Translate this page into:
Nobiletin alleviates brain injury in uremic mice and inhibits indoxyl sulfate-induced neurotoxicity in HT22 cells through the phosphatidylinositol 3-kinase/protein kinase B signaling pathway

*Corresponding author: Ruyi Zhang, Department of Internal Medicine, The Affiliated Kangning Hospital of Wenzhou Medical University Zhejiang Provincial Clinical Research Center for Mental Disorder, Wenzhou, China. 15968765927@163.com
-
Received: ,
Accepted: ,
How to cite this article: Xu L, Zhang R. Nobiletin alleviates brain injury in uremic mice and inhibits indoxyl sulfate-induced neurotoxicity in HT22 cells through the phosphatidylinositol 3-kinase/protein kinase B signaling pathway. CytoJournal. 2025;22:27. doi: 10.25259/Cytojournal_233_2024
Abstract
Objective
Uremic encephalopathy presents as central nervous system symptoms in acute and chronic renal failure. Nobiletin (NOB), an extract from chenpi, has demonstrated anti-inflammatory bioactivity and potential neuroprotective effects without remarkable toxicity. This study aims to evaluate the pharmacological effects of NOB on treating uremic brain injury and elucidate its underlying mechanisms.
Material and Methods
A uremic encephalopathy mouse model was established by inducing renal failure with cisplatin (DDP). The therapeutic effects of NOB were investigated by assessing its effect on brain damage and neuronal viability. HT22 murine hippocampal neurons were also treated with DDP to induce neurotoxicity, and the effects of NOB on cell viability, apoptosis, and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway were examined. The PI3K inhibitor LY294002 was used to further investigate the involvement of the PI3K/Akt pathway in the neuroprotective effects of NOB.
Results
NOB alleviated uremia-induced brain damage in mice, and this function was associated with the activation of the PI3K/Akt signaling pathway. In vitro, NOB improved the DPP-suppressed cell viability in HT22 neurons and restored apoptosis. NOB treatment also restored the phosphorylation levels of PI3K, Akt, and Pyruvate dehydrogenase kinase 1. These effects were partially blocked by the PI3K inhibitor LY294002.
Conclusion
NOB exerts potent neuroprotective effects by activating the PI3K/Akt pathway, mitigating uremia-induced brain injury and preventing DDP-induced neurotoxicity. These findings support the potential therapeutic application of NOB for uremic encephalopathy and provide insights into its underlying mechanisms.
Keywords
Neuroprotection
Nobiletin
Oxidative stress
Uremic toxins
INTRODUCTION
Chronic renal failure is the irreversible decline of renal function resulting from the ongoing progression of various chronic kidney diseases (CKDs). It impacts multiple organs and systems throughout the body. Uremia, also known as end-stage renal disease (ESRD), is the terminal stage of CKD, where the renal function deteriorates to such an extent that dialysis or a kidney transplant becomes necessary for survival.[1] Uremic encephalopathy, or renal encephalopathy, is a frequent complication of acute and chronic renal failure.[2] When renal failure occurs, most metabolic products normally excreted by the kidneys, such as urea, creatinine, uric acid, and phenols, accumulate in the body. In particular, the levels of these substances significantly increase in the serum, cerebrospinal fluid, and brain tissue of patients with renal failure, leading to neurological abnormalities such as cognitive decline, cerebrovascular events, and neuropathy.[3] Lee et al. reported that in a murine CKD model, uremic toxins are released into the brain tissues, resulting in elevated reactive oxygen species (ROS) levels in the hippocampus.[4] In addition, uremic toxins elevate ROS levels, which accumulate in brain cells and induce apoptosis. However, the neuronal injury caused by uremic toxins and the underlying mechanisms remain unclear.
Indoxyl sulfate (IS) is a protein-bound toxin metabolized from tryptophan derivatives, and over 90% of this toxin binds to proteins.[5] IS primarily exists in the bloodstream bound to albumin and is the most abundant indole compound accumulated in the body of uremic patients. When substantial renal damage occurs, the number of functional tubular cells decreases dramatically, leading to the significant accumulation of IS in the brainstem and hippocampus. Due to its protein-binding nature, this compound exceeds the pore size of dialysis membranes, making hemodialysis or peritoneal dialysis ineffective in removing IS.[6] Studies have shown a correlation between elevated IS levels in the blood of uremic patients and neurological complications.[7] In addition, the IS levels in patients with CKD are correlated with mortality rates.[8-10]
Nobiletin (NOB) is a bioactive compound classified as a polymethoxylated flavonoid and primarily derived from the peel of citrus fruits, particularly those belonging to the Rutaceae family, such as oranges, tangerines, and grapefruits.[11] NOB possesses anti-inflammatory, antioxidant, anticancer, and anti-atherosclerotic properties and the ability to regulate blood glucose levels, highlighting its potential in managing various clinical diseases.[12-15] It also exhibits notable neurotrophic activity, inhibiting the neurotoxicity of β-amyloid protein in brain tissue, reducing oxidative damage to neural cells caused by H2O2, and improving cognitive impairments associated with cerebral ischemia.[11,12,16] Various studies have collectively confirmed the neuroprotective effects of NOB and its effectiveness in mitigating neural damage. NOB exhibits significant antioxidant activity, with its antioxidant capacity positively correlated with its concentration. It exerts antioxidant effects by enhancing glutathione (GSH), GSH-peroxidase, and superoxide dismutase (SOD) activity while reducing malondialdehyde (MDA) levels, thereby mitigating oxidative damage from isoflurane-induced cognitive impairment in aging rats.[17] NOB effectively suppresses ROS production by enhancing the expression of Protein kinase B (Akt), B-cell lymphoma-2 (Bcl-2), and SOD while reducing the levels of Bcl-2-associated X protein (Bax), cleaved Caspase-3, and MDA, thereby effectively inhibiting the oxidative stress mediated by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway.[18] Through the activation of the SIRT-1/FOXO3a and PGC-1α pathways, NOB enhances autophagy, boosts mitochondrial function, and reduces oxidative damage, providing antioxidant effects in liver ischemic reperfusion injury.[19]
NOB has garnered increasing attention due to its broad pharmacological effects and bioactivity beneficial to human health. It exhibits significant anti-inflammatory and antioxidant properties and provides neuroprotection, memory enhancement, and cognitive improvement. NOB has also demonstrated notable neuroprotective effects and the ability to enhance brain function in conditions such as cerebral infarction, Parkinson’s disease, and diabetes-induced neural damage.[20] However, the neuroprotective mechanisms underlying its effects are not fully understood. Cisplatin (DDP) is a first-line clinical antineoplastic agent widely used to treat various solid malignancies but is associated with nephrotoxicity during chemotherapy.[21] This study employed DDP-induced renal failure in mice to explore NOB’s protective role in uremia-induced brain injury. We also investigated the role and underlying mechanisms of NOB in mitigating neuronal cell apoptosis induced by uremic toxins. The findings provide new evidence supporting the therapeutic potential of NOB in treating uremic encephalopathy.
MATERIAL AND METHODS
Animals
C57BL/6J male mice aged 8 weeks and with a body weight of 20–25 g were sourced from Beijing HFK Biotechnology Co., Ltd., Beijing, China. They were housed in an SPF environment with a 12-h light/dark cycle, appropriate humidity, temperature, and cleanliness, and had free access to food and water. After a week of acclimatization, the mice were randomly assigned to four groups (n = 8): control, NOB, DDP, and DDP + NOB. They were administered with DDP (500 mg/kg/d, G5388, Sigma, St. Louis, MI, USA) or NOB (100 mg/kg/d, HY-N0155, MedChemExpress, Monmouth Junction, NJ, USA) through intraperitoneal injection.[22] Blood, kidney, and brain tissue samples were collected after 1 week of treatment, and blood serum creatinine (SCr), urea nitrogen, kidney injury molecule 1 (Kim-1), and neutrophil gelatinase-associated lipocalin (Nagl) levels were measured using an automated biochemical analyzer (Cobas 8000 c702, Roche, Basel, Switzerland). LY294002 was purchased from MedChem Express (HY-10108, Monmouth Junction, NJ, USA). At the end of the experiment, the mice were euthanized by cervical dislocation under deep anesthesia induced by the intraperitoneal injection of 50 mg/kg sodium pentobarbital (P3761; Sigma-Aldrich; Merck KGaA). The animal experiments were approved by the Animal Ethics Committee of Guangzhou Miers Biotechnology Co., Ltd. (IACUC-MIS20230042) and were conducted in accordance with the Institutional and International Guidelines for Animal Care and use to ensure the ethical treatment of animals.
Hematoxylin and eosin (H&E) staining and tissue damage score
Kidney tissues were treated with 4% paraformaldehyde for fixation, followed by dehydration and embedding in paraffin. Sections of 4 μm thickness were cut and incubated overnight at 37°C, followed by dewaxing and rehydration. The kidney tissues were observed by H&E staining (C0105S, Beyotime, Shanghai, China). Consecutive fields were evaluated at a ×100 magnification under an optical microscope (IXplore Standard, Olympus, Tokyo, Japan).
Cell culture and grouping
HT22 cells were purchased from Procell (Wuhan, China, CL-0697) and authenticated by short tandem repeat profiling to confirm their origin. Mycoplasma contamination testing was also performed to ensure the authenticity and contamination-free status of the cell cultures. The HT22 mouse hippocampal neuronal cell line was cultured in a medium containing 10% FBS (A5256701, Gibco, Grand Island, NY, USA) and 1% antibiotics (15140148, Gibco, Grand Island, NY, USA) at 37°C with 5% carbon dioxide. The HT22 cells were plated and allowed to adhere, followed by treatment with DDP at concentrations ranging from 1 mm to 70 mm or NOB for 24 h. The cells were cocultured with DDP and NOB for 24 h to evaluate the effects of NOB (Sigma, St. Louis, MO, USA) on neurons. LY294002 (a specific PI3K inhibitor) was purchased from MedChemExpress (HY-10108, Shanghai, China). To investigate the effects of NOB on DDP-induced HT-22 cells, we treated the cells with NOB at concentrations of 25, 50, and 100 μM. The experimental groups were as follows: the untreated control group, the NOB (50 μM) alone group, the DDP (5 μM) alone group, the DDP + NOB 25 μM group, the DDP + NOB 50 μM group, and the DDP + NOB 100 μM group, totaling six groups. In Experiment 4, the PI3K inhibitor LY294002 was employed to examine the underlying mechanism of PI3K. The corresponding cell groups in this experiment included the control group, the NOB (50 μM) alone group, the DDP (5 μM) alone group, the DDP + NOB (50 μM) group, and the DDP + NOB (50 μM) + LY294002 (10 μM) group, also consisting of six groups in total.
Cell viability assay
Cell viability was assessed using CKK-8 assay (C0038, Beyotime, Shanghai, China) after treatment with various concentrations of DDP (0–20 mm) or NOB (0–100 mm) following the manufacturer’s instructions. Cell viability was measured according to the instructions of the cell counting kit-8 (CCK-8) assay kit. In brief, 100 μL of cell suspension was seeded in a 96-well plate and cultured overnight at 37°C. The next day, the medium was replaced, and different drugs were added for coculturing for 24 h. The medium was replaced with a fresh one, and 10 μL of CCK-8 solution was added to each well. The plate was then incubated for 30 min in the incubator. Absorbance at 450 nm was measured using a microplate reader (Thermo Scientific™ Multiskan™ FC) to assess cell viability.
ROS detection
Cell apoptosis was determined using the ROS assay kit (S0033S, Beyotime, Shanghai, China). The cells were pre-incubated at 37°C with 10 μM Dichlorodihydrofluorescein diacetate, followed by suspension and incubation in the dark for 20 min. After incubation, the cells were observed by fluorescence microscopy (FV4000, Olympus, Tokyo, Japan).
Western blot
Tissues or cells were homogenized in RIPA buffer (P0013 C, Beyotime, Shanghai, China) containing 1 mM PMSF (ST 507, Beyotime, Shanghai, China). After centrifugation (13,000 rpm, 10 min, 4°C), the supernatant was collected for Western blot analysis. The total protein concentration was measured using a BCA assay kit (P0012, Beyotime, Shanghai, China). In brief, 20 μg of proteins per lane were subjected to electrophoresis on a 10–15% SDS-PAGE gel and then transferred to a PVDF membrane (IPVH00010, Merck Millipore, Billerica, MA, USA). The equipment for electrophoresis and transfer was obtained from Bio-Rad (Hercules, CA, USA). The membrane was then blocked using 5% nonfat milk at room temperature for 1 h and subsequently incubated overnight at 4°C with primary antibodies targeting α-tubulin (2144), Bcl2 (15071), poly ADP-ribose polymerase (PARP) (9532), PI3K (4292), pyruvate dehydrogenase kinase 1 (PDK1) (3062), Akt (4691), p-PI3K (17366), p-PDK1 (3438), p-Akt (4060), and Bax (5023) (1:1000 each, Cell Signaling Technology, Danvers, MA, USA). Afterward, the membrane was incubated with HRP-conjugated anti-rabbit Immunoglobulin G (IgG) secondary antibody (1:2000, A0208, Beyotime, Shanghai, China), and HRP-conjugated anti-mouse IgG secondary antibody (1:2000, A0216, Beyotime, Shanghai, China). An appropriate amount of ECL (P0018S, Beyotime, Shanghai, China) (prepared by mixing Solution A and Solution B in a 1:1 volume ratio) was applied to the PVDF membrane to ensure that it was completely covered with the substrate. The membrane was then placed into a ChemiDoc XRS + chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA) for detection. Semi-quantitative analysis of band intensity was performed using ImageJ software (version 1.5f, NIH, Maryland, USA).
Statistical analysis
The experimental data were analyzed using the Statistical Package for the Social Sciences (SPSS) 20.0 software (SPSS Inc., Chicago, IL, USA). All experiments were conducted in at least three replicates, and data from animal and cell model experiments were expressed as the mean ± Standard deviation. The normality of the data was assessed using the Shapiro–Wilk test. If the data in each group were normally distributed or approximately normal with homogeneous variances, comparisons between the two groups were performed using the independent samples t-test. For comparisons involving three or more groups, one-way analysis of variance was used. P < 0.05 was considered statistically significant.
RESULTS
NOB attenuates DDP-induced brain damage in uremic mice
To verify that DDP can induce uremia in mice, we first observed the overall condition of the mice. The mice in the DDP group exhibited significant weight loss, lethargy, rapid breathing, decreased body temperature, refusal to eat or drink, disheveled and dull fur, and minimal response to touch. By contrast, the mice in the DDP + NOB group showed reduced appetite, depressive behavior, and a sluggish response to touch, indicating that NOB can improve the overall health status of the mice. The changes in body weight are shown in Figure 1a. Throughout the experiment, the mice in the control and NOB groups tended to gain weight, and those in the DDP group tended to lose weight. In particular, the DDP + NOB group experienced minimal weight loss. The kidney coefficients in the mice are presented in Figure 1b. Compared with the control group, the DDP group showed a significant reduction in kidney coefficients (P < 0.001). However, the kidney coefficients of the DDP + NOB group were significantly higher than those of the DDP group, suggesting that NOB can alleviate the DDP-induced reduction in kidney coefficient in mice (P < 0.01).
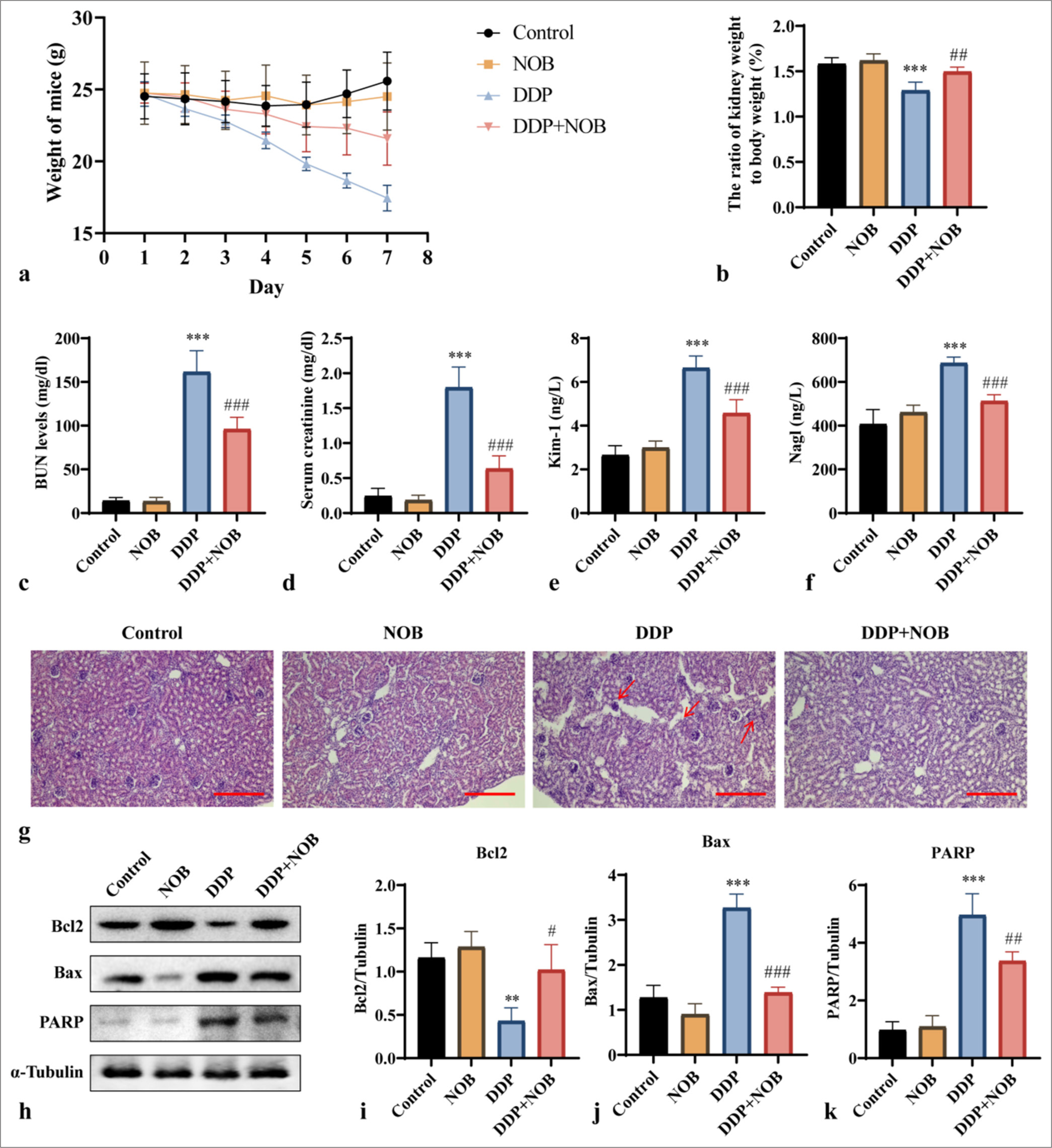
- NOB alleviates DDP-induced brain damage in uremic mice. (a) Body weight of mice. (b) Kidney coefficient of mice. (c-f) Serum levels of BUN, SCr, Kim-1, and Nagl in mice. (g) Representative images of hematoxylin & eosin staining morphological analysis of kidney tissue, showing renal injury features including cellular debris, tubular necrosis, and inflammatory cells ×100. Scale bar = 100 μm (h-k) WB analysis and quantification of Bcl-2, Bax, and PARP protein expression in mouse brain tissue. n = 3; ✶✶✶P < 0.001, ✶✶P < 0.01 versus Control, ###P < 0.001, ##P < 0.01, #P < 0.05 versus DDP. The bar represents mean ± S.D. NOB: Nobiletin, BUN: Blood urea nitrogen, SCr: Serum creatinine, Bcl2: B-cell lymphoma-2, Bax: Bcl-2-associated X protein, PARP: Poly ADP-ribose polymerase, DDP: Cisplatin, WB: Western blot, S.D.: Standard deviation.
Next, we assessed kidney function by measuring SCr, blood urea nitrogen (BUN), Kim-1, and Nagl levels. As shown in Figures 1c-f, the DDP-treated mice exhibited significantly higher levels of BUN in plasma and SCr, Kim-1, and Nagl in serum compared with the control group, indicating their impaired renal function due to DDP (P < 0.001). By contrast, the DDP + NOB group showed significantly lower levels of BUN, SCr, Kim-1, and Nagl than the DDP group, suggesting that NOB improves kidney function (P < 0.001). Examination of kidney tissues with H&E staining indicated that DDP treatment resulted in damage to the tubular interstitium, injury to tubular epithelial cells, and interstitial inflammation, all of which notably improved with NOB treatment [Figure 1g]. The above mouse renal injury phenotype results showed that DDP can induce uremia in mice, and NOB can improve the kidney injury of uremic mice. To assess cellular damage in the brains of uremic mice, we evaluated the expression of Bcl-2, Bax, and PARP (85 kDa) in brain tissues. Compared with the control group, the DDP-treated mice exhibited higher levels of Bax (P < 0.001) and PARP (P < 0.001) and lower levels of Bcl-2 (P < 0.01) in brain tissues [Figures 1h-k]. These levels indicated an increase in apoptosis, which was alleviated by NOB treatment (P < 0.05). Hence, brain cell damage in uremic mice possibly occurs through the enhancement of the apoptotic pathway, with NOB treatment potentially reducing this damage by influencing the expression of Bcl-2, Bax, and PARP.
Activation of PI3K/Akt signaling pathway by NOB
The regulation of apoptosis involves several signaling pathways, including the PI3K/Akt pathway that is involved in memory, learning, and the survival, differentiation, and axonal growth of neurons in the brain.[23,24] To assess whether NOB alleviates DDP-induced brain tissue damage through the PI3K/Akt signaling pathway, we studied the activation of this pathway by conducting Western blot analysis. This method allowed us to measure the expression levels of key proteins involved in the PI3K/Akt signaling cascade, providing insights into the molecular mechanisms underlying the protective effects of NOB. Compared with that in the control group, DDP treatment caused a decline in p-PI3K (P < 0.01), p-PDK1 (P < 0.01), and p-Akt (P < 0.001) levels, but NOB treatment significantly restored their expression (P < 0.05) [Figures 2a-d]. These findings demonstrated that the protective effects of NOB against DDP-induced brain damage in uremic mice may be associated with the PI3K/Akt signaling pathway.
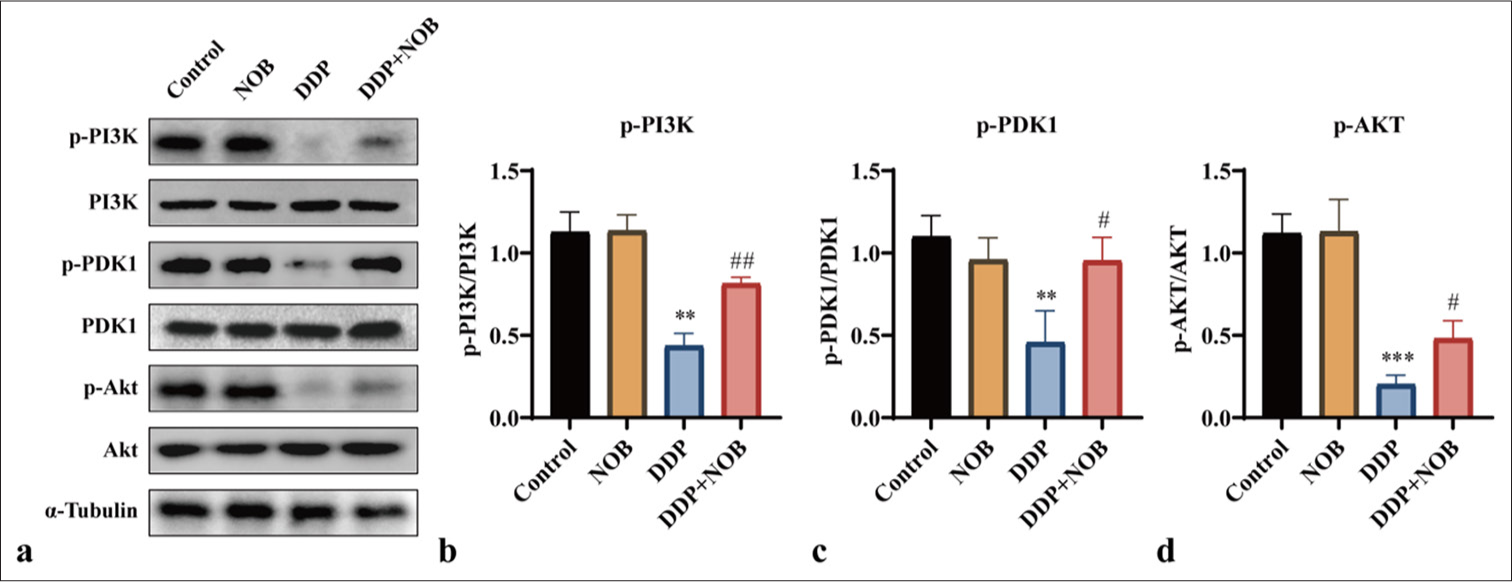
- NOB activates the PI3K/Akt signaling pathway. (a-d) WB analysis and quantification of p-PI3K, p-PDK1, and p-Akt protein expression in mouse kidney tissue. n = 3; ✶✶✶P < 0.001, ✶✶P < 0.01 versus Control, ###P < 0.001, ##P < 0.01, #P < 0.05 versus DDP. The bar represents mean ± S.D. NOB: Nobiletin, PI3K: Phosphatidylinositol 3-kinase, PDK1: Pyruvate dehydrogenase kinase 1, Akt: Protein kinase B, DDP: Cisplatin, WB: Western blot.
Anti-apoptotic effect of NOB on DDP-induced HT22 cells
To further investigate the mechanism by which NOB ameliorates brain damage in uremic mice, we treated HT22 mouse hippocampal neurons with DDP to simulate uremia-induced brain damage in vitro. HT22 cells were exposed to DDP at varying concentrations (0–20 mm) for 24 h, and cell viability was subsequently evaluated. Compared with that in the control, high DDP concentrations significantly reduced cell viability after 24 h (P < 0.001) [Figure 3a]. No significant differences in cell viability were observed when the HT22 cells were treated with NOB at concentrations ranging from 5 μM to 100 μM, indicating that NOB is nontoxic to these cells [Figure 3b]. When NOB was introduced to the DDP-treated group, their cell viability was markedly improved compared with that of the DDP-only group (P < 0.05), indicating that NOB can mitigate the toxicity caused by DDP in HT22 cells [Figure 3c].
We also examined the effects of NOB on DDP-induced apoptosis by measuring the expression of apoptosis-related proteins Bcl-2, Bax, and PARP (85 kDa). Compared with the control group, the group treated with DDP exhibited higher levels of Bax and PARP and lower levels of Bcl-2 (P < 0.001) [Figure 3d-g]. In contrast, NOB treatment markedly improved these apoptotic markers (P < 0.05) [Figure 3d-g]. Considering that ROS regulates apoptosis through oxidative stress, we measured the intracellular ROS levels. The findings revealed that NOB effectively decreased the oxidative stress induced by DDP (P < 0.01) [Figure 3h and i], verifying its potential to mitigate the harm to neurons resulting from uremic toxins.
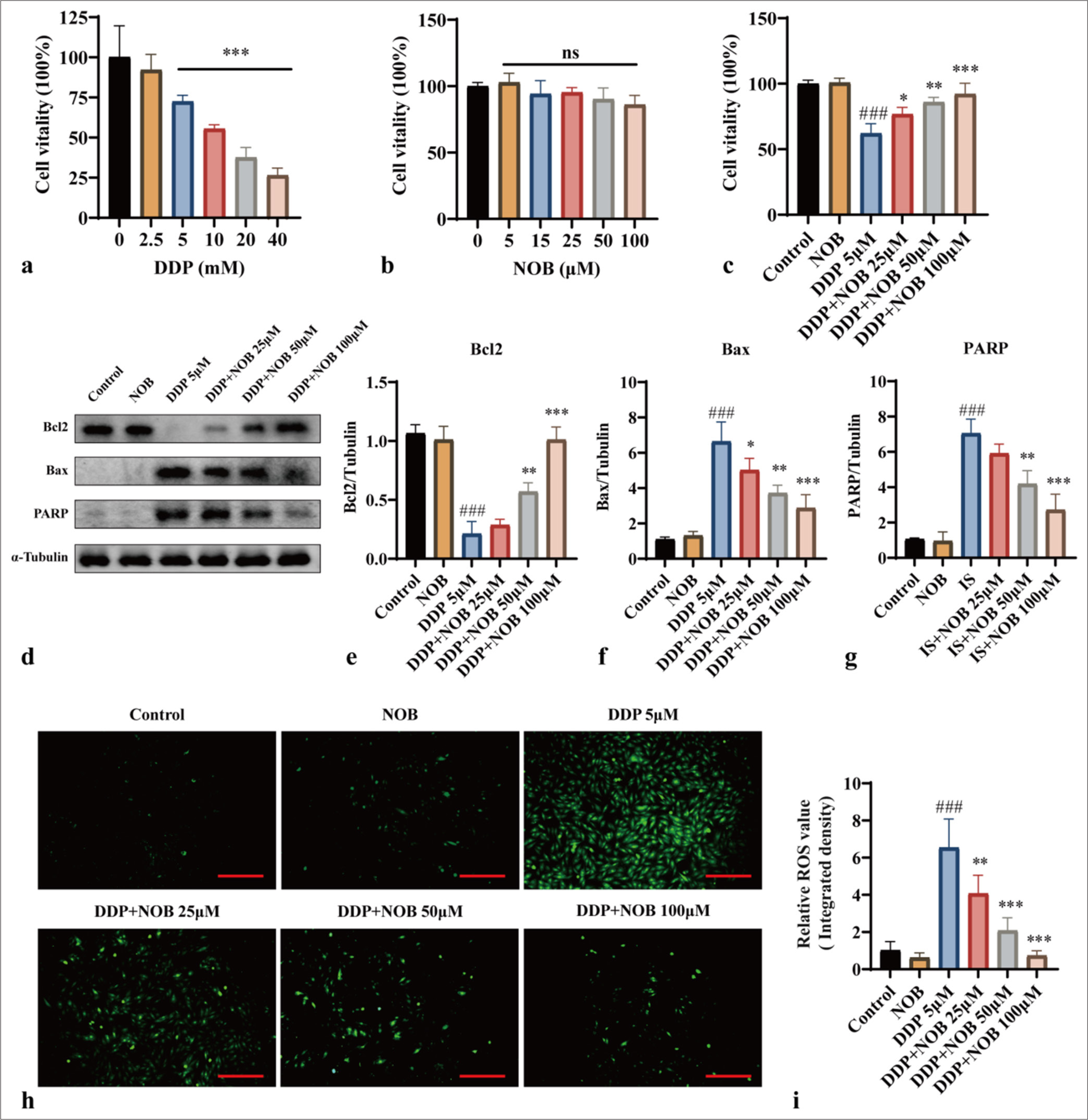
- NOB exhibits anti-apoptotic effects on DDP-induced HT22 cells. (a and b) CCK-8 assay evaluating the impact of DDP and NOB on HT22 cell viability. ✶✶✶P < 0.0001, ✶✶P < 0.001, ✶P < 0.01 versus 0 (c) CCK-8 assay assessing the effect of various concentrations of NOB on DDP-induced HT22 cell viability. (d-g) WB analysis and quantification of Bcl-2, Bax, and PARP protein expression in HT22 cells across different groups. (h-i) DCFH-DA probe measurement of intracellular ROS levels in different groups. ×100 Scale bar = 100 μm. n = 3; ✶✶✶P < 0.001, ✶✶P < 0.01, ✶P < 0.05 versus DDP; ###P < 0.001 versus Control. The bar represents mean ± S.D. NOB: Nobiletin, DDP: Cisplatin, CCK-8: Cell counting kit-8, Bcl2: B-cell lymphoma-2, Bax: Bcl-2-associated X protein, PARP: Poly ADP-ribose polymerase, WB: Western blot, DCFH-DA: Dichlorodihydrofluorescein diacetate, ROS: Reactive oxygen species.
NOB inhibits apoptosis through the PI3K/Akt signaling pathway
We further explored the mechanism by which NOB mitigates DDP toxicity by examining the expression changes of p-PI3K, p-PDK1, and p-Akt through WB analysis. Compared with those in the control group, the relative levels of p-PI3K, p-PDK1, and p-Akt were reduced by DDP but significantly restored by NOB treatment (P < 0.05) [Figure 4a-d]. To further confirm whether NOB protects against DDP-induced neurotoxicity by activating the PI3K/Akt signaling pathway, we treated HT22 cells with NOB and LY294002 (10 μM), a specific inhibitor of the PI3K/Akt pathway. Figures 4e-h show that after treatment with LY294002, the expression of the PI3K/Akt pathway was reduced (P < 0.05). According to the apoptotic-related proteins and ROS levels, the apoptosis inhibition induced by NOB was partially reversed after LY294002 treatment (P < 0.05) [Figure 4i-n]. Similarly, the CCK-8 assay showed that compared with the DDP + NOB group, the DDP + NOB + LY group exhibits decreased cell viability (P < 0.05) [Figure 4o]. These experimental results suggested that the protective effect of NOB on HT22 cells is partially blocked by the PI3K/Akt pathway inhibitor, indicating that NOB may alleviate DDP-induced HT22 cell apoptosis through the PI3K/Akt signaling pathway and exert a protective effect.
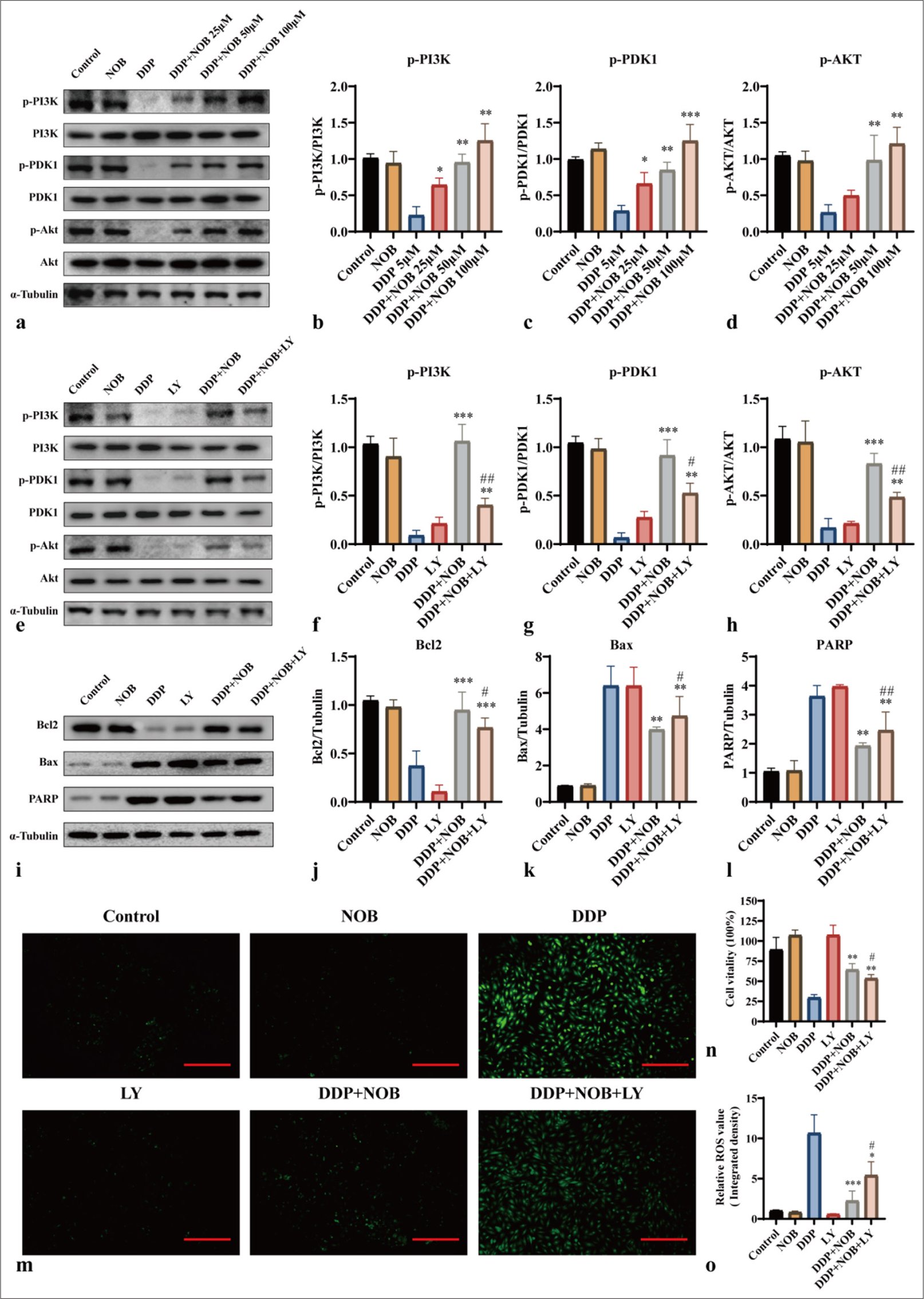
- NOB inhibits apoptosis through the PI3K/Akt signaling pathway. (a-h) WB detection and quantitative analysis of the protein expression of p-PI3K, p-PDK1, and p-Akt in different groups of HT22 cells. (i-l) WB detection and quantitative analysis of the protein expression of Bcl2, Bax, and PARP in different groups of HT22 cells. (m and n) DCFH-DA probe measurement of intracellular ROS levels in different groups. ×100 Scale bar = 100 μm (o) CCK-8 assay assessing the effect of various LY concentrations on HT22 cell viability. n = 3; ✶✶✶P < 0.001, ✶✶P < 0.01, ✶P < 0.05 versus DDP; ##P < 0.01, #P < 0.05 versus DDP + NOB. The bar represents mean ± S.D. NOB: Nobiletin, DDP: Cisplatin, PI3K: Phosphatidylinositol 3-kinase, PDK1: Pyruvate dehydrogenase kinase 1, Akt: Protein kinase B, Bcl2: B-cell lymphoma-2, Bax: Bcl-2-associated X protein, PARP: Poly ADP-ribose polymerase, WB: Western blot, DCFH-DA: Dichlorodihydrofluorescein diacetate, ROS: Reactive oxygen species, LY: LY294002, CCK-8: Cell counting kit-8.
DISCUSSION
Accelerated by hypertension, diabetes, obesity, and primary kidney disease, CKD affects approximately 8–16% of the global population, posing a significant global health burden.[25] Complications from renal failure are common causes of increased mortality, with uremic encephalopathy being a major neurological complication of ESRD. The high risk of cardiovascular complications associated with renal failure further increases the incidence of uremic encephalopathy.[26] However, treatment options for CKD are limited. CKD progressing to uremia requires kidney replacement therapy and transplantation, which entail lifelong treatment and substantial economic burdens. Despite advances in the medical management of late-stage CKD, including dialysis and transplantation, patients continue to exhibit numerous neurological symptoms. Therefore, further research into the underlying pathophysiological mechanisms is necessary,[27] and developing new therapeutic strategies to alleviate CKD and associated brain damage is an urgent task.
This study delves into the mechanisms underlying the effects of NOB on uremic encephalopathy in mice. First, by utilizing DDP on HT22 mouse hippocampal neurons as an in vitro model, we confirmed that DDP induces renal failure and subsequent brain tissue damage. Our findings demonstrated that NOB alleviates brain damage induced by DDP in uremic mice, a process linked to the PI3K/Akt pathway. In addition, NOB exhibits therapeutic effects against DDP-induced cytotoxicity in hippocampal neurons by inhibiting apoptosis through the PI3K/Akt pathway, thereby protecting the brain from the harmful effects of uremic toxins. These results provide theoretical and experimental evidence for understanding the pathological mechanisms of DDP-induced uremic brain damage and confirm the significant therapeutic potential of NOB. This work offers a new target for the effective prevention and management of uremic encephalopathy, a serious complication of CKD. Targeting this pathway opens up new avenues for developing effective treatments, potentially improving the cognitive function and overall well-being of patients. The kidneys are the excretory organs of the body and the primary sites of injury from various toxic chemicals. DDP, a commonly used chemotherapeutic agent, is frequently employed to treat solid tumors. However, its side effects, including nephrotoxicity, cardiotoxicity, and myelosuppression, are significant, with nephrotoxicity being a common adverse reaction.[28] In this study, we used a mouse model of DDP-induced chronic renal failure and observed abnormal serum creatinine and BUN levels, structural changes in kidney tissue, and an increase in apoptosis-related proteins in brain tissues, confirming brain damage in uremic mice. To investigate the effects of DDP on brain tissue, we simulated uremic brain damage in vitro by treating hippocampal HT22 cells with DDP, providing insights into the cellular mechanisms underlying uremic encephalopathy. Our experimental results revealed dose-dependent cell death in HT22 cells following DDP treatment. These findings validate the establishment of the in vivo and in vitro models of uremic brain damage.
The benefit of using natural products lies in their integration into the human diet, reducing the likelihood of side effects of synthetic medications and allowing for safe and gradual therapeutic effects. Citrus peel, a byproduct of fruit and a staple in traditional Chinese herbal medicine has been widely used since ancient civilizations. NOB is the main active component of citrus peel and is abundant in the stems, leaves, and peels of citrus fruits. It has shown significant neuroprotective effects and has improved brain functions in various diseases such as cerebral infarction, Parkinson’s disease, and diabetes-related neurological damage.[11,29] However, only a few studies have focused on the preventive and therapeutic effects of NOB on uremic encephalopathy. In the present work, we used a mouse model of DDP-induced uremic encephalopathy and confirmed that NOB significantly improves brain damage in uremic mice. In vitro experiments using DDP to treat HT22 cells demonstrated that NOB has similar therapeutic effects. The results suggested that the protective effect of NOB is linked to PI3K/Akt pathway-mediated apoptosis, highlighting its potential as an intervention to reduce the incidence of uremic encephalopathy.
The PI3K/Akt pathway is vital in managing cell proliferation and apoptosis, acting as a prominent anti-apoptotic route.[30] On activation by extracellular stimuli, PI3K activates the downstream protein kinase AKT. AKT is a critical regulator in the apoptosis pathway, influencing cell proliferation, differentiation, and apoptosis by phosphorylating and subsequently activating or inhibiting downstream target proteins such as Bax, Bcl-2, and caspase.[31] Extensive research has highlighted the PI3K/Akt signaling pathway as a crucial pathway for the survival of neurons.[32,33] For instance, the PI3K/Akt pathway mitigates neuronal apoptosis by increasing anti-apoptotic Bcl-2 and decreasing pro-apoptotic Bax, thus minimizing neurological damage after intracerebral hemorrhage.[34] Another study demonstrated that uremic toxin induces brain damage and cell death in CKD, and omega-3 polyunsaturated fatty acids protect against this damage through the PI3K-Akt signaling pathway.[35] Curcumin enhances neuron protection by promoting the release of NGF, which activates the PI3K/Akt survival pathway, leading to a significant reduction in H2O2-induced apoptosis.[36] Similarly, our study indicated that NOB can modulate the PI3K/Akt signaling pathway to alleviate brain damage in mice with uremic encephalopathy and significantly reverse DDP-induced hippocampal neuron apoptosis.
SUMMARY
This study demonstrates that NOB can alleviate DDP-induced uremic encephalopathy, enhance neuronal cell viability, inhibit neuronal apoptosis, and mitigate apoptosis and proliferation suppression caused by DDP-induced neurotoxicity in mice. The activation of the PI3K/Akt pathway is involved in the neuroprotective effects of NOB. This research further supports the protective effects of NOB on the nervous system. Tangerine peel is a widely available and inexpensive traditional Chinese medicine, and its extract NOB possesses favorable characteristics such as relative safety and confirmed efficacy, making it a promising anti-inflammatory molecule. This study provides a new research approach for treating clinical conditions, such as uremic brain damage. Subsequent research will include further in vivo studies and clinical trials to uncover the neuroprotective benefits and underlying mechanisms of NOB.
AVAILABILITY OF DATA AND MATERIALS
The data and materials that support the findings of this study are available from the corresponding author on reasonable request.
ABBREVIATIONS
BUN: Blood urea nitrogen
CAT: Catalase
CKD: Chronic kidney diseases
DDP: Cisplatin
ESRD: End-stage renal disease
H&E: Hematoxylin and eosin
IS: Indoxyl sulfate
Kim-1: Kidney injury molecule 1
MDA: Malondialdehyde
Nagl: Neutrophil gelatinase-associated lipocalin
NOB: Nobiletin
ROS: Reactive oxygen species
SCr: Serum creatinine
SOD: Superoxide dismutase
ACKNOWLEDGMENTS
We are grateful to the Experimental Center for its assistance.
AUTHOR CONTRIBUTIONS
LSX: Literature search, experimental studies, statistical analysis, and manuscript preparation; RYZ: Design, data analysis, manuscript preparation, manuscript editing, and review.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Animal Ethics Committee of Guangzhou Miers Biotechnology Co., Ltd. (IACUCMIS20230042). Informed consent from patients is not required as this study does not involve human experiments.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: Not applicable.
References
- Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat Rev Nephrol. 2019;15:301-16.
- [CrossRef] [PubMed] [Google Scholar]
- Crosstalk between the nervous system and the kidney. Kidney Int. 2020;97:466-76.
- [CrossRef] [PubMed] [Google Scholar]
- Uremic toxin indoxyl sulfate promotes macrophage-associated low-grade inflammation and epithelial cell senescence. Int J Mol Sci. 2023;24:8031.
- [CrossRef] [PubMed] [Google Scholar]
- TUDCA-treated mesenchymal stem cells protect against ER stress in the hippocampus of a murine chronic kidney disease model. Int J Mol Sci. 2019;20:613.
- [CrossRef] [PubMed] [Google Scholar]
- Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J. 2019;12:258-61.
- [CrossRef] [PubMed] [Google Scholar]
- Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016;90:77-89.
- [CrossRef] [PubMed] [Google Scholar]
- The dopamine system: Insights between kidney and brain. Kidney Blood Press Res. 2022;47:493-505.
- [CrossRef] [PubMed] [Google Scholar]
- Tryptophan metabolites suppress the Wnt pathway and promote adverse limb events in chronic kidney disease. J Clin Invest. 2022;132:e142260.
- [CrossRef] [PubMed] [Google Scholar]
- The chronic kidney disease-Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone. 2017;100:80-6.
- [CrossRef] [PubMed] [Google Scholar]
- Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-Notch signaling. Circulation. 2019;139:78-96.
- [CrossRef] [PubMed] [Google Scholar]
- A narrative review of the effects of Citrus peels and extracts on human brain health and metabolism. Nutrients. 2022;14:1847.
- [CrossRef] [PubMed] [Google Scholar]
- Nobiletin prevents amyloid β1-40-induced cognitive impairment via inhibition of neuroinflammation and oxidative/nitrosative stress. Metab Brain Dis. 2022;37:1337-49.
- [CrossRef] [PubMed] [Google Scholar]
- Nobiletin ameliorates cellular damage and stress response and restores neuronal identity altered by sodium arsenate exposure in human iPSCs-derived hNPCs. Pharmaceuticals. 2022;15:593.
- [CrossRef] [PubMed] [Google Scholar]
- Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid Med Cell Longev. 2022;2022:9966750.
- [CrossRef] [PubMed] [Google Scholar]
- Nobiletin prevents D-galactose-induced C2C12 cell aging by improving mitochondrial function. Int J Mol Sci. 2022;23:11963.
- [CrossRef] [PubMed] [Google Scholar]
- Preventive action of nobiletin, a constituent of AURANTII NOBILIS PERICARPIUM with anti-dementia activity, against amyloid-beta peptide-induced neurotoxicity expression and memory impairment. Yakugaku Zasshi. 2010;130:517-20.
- [CrossRef] [PubMed] [Google Scholar]
- Nobiletin ameliorates isoflurane-induced cognitive impairment via antioxidant, anti-inflammatory and anti-apoptotic effects in aging rats. Mol Med Rep. 2016;14:5408-14.
- [CrossRef] [PubMed] [Google Scholar]
- Analgesic effect of nobiletin against neuropathic pain induced by the chronic constriction injury of the sciatic nerve in mice. Phytother Res. 2022;36:3644-61.
- [CrossRef] [PubMed] [Google Scholar]
- Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp Mol Med. 2019;51:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- A review on recent advances on nobiletin in central and peripheral nervous system diseases. Eur J Med Res. 2023;28:485.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826.
- [CrossRef] [PubMed] [Google Scholar]
- Nobiletin mitigates D-galactose-induced memory impairment via improving hippocampal neurogenesis in mice. Nutrients. 2023;15:2228.
- [CrossRef] [PubMed] [Google Scholar]
- Autophagy and apoptosis cascade: Which is more prominent in neuronal death? Cell Mol Life Sci. 2021;78:8001-47.
- [CrossRef] [PubMed] [Google Scholar]
- Reelin through the years: From brain development to inflammation. Cell Rep. 2023;42:112669.
- [CrossRef] [PubMed] [Google Scholar]
- Renal-cerebral pathophysiology: The interplay between chronic kidney disease and cerebrovascular disease. J Stroke Cerebrovasc Dis. 2021;30:105461.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13:823-33.
- [CrossRef] [PubMed] [Google Scholar]
- Drug delivery strategies for platinum-based chemotherapy. ACS Nano. 2017;11:8560-78.
- [CrossRef] [PubMed] [Google Scholar]
- Nobiletin promotes antioxidant and anti-inflammatory responses and elicits protection against ischemic stroke in vivo. Brain Res. 2016;1636:130-41.
- [CrossRef] [Google Scholar]
- AKT-dependent and-independent pathways mediate PTEN deletion-induced CNS axon regeneration. Cell Death Dis. 2019;10:203.
- [CrossRef] [PubMed] [Google Scholar]
- P-TEFb promotes cell survival upon p53 activation by suppressing intrinsic apoptosis pathway. Nucleic Acids Res. 2023;51:1687-706.
- [CrossRef] [PubMed] [Google Scholar]
- Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol Sin. 2018;39:1259-72.
- [CrossRef] [PubMed] [Google Scholar]
- A new therapeutic trend: natural medicine for ameliorating ischemic stroke via PI3K/Akt signaling pathway. Molecules. 2022;27:7963.
- [CrossRef] [PubMed] [Google Scholar]
- TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2020;17:168.
- [CrossRef] [PubMed] [Google Scholar]
- Omega-3 polyunsaturated fatty acid attenuates uremia-induced brain damage in mice. Int J Mol Sci. 2021;22:11802.
- [CrossRef] [PubMed] [Google Scholar]
- NGF and PI3K/Akt signaling participate in the ventral motor neuronal protection of curcumin in sciatic nerve injury rat models. Biomed Pharmacother. 2018;103:1146-1153.
- [CrossRef] [PubMed] [Google Scholar]








