Translate this page into:
Utility and diagnostic accuracy of endobronchial ultrasound-guided transbronchial fine-needle aspiration cytology of mediastinal lesions: Saudi Arabian experience
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The objective of this study is to evaluate the cytological accuracy of endobronchial ultrasound-guided transbronchial fine-needle aspiration (EBUS-TFNA) of the mediastinal mass/nodular lesions.
Study Design:
Over 3½ years from inception at King Khalid University Hospital, a retrospective analysis of the cytological diagnoses of all the EBUS-TFNA procedures performed in 80 patients who had mediastinal mass/nodular enlargement. Cytology results were reviewed and correlated with the histologic follow-up.
Results:
Of the 80 patients who underwent EBUS-TFNA, 15 cases (18.75%) were positive for malignancy, 48 cases (60%) negative for malignancy and 17 cases (21.25%) unsatisfactory. Of the 48 cases, which were negative for malignancy, 24 (50%) cases were of granulomatous inflammation. The overall diagnostic yield of our EBUS-TFNA specimen was 78.75%. Forty-seven cases (58.75%) of 80 cases had histological follow-up biopsies. Among them, 32 cases (68%) had the same cytological and histological diagnosis and 15 cases (31.09%) had discordance between the cytology and the follow-up histological diagnosis. The sensitivity, specificity, and positive and negative predictive values for diagnosing granulomas by EBUS-TFNA are 77%, 82%, 83%, and 75% and for diagnosing malignancy are 71%, 100%, 100%, and 82%, respectively.
Conclusion:
Preliminary results show that cytological samples obtained through EBUS-TFNA are accurate and specific in making a diagnosis of the mediastinal mass/nodular lesions. Its optimum use depends on the effective collaboration between the cytotechnologist, pathologist, and the bronchoscopist.
Keywords
Cytology
endobronchial ultrasound-transbronchial fine-needle aspiration
fine-needle aspiration
INTRODUCTION
Although mediastinoscopy and video-assisted thoracoscopy have been the primary diagnostic techniques for obtaining samples of any suspicious lesions on imaging, particularly in the mediastinum, minimally invasive techniques like endobronchial ultrasound-guided transbronchial fine-needle aspiration (EBUS-TFNA) have gained popularity because of the ability to obtain tissue with less potential complications. It is a relatively new procedure for sampling mediastinal and hilar lymph nodes.[1234] This procedure enables direct and real-time aspiration of the mediastinal and hilar lymph nodes and is a less invasive alternative to mediastinoscopy.[5]
Endobronchial ultrasound-TFNA using a novel endobronchoscope with needle has been noted to have higher yields than those typically associated with conventional TBNA (85% vs. 66%).[678] Some of the early studies on conventional TBNA; however, did not use on-site evaluation of adequacy of the specimens,[9] which may have improved the sensitivity of the procedure by increasing the rate of adequate specimens.[1011]
The diagnostic yield of EBUS-TFNA procedure is better compared with conventional transbronchial needle aspiration because of the ability to observe the needle in the target of interest, and it has been demonstrated that the reproducibility of the diagnosis is excellent in experienced hands.[11213] In addition, EBUS-TFNA allows for broader access to mediastinal lymph nodes than mediastinoscopy.[12131415]
Several types of specimens can be obtained with EBUS guidance, including needle aspiration, brushing, washing, lavage, and core needle biopsy.[12416] Direct smears can be made from needle aspiration and brushing specimens to allow on-site evaluation. The other materials are usually processed in the cytology laboratory and subsequently, evaluated by the cytology personnel. Shidham's method can be applied to the remaining cytology specimen to improve the quality of diagnostic material in cell block sections.[17]
These cytological materials provide vital diagnostic information for appropriate patient management. In the English literature, only few studies have investigated the cytological features of EBUS-TFNA specimens.[12416] The objective of this study was to review the diagnostic utility of EBUS-TFNA specimens from a cytological perspective, in addition to correlate these findings with the histological follow-up.
MATERIALS AND METHODS
We performed a computerized search of the cytopathology electronic database from our institution for all aspirations performed by EBUS-TFNA from May 1, 2010 to December 15, 2013 for a variety of clinical indications. Procedure conducted by one experienced pulmonologist in the bronchoscopy suite on all patients with mediastinal lymphadenopathy based on chest computed tomography (CT). All patients understood the procedure required to establish the diagnosis, and written informed consent form was obtained from all participants. Local anesthesia with mild conscious sedation was used for the procedure.
Endobronchial ultrasonography was performed using fiberoptic ultrasound bronchoscope with a linear scanning probe (BF-UC260FW, Olympus Medical System, Tokyo, Japan). A dedicated ultrasound scanner (ALOKA SSD-Alpha 10, ALOKA Ultrasound System, Japan) was used for image processing. Targeted lymph node was sequentially sampled with a dedicated 21-gauge needle (NA-201SX-4021, Olympus Medical System, Tokyo, Japan).
Smears were made at the bedside in the bronchoscopy suite.
Preparation of smears
The aspirated material from the needle was expelled onto glass slides by the operating bronchoscopist and smeared by a cytotechnologist. 2-4 slides were prepared from each pass, taking care that any clotted material is preserved for cell block. Air-dried (for diff-quick staining) and fixed smears (fixed immediately in 95% ethyl alcohol for subsequent Papanicolaou staining) were prepared in an almost equal ratio with more emphasis on fixed smears. Pass number (indicating a specific site of the collection) was marked on each slide and site of collection of each pass was noted on a separate paper.
One or two representative of the air-dried smears from each pass were immediately stained with rapid Romanowsky (diff-quick stain from Shandon) and examined under the microscope for specimen adequacy assessment, preliminary diagnostic interpretation if necessary and to suggest additional studies if indicated.
Additional material was collected for ancillary studies if necessary (in cases of lymphomas, tuberculosis [TB]) and was preserved in normal saline (for microbiology) or RPMI cell preservative solution (for flow cytometry), if and as required. RPMI cell preservative solution was used as cell collection/preservation and transport medium for cell block and Thin-Prep slides preparation.
Material collected for cell block was grossly examined before the end of the procedure and if the collected material was still not enough to make a good cell block, an additional pass was dedicated to cell block only.
Cytology request form was completed by the consultant bronchoscopist, including the details of the procedure and site of specimen for each pass.
Number of fixed and air dried slides and description of any other material being sent to laboratory was noted on the request form. Sample was transported to the laboratory as early as possible and processed for staining of remaining slides, preparation of Thin-Prep slides and cell block.
Preparation of thin-prep slides
RPMI needle wash was centrifuged immediately and an aliquot was separated for Thin-prep processing and processed on Thin-prep 2000 according to manufacturer's instructions (Thin Prep processing manual).
Preparation of the cell block
Remaining sediment including any clotted material was fixed immediately in cell block fixative (10% alcohol formalin) for 30 min to 1 h depending on the size of the cell block, centrifuged and material transferred to a histology embedding cassette, and processed for routine histologic examination using standard techniques.
Appropriate immunohistochemical and special stains were performed on the cell block recut slides as required/needed according to microscopic findings and clinical suspicions, following the manufacturer specifications.
Microscopy
All smears, Thin-Prep slides and cell block sections were reviewed by two pathologists (Dr. ER and Dr. MA) with emphasis on the presence of lymphocytes, evaluation of cytomorphologic features, background of the smears, necrosis, overall cellularity, immunohistochemical, and special stains results. Correlations, if necessary, were made with histopathologic findings and other ancillary studies such as flow cytometry and TB culture, etc.
In cases with subsequent trans-bronchial core biopsy, tissues were fixed in 10% formalin and thin histologic sections were cut from paraffin-embedded tissue blocks followed by hematoxylin and eosin staining. A review of all available histologic slides was performed and correlated with the respective cytological diagnoses. Then 2 × 2 tables were prepared to calculate the sensitivity, specificity, and positive and negative predictive values, and the Fisher exact test was used to compare differences between groups. Calculations were performed using SPSS 14.0 for Windows (SPSS, Chicago, IL).
RESULTS
This study included a total of 80 EBUS-TFNA specimens obtained from 50 males (62.5%) and 30 females (37.5%). The male: Female was 1.6:1. The patients’ age ranged from 16 to 80 years old with a mean age of 46.5 years. Of the 80 consecutive cases, 73 (91.2%) had lymph node sampling, three had lung sampling and five had mediastinal mass sampling.
The cytological diagnosis included 15 cases (18.75%) positive for malignancy, 48 cases (60%), negative for malignancy and 17 cases (21.25%) unsatisfactory/nondiagnostic as shown in Table 1. Of the 48 cases which were negative for malignancy, 24 (50%) cases were granulomatous inflammation [Figure 1]. Of the 15 cases with a positive diagnosis, there were five (33.3%) nonsmall cell carcinoma [Figure 2], three (20%) nonHodgkin's lymphoma [Figure 3], one (6.6%) small cell carcinoma [Figure 4], one (6.6%) neuroendocrine carcinoma, one (6.6%) poorly differentiated carcinoma and three (20%) nonpulmonary metastatic carcinoma (one upper gastrointestinal tract [Figure 5], nasopharynx [Figure 6], and one poorly differentiated carcinoma with unknown primary).
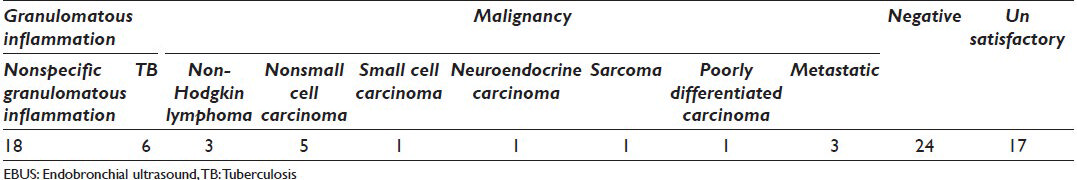

- Granulomatous inflammation, cytology smear shows well-defined epithelioid cell granuloma (diff-quick stain, original magnification ×500)

- Cytology smear from lung adenocarcinoma metastatic into mediastinal lymph node (Papanicolau stain, original magnification ×500)

- NonHodgkin's lymphoma, cell block preparation obtained from mediastinal lymph node by endobronchial ultrasound-fine needle aspiration (Hematoxylin and Eosin stain, original magnification ×400)
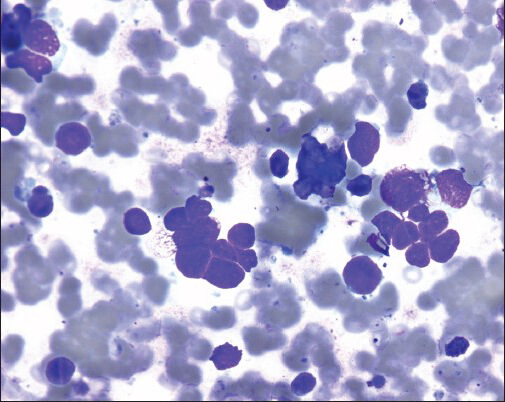
- Cytology smear from small cell carcinoma of lung primary, metastatic into mediastinal lymph (diff quick stain, original magnification ×500)
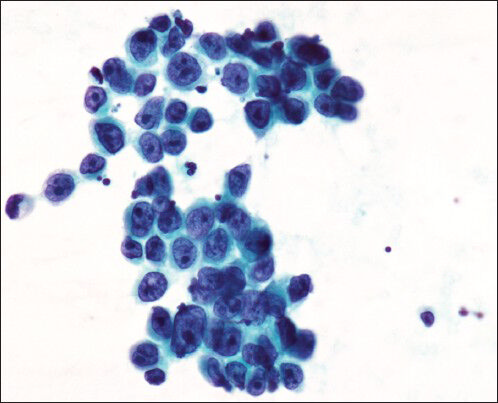
- Cytology smear from mediastinal lymph node reveals metastatic Adenocarcinoma of gastric origin (Papanicolau stain, original magnification ×500)
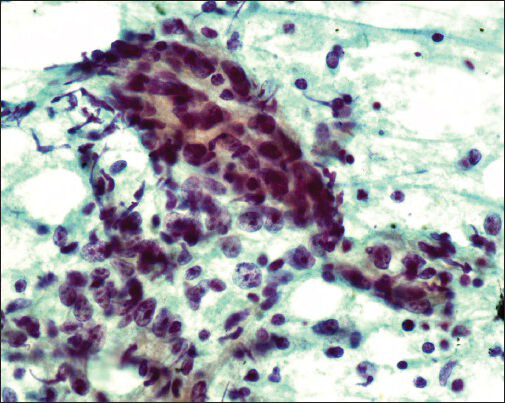
- Cytology smear from mediastinal lymph node reveals metastatic nasopharyngeal carcinoma (Papanicolau stain, original magnification ×500)
The overall diagnostic yield of our EBUS-TFNA specimen was 78.75% with 17 cases, which were considered unsatisfactory.
Histological correlation
Forty-seven cases (58.75%) of 80 cases had corresponding core biopsies or follow-up surgeries. Of the 17 unsatisfactory specimens on EBUS-TFNA, 15 cases had corresponding histopathology follow-up. Of these cases on histology, six cases were negative, two unsatisfactory, two cases of granulomas (TB), three cases were of adenocarcinoma, one case of Hodgkin's lymphoma, nodular sclerosis subtype and one case of leiomyoma. Of the 24 negative cases, 14 cases had corresponding histology follow-up. Of these, nine cases were negative, three cases of granulomatous inflammation (two TB and one sarcoidosis), one case of adenocarcinoma and one Hodgkin's lymphoma. Of the 24 cases of granulomatous inflammation diagnosed on EBUS-TFNA, 12 had corresponding histology confirmation. 10 cases were granulomatous inflammation (seven sarcoidosis and three TB) and two were negative for granuloma or malignancy. Of the 15 cases of malignancy, six cases had corresponding histologic follow-up. Of these five cases were malignant (two were adenocarcinoma, one synovial sarcoma, one non-Hodgkin's lymphoma and one metastatic adenocarcinoma from upper gastrointestinal tract) and one case was negative. This data is summarized in Table 2.
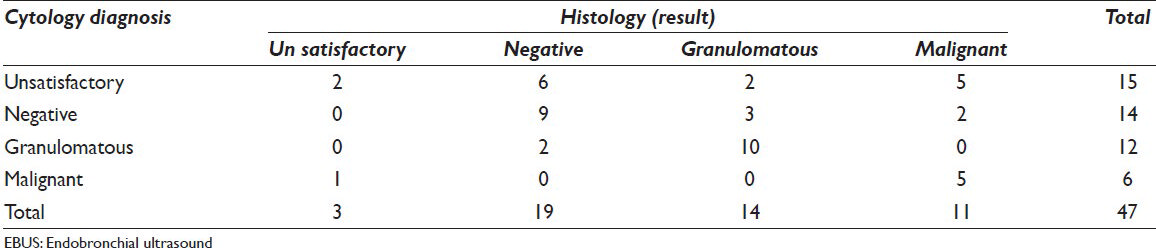
Thus, in our study, 32 cases (68%) had the same cytological and histological diagnosis and 15 cases (31%) had cytological diagnosis with discordant histological diagnosis. This data is summarized in Table 3.

When the histological diagnosis was taken as the gold standard, the sensitivity, specificity, positive predictive value, and negative predictive values for diagnosing granulomas by EBUS-TFNA are 77%, 82%, 83%, and 75% and for diagnosing malignancy are 71%, 100%, 100%, and 82%, respectively.
DISCUSSION
The investigation for mediastinal masses or nodular lesions etiology is a subject that the clinicians are commonly faced with. However, the detection of enlarged mediastinal lymphadenopathy and accurate staging of extrathoracic malignancy is important to plan further treatment. Mediastinoscopy is considered the gold standard procedure in this clinical scenario. However, it requires general anesthesia, and there is the possibility of significant complications.[18] In addition, mediastinoscopy has limitations in accessing hilar nodal stations, can be difficult in patients of previous radiations and the procedure cannot be repeatedly used on the same patient.[19]
There are various fine-needle aspiration techniques exist for diagnosing lung and mediastinal lesions, including trans-esophageal EUS-FNA, CT-guided transthoracic FNA, conventional TBNA, and EBUS-TFNA. Among them, EBUS-TFNA is a relatively new modality for sampling mediastinal lesions. It has been shown to be a safer, more sensitive, and cost-effective diagnostic procedure, which shows potential to avoid explorative thoracoscopies.[1234122021] It is usually performed under local anesthesia, using moderate sedation in an outpatient setting.
There are a few studies published regarding the clinical usefulness of EBUS-TFNA from the United States,[22] specialized centers in Europe, the United Kingdom and Japan.[71523] These studies showed high diagnostic rates for EBUS-TFNA with sensitivities and positive predictive values of more than 90% and specificities of 100%.
The present retrospective study from Saudi Arabia showed that EBUS-TFNA is a sensitive, accurate and safe modality for the evaluation of mediastinal masses and nodular lesions. EBUS-TFNA was introduced in recent years to the pulmonology and thoracic surgery practice in our hospital as a new modality to diagnose and stage lung cancer and to evaluate mediastinal diseases. In our study, diagnostic material was obtained in 78.5% of sites sampled (63/80). The unsatisfactory specimen rate was 21% (17/80). An accurate diagnosis was made in 32 (68%) of 47 satisfactory aspirates with histological follow-up. The sensitivity, specificity, and positive and negative predictive values for diagnosing granulomas by EBUS-TFNA were 77%, 82%, 83%, and 75% and for diagnosing malignancy are 71%, 100%, 100%, and 82%, respectively. This number is slightly lower than other retrospective studies who reported sensitivity of EBUS-TFNA between 85% and 92%, respectively.[234212224252627]
Alsharif et al. have reported a study of 100 patients who underwent EBUS-TFNA, a nondiagnostic rate of 15.7%.[4] In their study, 28% of cytology specimens had corresponding histology, yielding sensitivity and specificity of 86% and 100%, respectively. Jacob-Ampuero et al. reported 48 cases with a diagnostic yield of 77% and both sensitivity and specificity of 100%.[2] Feller-Kopman et al. have reported a study of 135 cases of lymph node and lung lesion biopsy by EBUS-TFNA with sensitivity and specificity of 85% and 100%.[26] The overall nondiagnostic rate reported by most authors range from 4% to 23%.[2426] Our data shows nondiagnostic rate of 21.25%. There are various factors, which contribute to the diagnostic yield. Some of them are skill of the endoscopist, size and station of the lymph nodes, difficult target with fibrotic tissue and experience of the cytopathology personnel performing on site material adequacy.
Until date, pathology criteria for representativeness of an aspirate are not well-established for EBUS-TFNA and may vary among pathologists.[1] There has been little focus on this area and definition of adequacy can be improved by setting up commonly agreed pathology criteria. The cellularity plays an important role in making the diagnosis. For our study, we found that the cellularity varied widely and the adequate sample was defined as the presence of either site specific diagnostic material or sufficient lymphoid cells with at least 40 lymphocytes per high power field in the most cellular areas, as is used in other studies.[2829] The amount of lymphoid tissue is likely not only dependent on the bronchoscopist's skill, but also on the degree of nodal involvement by the disease process, particularly in metastatic lesions or granuloma. Therefore, the lymphoid tissue may not be seen in a lymph node that has been entirely replaced by granulomata or metastasis. When there are very few lymphocytes, they most likely come from peripheral blood rather than a lymph node. We consider that the presence of moderate to abundant numbers of lymphocytes and/or pigmented macrophages is a good indicator of adequate sampling of lymph node and lung parenchyma in most cases. Hence, as the cytopathological adequacy criteria for EBUS-TFNA are not well-established, future studies are needed to help standardize these criteria because of its importance in maximizing the diagnostic performance of the procedure and defining an adequate specimen, which can minimize false-negative diagnoses.
An early study by Baker et al.[9] have reported a significant difference in the predictive values of negative transbronchial aspirates with and without lymphocytes (78% vs. 36%). Later Alsharif et al.[4] found that the presence and quantity of bronchial cells had no bearing on the adequacy because these cells were found in the majority of their samples, without correlation with the number of lymphocytes. Excess contamination with normal cells can obscure the scant malignant cells or, in cases with reactive respiratory cells, mimic a well-differentiated adenocarcinoma. The cell block is extremely helpful in such cases and immunohistochemical analysis can be performed when necessary.
SUMMARY
Endobronchial ultrasound- TFNA is minimally invasive, cost-effective, accurate, safe, and promising technique for the diagnosis of mediastinal masse/lesions. It is usually performed under local anesthesia, using moderate sedation in an outpatient setting. It has a good sensitivity and high specificity with a high diagnostic yield. Cell block for histology and immunohistochemistry provides supportive evidence for the diagnosis. The optimum use of EBUS-TFNA depends on the effective collaboration between the cytotechnologist, pathologist and the bronchoscopist.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
Declare that we have no competing interests. The authors would like to emphasize that we have not currently or in the past received financial or any other form of support from any of the companies and/or manufacturers mentioned in the article.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article; utility and diagnostic accuracy of endobronchial ultrasound-guided transbronchial fine-needle aspiration cytology: Saudi Arabian experience. Declare that we qualify for authorship as defined by ICMJE. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. AB carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. JY carried out the immunoassays. MT participated in the sequence alignment. ES participated in the design of the study and performed the statistical analysis. FG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia. Author takes responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Cytopathologic diagnoses of fine-needle aspirations from endoscopic ultrasound of the mediastinum: Reproducibility of the diagnoses and representativeness of aspirates from lymph nodes. Cancer. 2007;111:234-41.
- [Google Scholar]
- Cytologic accuracy of samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration at Thomas Jefferson University Hospital. Acta Cytol. 2008;52:687-90.
- [Google Scholar]
- Endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes: A single institution's early learning curve. Ann Thorac Surg. 2008;86:1104-9.
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial fine-needle aspiration: The University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. Am J Clin Pathol. 2008;130:434-43.
- [Google Scholar]
- The role of endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) for qualitative diagnosis of mediastinal and hilar lymphadenopathy: A prospective analysis. BMC Cancer. 2011;11:100.
- [Google Scholar]
- Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795-8.
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. 2006;28:910-4.
- [Google Scholar]
- Preliminary experience with a new method of endoscopic transbronchial real time ultrasound guided biopsy for diagnosis of mediastinal and hilar lesions. Thorax. 2003;58:1083-6.
- [Google Scholar]
- Transbronchial fine needle aspiration of the mediastinum. Importance of lymphocytes as an indicator of specimen adequacy. Acta Cytol. 1990;34:517-23.
- [Google Scholar]
- Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens. Cytojournal. 2011;8:20.
- [Google Scholar]
- Diagnostic difficulties and pitfalls in rapid on-site evaluation of endobronchial ultrasound guided fine needle aspiration. Cytojournal. 2010;7:9.
- [Google Scholar]
- Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: A randomized trial. Chest. 2004;125:322-5.
- [Google Scholar]
- Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122-8.
- [Google Scholar]
- Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006;130:710-8.
- [Google Scholar]
- Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: A combined approach in the evaluation of mediastinal lesions. Endoscopy. 2005;37:833-9.
- [Google Scholar]
- Endobronchial ultrasound-guided fine-needle aspiration and liquid-based thin-layer cytology. J Clin Pathol. 2007;60:388-91.
- [Google Scholar]
- Cell block preparation from cytology specimen with predominance of individually scattered cells. J Vis Exp. 2009;21:e1316.
- [Google Scholar]
- Nine-year single center experience with cervical mediastinoscopy: Complications and false negative rate. Ann Thorac Surg. 2006;82:1185-9.
- [Google Scholar]
- Mediastinal staging procedures in lung cancer: EBUS, TBNA and mediastinoscopy. Curr Opin Pulm Med. 2009;15:334-42.
- [Google Scholar]
- EUS-FNA and EBUS-TBNA; the pulmonologist's and surgeon's perspective. Endoscopy. 2006;38(Suppl 1):S105-9.
- [Google Scholar]
- Competition for EUS (a) EBUS-TBNA (b) video assisted thoracoscopy. Endoscopy. 2006;38(Suppl 1):S80-3.
- [Google Scholar]
- Real-time endobronchial ultrasound-guided transbronchial lymph node aspiration. Ann Thorac Surg. 2008;85:224-30.
- [Google Scholar]
- Advances in lung cancer diagnosis and staging: Endobronchial ultrasound. Intern Med J. 2008;38:85-9.
- [Google Scholar]
- Bronchoscopic evaluation of the mediastinum using endobronchial ultrasound: A description of the first 216 cases carried out at an Australian tertiary hospital. Intern Med J. 2011;41:815-24.
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration of undiagnosed mediastinal lymphadenopathy. Chin Med J (Engl). 2010;123:2211-4.
- [Google Scholar]
- Cytology of endobronchial ultrasound-guided transbronchial needle aspiration: A retrospective study with histology correlation. Cancer. 2009;117:482-90.
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration biopsy is useful evaluating mediastinal lymphadenopathy in a cancer center. Cytojournal. 2011;8:10.
- [Google Scholar]
- Evaluation of endobronchial ultrasound-guided fine-needle aspirations (EBUS-FNA): Correlation with adequacy and histologic follow-up. Cancer Cytopathol. 2014;122:23-32.
- [Google Scholar]
- Cytology of endobronchial ultrasound-guided transbronchial needle aspiration versus conventional transbronchial needle aspiration. Cancer Cytopathol. 2010;118:278-86.
- [Google Scholar]








