malignant mesothelioma accompanying an adenomatoid tumor-like lesion. Pathol Int. 2002;52:416-22.
- [Google Scholar]
- Histologic assessment and prognostic factors of malignant pleural mesothelioma treated with extrapleural pneumonectomy. Am J Clin Pathol. 2008;130:754-64.
- [Google Scholar]
- Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647-67.
- [Google Scholar]
- Mucin-positive epithelial mesotheliomas: A histochemical, immunohistochemical, and ultrastructural comparison with mucin-producing pulmonary adenocarcinomas. Ultrastruct Pathol. 1996;20:293-325.
- [Google Scholar]
- ‘Mucin-positive’ epithelial mesothelioma of the peritoneum: An unusual diagnostic pitfall. Histopathology. 2000;37:33-6.
- [Google Scholar]
- Epithelioid hemangioendothelioma of the lung (intravascular bronchioloalveolar tumor) in a young girl. Pediatr Pulmonol. 1991;11:181-6.
- [Google Scholar]
- Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J. 1998;12:89-96.
- [Google Scholar]
- Epithelioid hemangioendothelioma of the lung (IVBAT) - Clinicopathological and immunohistochemical analysis of 11 cases. Pathologe. 2006;27:106-15.
- [Google Scholar]
- Chordomas of the mediastinum: Clinicopathologic, immunohistochemical, and ultrastructural study of six cases presenting as posterior mediastinal masses. Hum Pathol. 1995;26:1354-62.
- [Google Scholar]








