Translate this page into:
Nonneoplastic Cervical Cytology

*Corresponding author: Meherbano Kamal, Department of Pathology, Government Medical College, Nagpur, Maharashtra, India. dr.mmkamal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kamal M, Topiwala F. Nonneoplastic cervical cytology. CytoJournal 2022;19:25.
Abstract
Diagnostic cytology of cervix can be made strong if normal cytology is known thoroughly. Cervical lining comprises three layers of squamous cells, the basal, intermediate, and superficial cells. Knowing the dimensions of these cells, especially the intermediate cells, helps to diagnose the squamous intraepithelial lesions accurately. Furthermore, recognizing the parabasal cells in the menopausal smears, either singly or as syncytial aggregates, is important to avoid overdiagnosis of squamous intraepithelial lesions. The other cell type in the cervical lining is the endocervical glandular epithelium. Exfoliated endocervical cells may at times resemble endometrial glandular cells. The morphology and differences between these two cell types have been highlighted. It is essential to recognize and report endometrial cells in women of 40 years and above according to the recent Bethesda System for Reporting Cervical Cytology. The squamous epithelium of cervix and vagina is highly sensitive to estrogen and progesterone hormones. Hence, the Pap smears, if desired, can help in evaluating the hormonal status of the woman. The ratio of parabasal, intermediate, and superficial squamous cells can help in calculating various maturation indices.
Keywords
Normal cervicovaginal cells
Normal endocervical cells
normal endometrial cells
Hormonal cytology
Maturation indices in cervical cytology

THE NORMAL SMEAR PATTERNS
The mucosal lining of the labia minora, vagina, and portio vaginalis of the cervix is normally a non-keratinized stratified squamous epithelium. The fullest development of the epithelium occurs during childbearing age. This multilayered squamous epithelium [Figure 1a and b] provides a barrier against external injuries and stores nutrients in the form of glycogen.
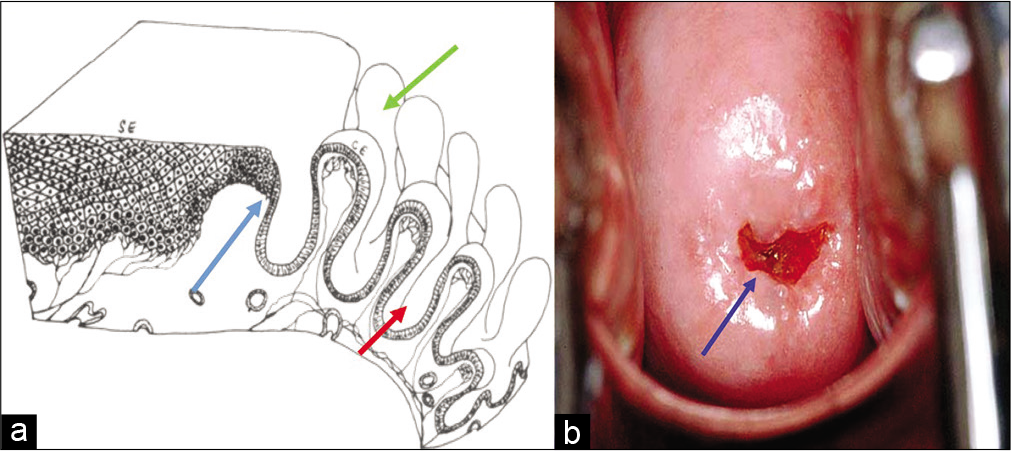
- (a) Diagrammatic representation showing squamocolumnar junction (SCJ) - blue arrow, crypts, and villi - green and red arrows. (b) Cervix showing SCJ.
HISTOLOGY AND CYTOLOGY OF THE FEMALE GENITAL TRACT EPITHELIA
Figure 2 shows histology of multilayered squamous epithelium (hematoxylin and eosin, 40×)
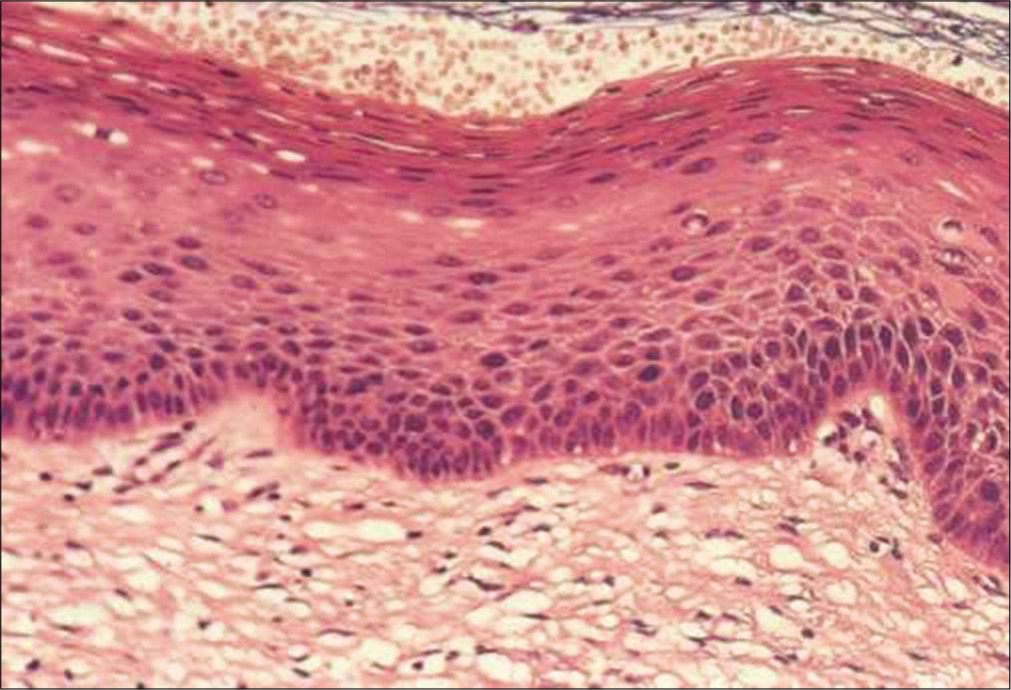
- Histology of multilayered squamous epithelium (hematoxylin and eosin, 40×)
The squamous epithelium is supported by basement membrane and is divided into basal/regenerative layer, the mid-zone forming the major thickness, and the superficial zone
The germinal/basal layer is composed of a single row of small regular cells adhering to a basement membrane and showing signs of active growth
The mid-zone comprises parabasal and intermediate cells. Parabasal cells are immature and crowded, lying just above the basal layer. The parabasal cell layer is two to three cells deep
These cells mature into an intermediate layer of variable thickness in which the cells have more cytoplasm, the nuclei still show a recognizable chromatin pattern and the cells are bound to each other by intercellular cytoplasmic bridges
In fully mature cervical squamous mucosa, there is a superficial layer consisting of cells that do not normally mature any further. Intercellular bridges are not highly developed at this level so that the cell bonds are loosely attached. Superficial cells are actually dead or dying and exfoliate spontaneously. The nuclei of the superficial cells are pyknotic.
Mucosal thickness depends on hormonal status as the parabasal, intermediate, and superficial layers are all hormonally responsive. Under the influence of estrogen, a superficial layer develops in about 4 days.
CYTOLOGIC DETAILS OF INDIVIDUAL CELLS
Two of the components of the Papanicolaou stain are cytoplasmic stains: Eosin, which stains superficial cells pink or orange, and light green, which is taken up by the cytoplasm of all of the less mature cells. Because the stains are alcohol based, the cytoplasmic staining is particularly delicate and translucent, unless keratinization is present, in which case, the staining becomes densely orangeophilic. The nuclei are stained blue by hematoxylin. Good fixation is an essential prerequisite for successful use of the Papanicolaou stain, particularly for the nuclei features.
Basal cells
Basal cells are small, undifferentiated cells that measure around 10–12 µm in diameter. These cells have central round to oval nucleus, with fine chromatin and occasional chromocenters and small delicate amount of cytoplasm. They resemble small histiocytes. Isolated basal cells are rarely seen in a Pap smear unless there is severe atrophy. In severe atrophy, they are present as syncytial aggregates or hyperchromatic crowded groups (HCGs).
Basal cells have following functions: They anchor the epithelium to the basement membrane by hemidesmosomes, they form the regenerative epithelium, they help in the formation of basement membrane by sending signals to the stroma. This basement membrane helps to separate the epithelium form the underlying mesenchyme.
Parabasal cells
Moderately large ranging from 15 µm to 30 µm in diameter. They have round to oval outlines with fairly dense green cytoplasm. Nuclei occupy about one half of the cell with a size slightly larger than the red blood cells (RBCs) nucleus. The N/C ratio is approximately 20% but it can be variable. The nuclei are round to oval with finely granular chromatin and occasional chromocenters. Nucleoli are inconspicuous. Immature parabasal cells lie in sheets while more mature cells are usually dissociated. Parabasal cells may predominate in atrophic smears from postmenopausal women. In younger women, postnatal atrophy or high-dose progesterone oral contraception produces similar changes [Figure 3].
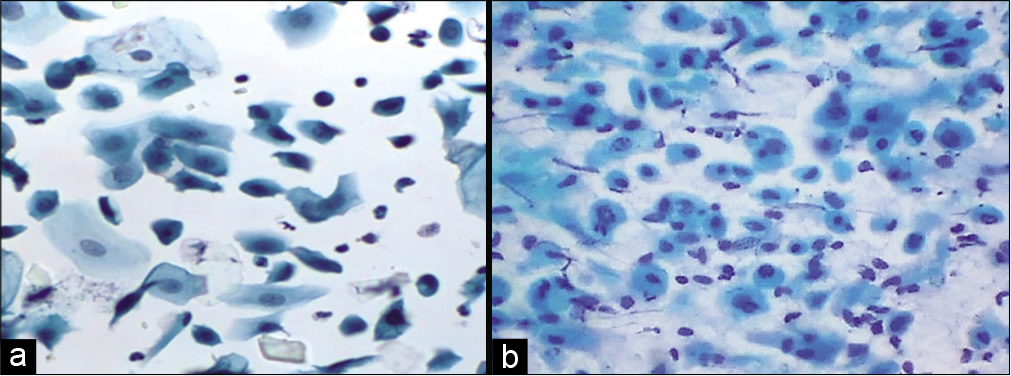
- (a) Liquid-based cytology preparation – smear in atrophy showing parabasal cells (40×). (b) Conventional smear showing parabasal cells and few basal cells, karyorrhexis is also seen in lower half of the field (40×).
Intermediate cells
Intermediate cells are large polygonal ranging from 35 µm to 40 µm in diameter, depending on their level of maturation. Low intermediate cells are about the size of parabasal cells while high intermediate cells are about the size of superficial cells. These cells are having ample pale green filmy cytoplasm often folded at periphery. Nucleus is round or ovoid, centrally located and the size ranges from 7 µm to 8 µm in diameter [Figure 4]. The chromatin pattern is fine and vesicular. Barr body is easily seen at the nuclear membrane. The N/C ratio is very low (about 3–5%). The nucleus of intermediate cells is a benchmark. It is used to gauge nuclear size, intensity of chromatin staining, and chromatin texture. The intermediate cell nucleus is key reference in evaluation of dysplasia. A dysplastic cell has 3–5 times the area of an intermediate nucleus. Cytoplasmic disintegration (cytolysis) is essentially a feature of intermediate cell.
- Liquid-based cytology preparation – smear showing intermediate squamous cells with translucent cytoplasm and vesicular nucleus (40×).
Superficial cells
During child bearing age group of normal women, the bulk of cells seen in cervical cytology is of superficial squamous cells. They usually occur singly and rarely in clusters. These cells are large polygonal with flat delicate, transparent cytoplasm, and measure about 45–50 µm in diameter [Figure 5]. In Papanicolaou stain, the cytoplasm of the majority of superficial cells stains predominantly a delicate pink and has keratohyalin granules [Figure 6] in their cytoplasm. The superficial cells have a centrally placed small pyknotic nucleus. There may be a small clear halo around the pyknotic nucleus as a reminder of its former size. The size of nucleus is less than that of the RBC. If it is larger think of dysplasia. The N/C ratio is about 2–3%. The key distinguishing feature between intermediate and superficial cells is the vesicular chromatin of the earlier and pyknotic of the latter.
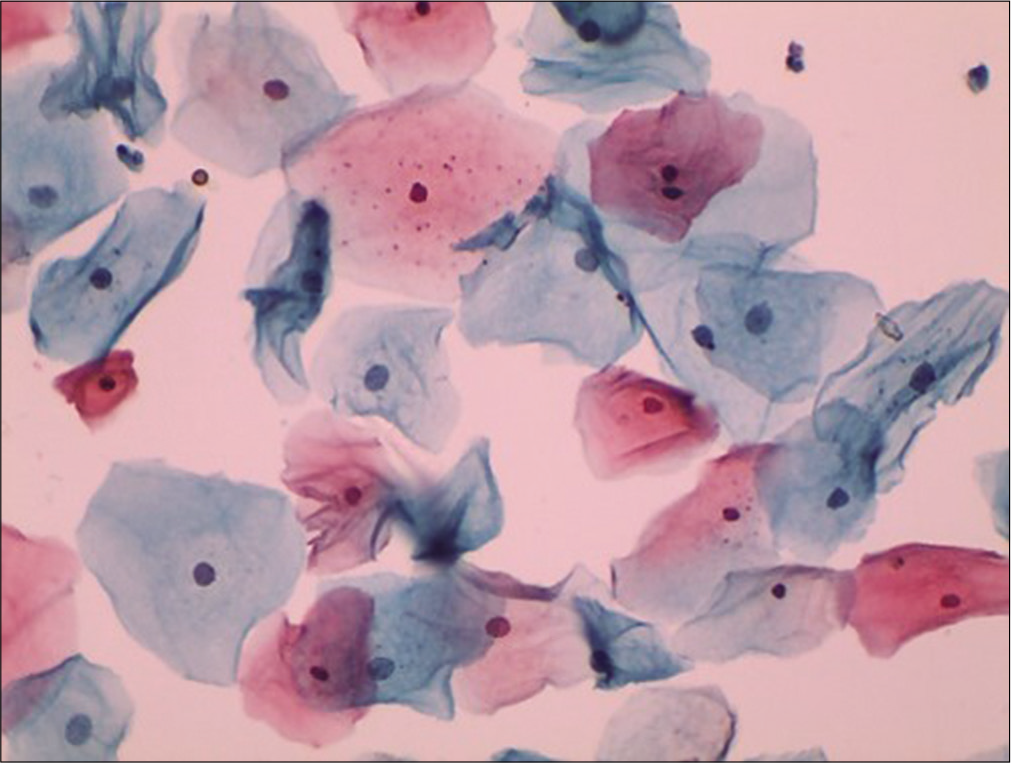
- Liquid-based cytology preparation: Smear shows superficial squamous cells with pyknotic nuclei (40×).
- Conventional Pap smear showing intermediate and superficial squamous cells with plenty keratohyalin granules. These keratin granules are blue-black, variable sized granules within the cytoplasm (40×).
Anucleate squames
They are mature superficial squamous cells with loss of nuclei, they stain orange or yellow with eosin [Figure 7].
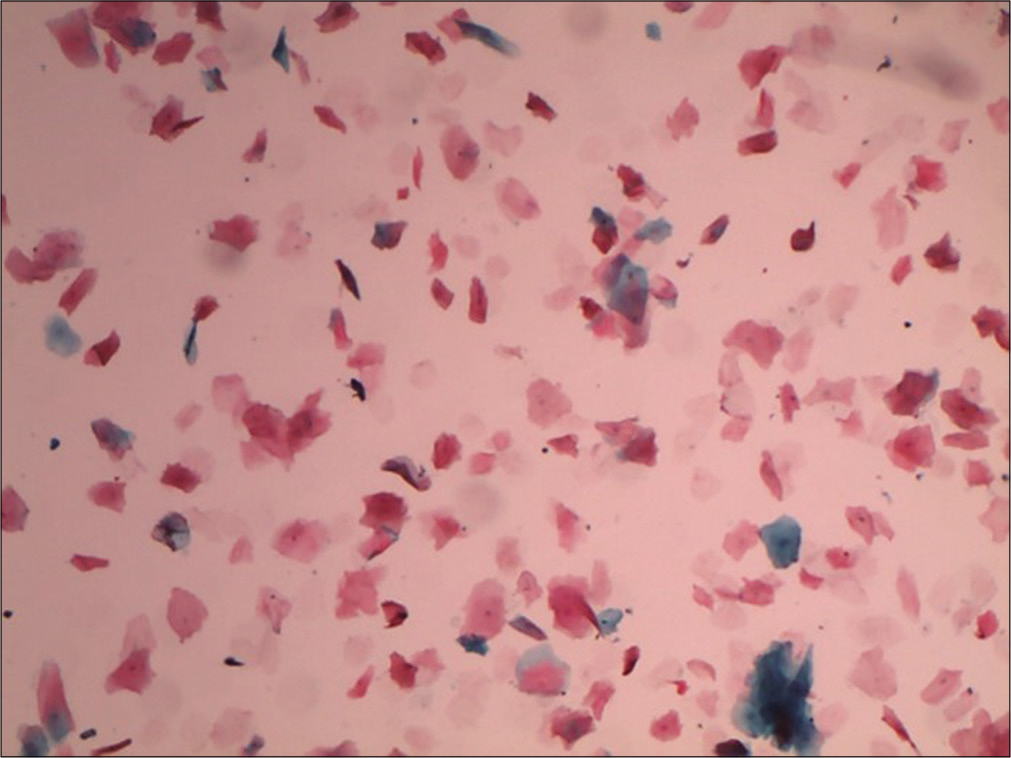
- Liquid-based cytology preparation showing plenty anucleate squames (10×).
Anucleate squames are often present with granular cells, indicating completion of keratinization. Less mature cells from the deeper layers may also become anucleate, usually in association with degenerative changes. Cakes or stacks of anucleate squames may be seen when the cervix or vagina harbors a leukoplakia. It may also represent the hyperkeratotic surface of a benign wart due to human papillomavirus 6, 11 and is a non-classic finding of low-grade squamous intraepithelial lesion.
Endocervical cells
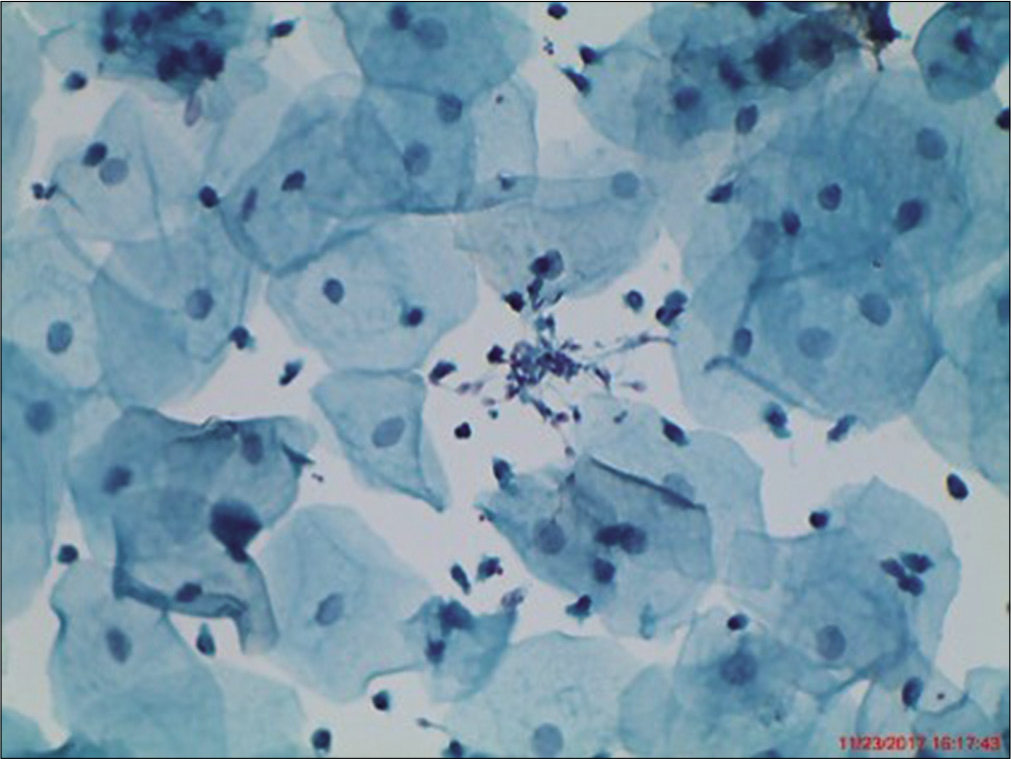
Endocervix is a series of grooves or clefts formed by deep infoldings of mucosa that may tunnel into endocervical stroma forming crypts. A single layer of endocervical columnar cells lines these mucosal folds. They are tall and columnar most are secretory but few are ciliated. Endocervical cells can occur singly, in strips or in sheets. Dissociation is more commonly seen with the LBC [Figure 8a]. Viewed from above, sheets have a honeycomb appearance. Sheets of endocervical cells when viewed from side line up like “picket-fence” palisades with basal nuclei and columnar cytoplasm [Figure 8b]. From above, nuclei appear rounded, from the side, the nuclei are oval of endocervical cells [Figure 8c]. They tend to round up or fold, particularly in a three-dimensional clusters or HCGs. Fine chromatin pattern, one or more small nucleoli are often seen near the nuclear membrane. Nuclei are usually single but occasionally binucleation or multinucleation occurs, particularly in reactive conditions. The nuclear membrane is usually smooth but sometimes there is a nipple-like protrusion seen in cases with estrogen excess. Bare nuclei may accompany groups of degenerate endocervical cells.
- (a) Liquid-based cytology (LBC) preparation showing endocervical columnar cells singly and in loose clusters. This is an effect of randomization of the specimen during vortexing of LBC vial (40×). (b) Conventional Pap smear showing strips of normal endocervical glandular cells (c) shows flat honeycomb sheets (40×).
Secretory endocervical cells
It has generous cytoplasm that contains fine mucin vacuole/s. The cells can be plump in the luteal phase, pregnancy, in response to OCP’S, hormone therapy, and endocervical polyp [Figure 9].
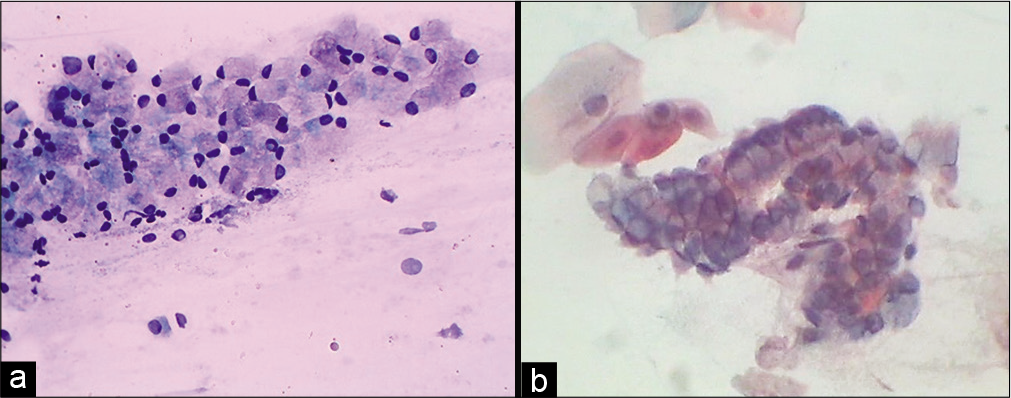
- (a) Conventional Pap smear showing endocervical glandular cells distended with mucin (goblet cells). The mucin takes a light pink color. (b) Smear showing strip of same cells (40×).
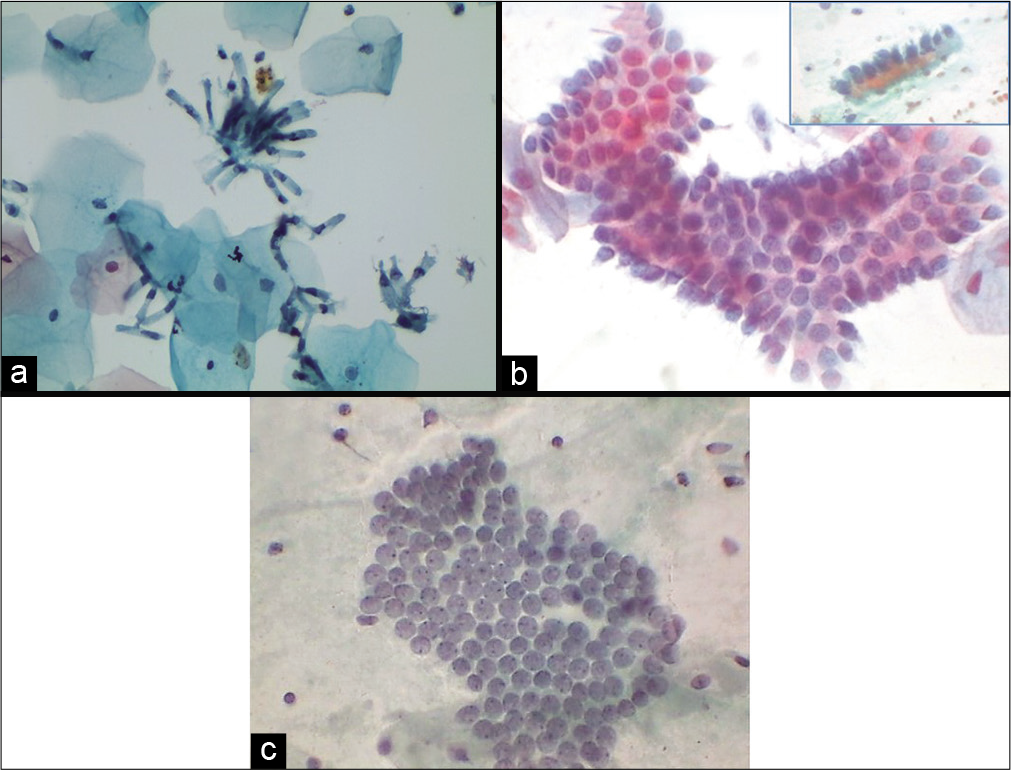
Ciliated endocervical cells
These cells show denser cytoplasm than secretory cells. They look like ciliated respiratory bronchiolar cells. These cells increase in the first phase of menstrual cycle [Figure 10 a and b].
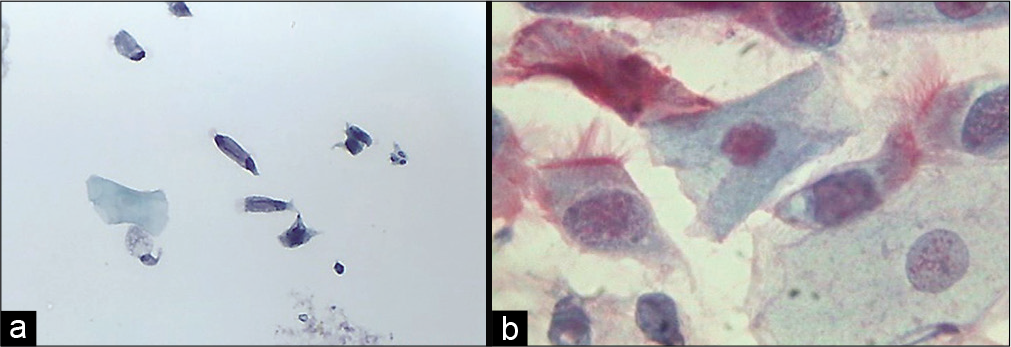
- (a) Liquid-based cytology preparation and (b) conventional smear showing ciliated columnar endocervical cells (40×).
High endocervical cells
It is seen high in the endocervix resembling more like endometrial cells. They can occur in crowded groups, high N/C ratio with scant cytoplasm but the nuclei are round and smooth with denser cytoplasm [Figure 11 a and b].
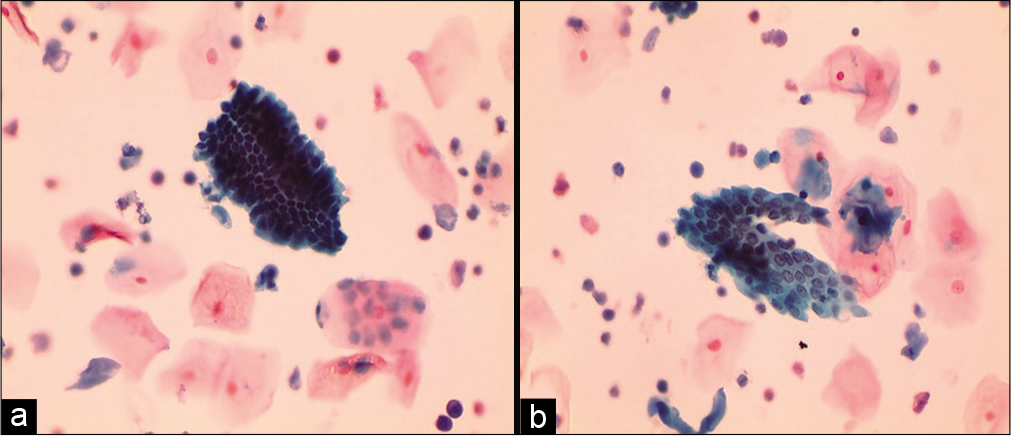
- Liquid-based cytology preparation: Smear is showing slightly smaller glandular cells in compact group (a) and flat sheet (b) (40×). These may represent high endocervical cells or cells from lower uterine segment collected by the tip of any brush that reaches high in the endocervical canal.
Endometrial cells
Endometrial cells which are spontaneously shed are usually in a 3-dimensional cluster with or without a central stromal core. Double contour cell balls or wreath with darkly staining stroma inside and lighter staining epithelium wrapped outside are the characteristic arrangement of endometrial cells [Figure 12 a]. It is generally seen in the first 6–10 days of menstrual cycle and known as “exodus” (meaning exit). Factors influencing their presence beyond this may reflect underlying endometrial pathology in a woman over 40 years of age or could be due to exogenous hormonal manipulation such as hormone replacement therapy or oral contraceptive use; tamoxifen use, intrauterine device carriage, and dysfunctional bleeding are further potential sources of endometrial cells being shed outside the allowed phase of the cycle. Endometrial stromal histiocytes commonly accompany the endometrial wreaths during exodus [Figure 12 b and c].
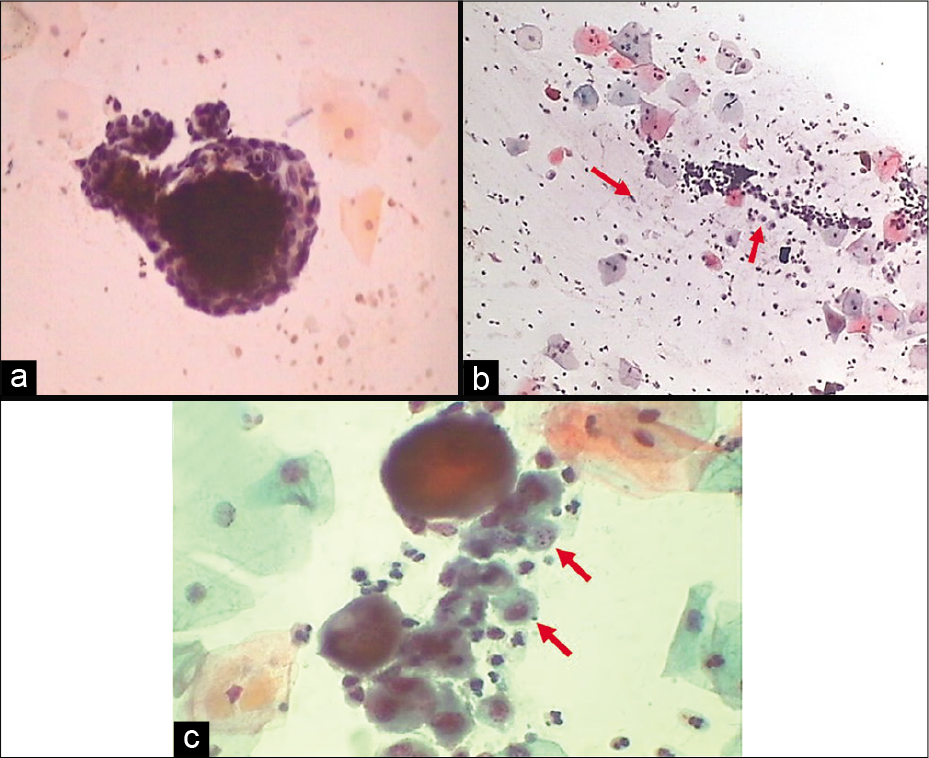
- (a) Exodus or wreath-like appearance of endometrial cells with central dark core formed by endometrial stromal cells surrounded by endometrial glandular cells (40×). (b and c) Endometrial cell balls in dirty background of histiocytes (red arrows) and inflammatory cells (b: 10×, c: 40×).
Endometrial cells also occur in 3-dimensional clusters without central stroma. The cells are small and crowded, and the nuclei usually are degenerated and hyperchromatic, appearing as HCG. These cells are generally seen in the second half of menstrual cycle. Distinguishing endometrial cells from endocervical cells is a common problem in Pap test interpretation [Table 1].[1]
| Endocervical cells | Endometrial cells |
|---|---|
| Larger | Smaller |
| More variable | Uniform |
| Flat 2-D sheets | Crowded, 3-D balls |
| Honeycombs, palisades | Double contour cell balls |
| Nuclei vary in size, but not in shape | Nuclei vary in shape, but not in size |
| Multinucleation | Multinucleation rare |
| Abundant cytoplasm and better preservation | Scant cytoplasm and degeneration |
Endometrial stromal cells: They are of two types, superficial stromal cells and deep stromal cells
Superficial stromal cells appear like histiocytes and tend to be loose clusters and are most prominent in the 6th–10th day of menstrual cycle along with exodus. Deep stromal cells are typically spindle or stellate shaped with scant cytoplasm having small oval nuclei and scant cytoplasm.
Abnormal shedding of the normal endometrial cells
Normal endometrial cells shed during the first half of the menstrual cycle. It is physiologic exfoliation. Exfoliation other than this time of the endometrial glandular or stromal cells is abnormal, known as “Out-of-phase shedding.” Postmenopausal (above 40 years of age) shedding is always abnormal. The Bethesda system 2001 recommends reporting the presence of normal appearing endometrial cells in women >40 years of age, regardless of the menstrual status.
The causes of abnormal shedding of endometrial cells are as follows: Endometriosis, endometritis, postpartum, abortion, intrauterine device, instrumentation, hormonal therapy, dysfunctional uterine bleeding, submucosal myoma, endometrial polyp, endometrial hyperplasia, and endometrial carcinoma.
The predominant cells seen during menstrual cycle, pregnancy, and menopause
During the 1st–5th days of menstrual cycle, there is predominance of intermediate cells but few superficial cells, endometrial glandular and stromal cells, blood, inflammatory cells, and debris are also seen. Endocervical cells are sparse. During proliferative phase, follicular phase, or estrogenic phase days 6th–14th, the maturation pattern shifts from intermediate predominant to superficial predominant pattern. During exodus days, that is, 6th–10th, there are many histiocytes present. The endometrial cells decrease in number by the 6th day and normally disappear by the 10th–12th day.
At ovulation, that is, approximately 14th day of the cycle, superficial cells predominate, endocervical cells become secretory, leukocytes are rare, and background becomes clean. Ferning of endocervical mucus may be seen. During the secretory phase or the luteal phase or the pro-gestational state, that is, 14th–28th day of the cycle, there is shift from the superficial cells to intermediate cells and after ovulation few navicular cells are also seen. Toward the end of the cycle, there is cytolysis of the cells along with increase in the number of bacteria and leukocytes giving a dirty background [Figure 13a and b].
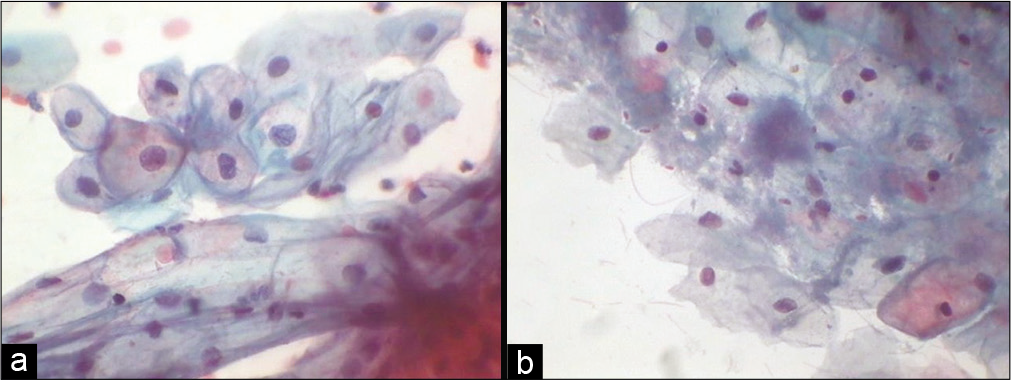
- (a) Conventional Pap smear showing predominance of intermediate cells in smear taken during pregnancy (40×). (b) Conventional smear showing cytolysis in the pre-menstrual phase along with commensal Candida group of organisms (40×).
Just before menstruation, there is shift of cells to superficial type.
Pregnancy, postpartum, and lactation
During the 1st few weeks of pregnancy, there is a premenstrual pattern. As the pregnancy progresses, the maturation index changes to intermediate cell predominance. By the end of the third trimester, there is predominance of navicular cells (glycogenated intermediate cells), lush lactobacilli, and extensive cytolysis. Endocervical cells show Arias Stella reaction. In the postpartum period with loss of placental function, navicular cells are rapidly replaced by parabasal cells. If the mother is nursing her child, postpartum atrophy gradually changes to a mature pattern but atrophy persists as long as the ovarian cycle is suppressed by lactation.
Menopause
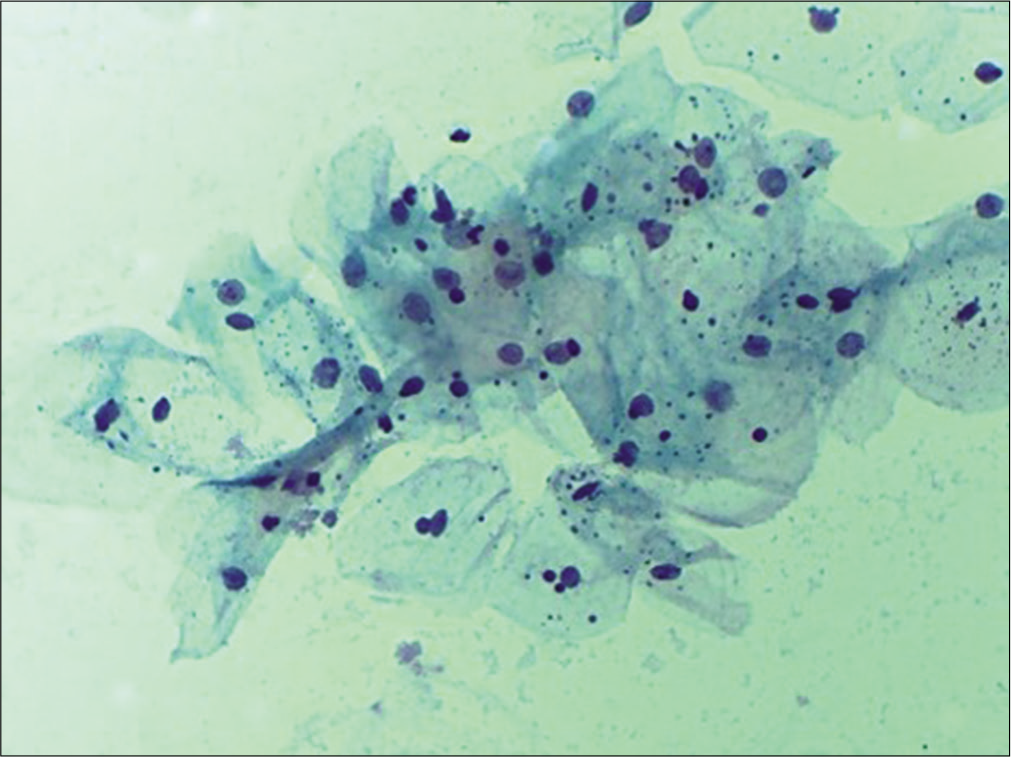
In early menopause, there is typically an intermediate cell predominance [Figure 14]. This remains till women are sexually active. With time, there is a shift in maturation pattern to parabasal predominance. Cells from all the three layers may be present at times but rarely. Finally, in longstanding atrophy, the superficial and intermediate cells are completely replaced by syncytial aggregates of parabasal and basal cells [Figure 15]. Endocervical cells are usually sparse.
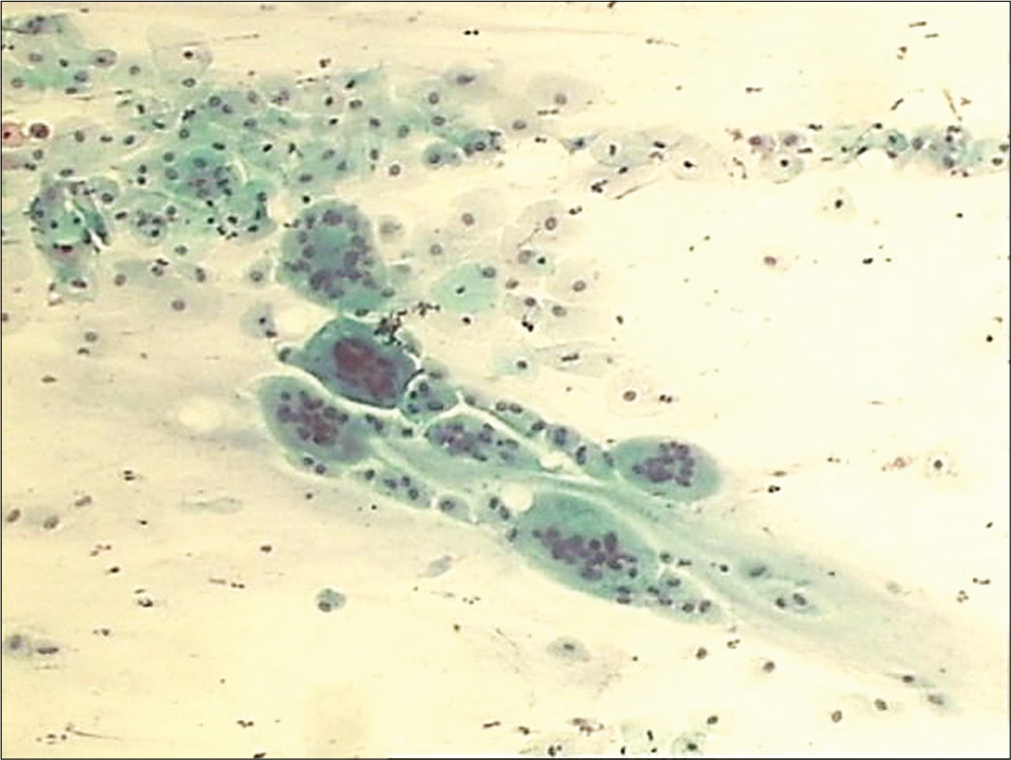
- Conventional smear showing predominance of intermediate cells that are also forming giant cells in early atrophy (10×).
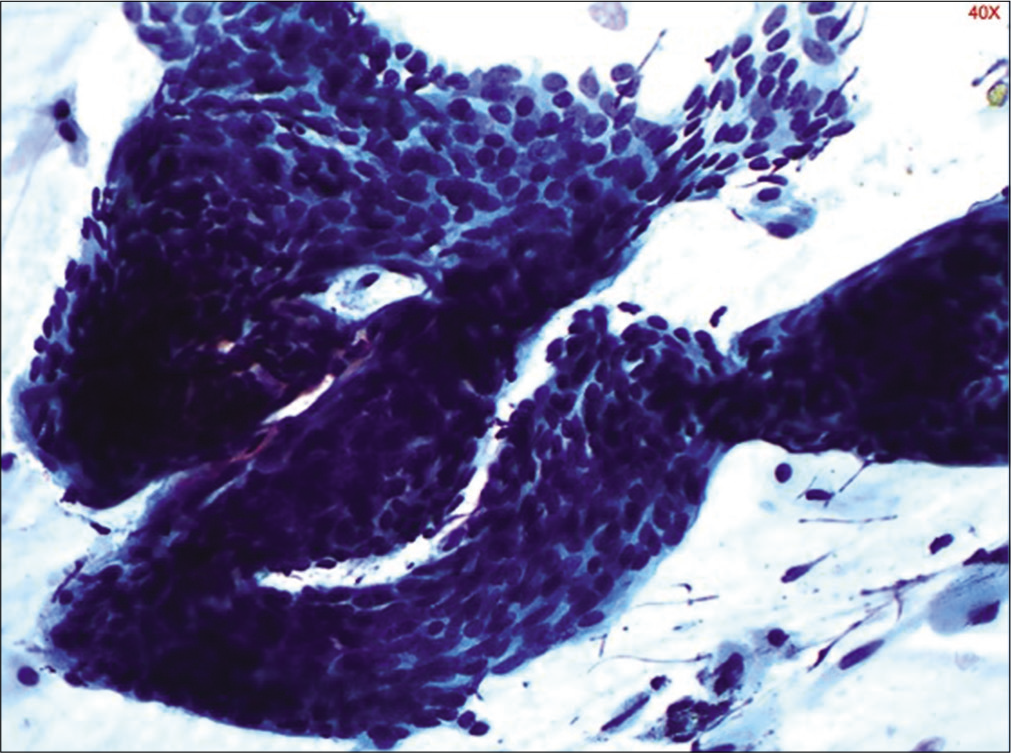
- Conventional Pap smear showing sheets of parabasal cells in an atrophic smear (40×).
HORMONAL CYTOLOGY
The squamous epithelium of cervix and vagina responds to a wide variety of stimuli, especially the hormones. Changes in the epithelium related to ovulation have been described. Because of the considerable variation from cycle to cycle in the same patient, daily variations and even variations between simultaneously obtained smears, it becomes difficult to evaluate the endocrinal status by cytology alone. Hormonal status is routinely done by direct measurement of the hormones in blood. Nevertheless, knowledge of hormonal cytology is helpful in evaluating Pap smears.[1,2]
Hormonal cytology could play an important role in the diagnosis of situations associated with
Infertility
Pregnancy
Polycystic ovarian disease
Functioning ovarian tumors
Fetal death in utero
Post-maturity
Threatened abortion
Premature onset of labor
Amenorrhea or other significant disturbances of the menstrual cycle
In determining the time of ovulation for artificial insemination or in vitro fertilization.
The squamous epithelium, especially of the vaginal mucosa, is not only sensitive to estrogen and progesterone but also to the retinoids, androgens, corticoids, thyroxin, vitamins, antibiotics, digitalis, etc.
During the follicular phase of menstrual cycle, squamous mucosa of cervix and vagina is primarily under the influence of estrogen. It promotes full maturation of epithelium to superficial cell layer by acting on the estrogen receptors which are present in the nuclei of basal cells. Estrogen receptor concentration shows cyclic variation and is reduced in atrophy.
During the luteal phase of menstrual cycle and during pregnancy, the epithelium is primarily under the influence of progesterone. It inhibits full squamous differentiation, with maturation only up to the intermediate cell layer. The thickness of the mucosa is maintained by the functional hyperplasia of intermediate zone.
Pap test is a biological assay that correlates and evaluates the ovarian function, time of ovulation, placental function, and hormonal therapy. A Pap smear dominated by intermediate cells is characteristic of late luteal and early follicular phases of the cycle. Predominance of superficial cells is at the time of ovulation. Parabasal cells predominance indicates thin and atrophic epithelium.
Prerequisites for hormonal cytology are as follows:
There should be absence of inflammation or cytolysis
No recent medication either topical or systemic
No history of radiotherapy or recent surgery to the vagina or cervix
An adequate baseline investigation must be performed in menstruating women, which include daily smears (at least one and preferably two) during complete cycles or their chronologic equivalent in non-menstruating patients two or three smears may suffice
The smears should be obtained from the proximal portion of the lateral wall of the vagina, care being taken to avoid contamination with material from the adjacent cervix. (The area of the vagina most accurately reflects the hormonal status. Squamous epithelium of distal vagina or of the cervix shows a lesser response to hormonal stimulation).
Although the routine cervicovaginal smears are less accurate for purposes of hormonal evaluation, their use cannot be condemned as the patterns of maturation of squamous cells provide useful information either by the presence of a marked estrogenic effect (dominance of mature superficial squamous cells in smears) or their complete absence (atrophic smear pattern).
There are different ways to express the degree of maturation of squamous epithelium which include:
Maturation index (MI)
Karyopyknotic index (KI)
Eosinophilic index (EI)
Maturation value (MV)
Folded cell index
Crowded cell index.
MI
It expresses the percentile relationship of parabasal cells to intermediate cells to superficial squamous cells. Cells in cell clusters are not counted, count is performed on single cells. In normal menstruating women, MI is 0:35:65 (indicates zero parabasal cells, 35% of intermediate cells, and 65% of superficial cells). MI of 90:10:0 indicates predominance of parabasal cells.
This shift to left of MI is seen in physiological conditions such as atrophy and postpartum period
Women taking oral contraceptive pills or hormone replacement therapy may show variation in this index depending on the proportion of estrogens and progesterone and the duration of consumption of such pills
A shift to left in this index is seen in women taking progestin-only contraceptives.
KI
It expresses the percentile relationship of superficial squamous cells with pyknotic nuclei to all mature squamous cells. 200–400 consecutive cells in three or four different fields on the smear are counted and evaluated. In a normally menstruating woman, the peak of KI (50–85%) usually coincides with the time of ovulation. KI is also high in pathological conditions like estrogen-secreting ovarian tumors [Figure 16].
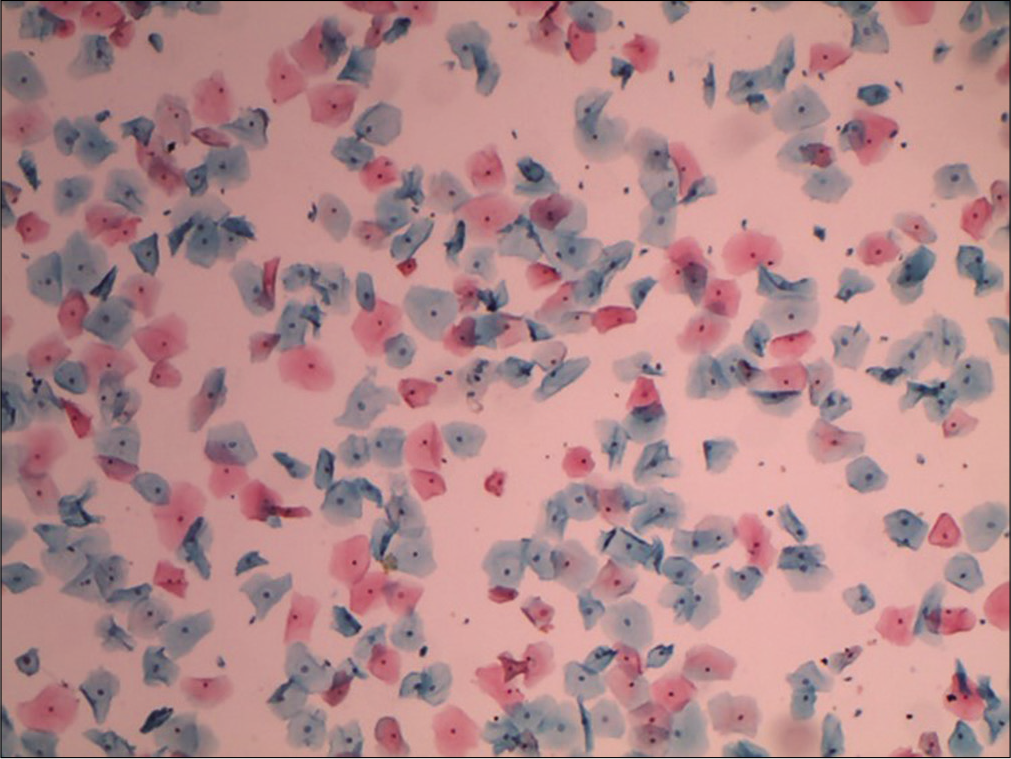
- Conventional smear showing mature superficial and intermediate squamous cells. Ratio of number of superficial squamous cells (with pyknotic nuclei) to total number of mature squamous cells counted will give the karyopyknotic index (10×).
EI
It expresses the percentile relationship of mature squamous cells with eosinophilic cytoplasm to all mature squamous cells, regardless of status of nucleus [Figure 17]. The peak of EI coincides with the KI and may reach maximum at the time of ovulation.
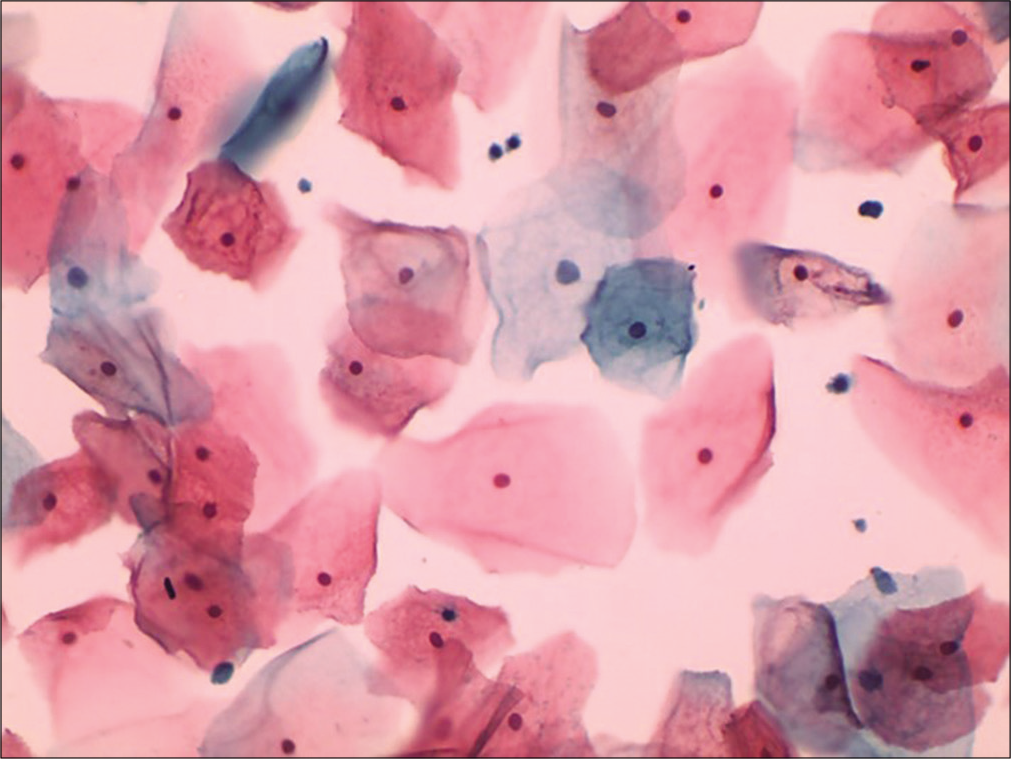
- Conventional smear showing plenty mature squamous cells with eosinophilic cytoplasm (40×).
MV
Meisels in 1967 suggested that a specific numerical value be given to the three types of squamous cells (value of 1.0 to superficial cells, value of 0.5 to intermediate cells, and value of 0.0 to parabasal cells). The MV is expressed by multiplying the percentage of each cell category by its assigned value.
For example, MI of normal menstruating women at the time of ovulation 0:35:65
0 × 0 = 00.00
35 × 0.5 = 17.50
65 × 1.0 = 65.00
MV = 82.5
This system gives figure from 0 to 100, which expresses the hormonal status of the patients.
MV of 100 indicates pure population of superficial cells. MV of 0 indicates pure parabasal cell population. For normal menstruating women, MV is in the range of 50–95. Whereas for women with varying degrees of atrophy, MV is below 50.
Folded cell index: It expresses relationship of mature superficial cells or intermediate cells with folded cytoplasm to all the mature squamous cells. This index peaks in the secretory phase of menstrual cycle and in pregnancy [Figure 18a and b]
Crowded cell index [Figure 19]: It represents relationship of mature squamous cells in clusters of four or more cells to all mature squamous cells. This index peaks in the secretory phase of the cycle and is also seen in early/ crowded menopause
Cornification index: This expresses relationship of superficial cells to all other cells. It peaks at ovulation.
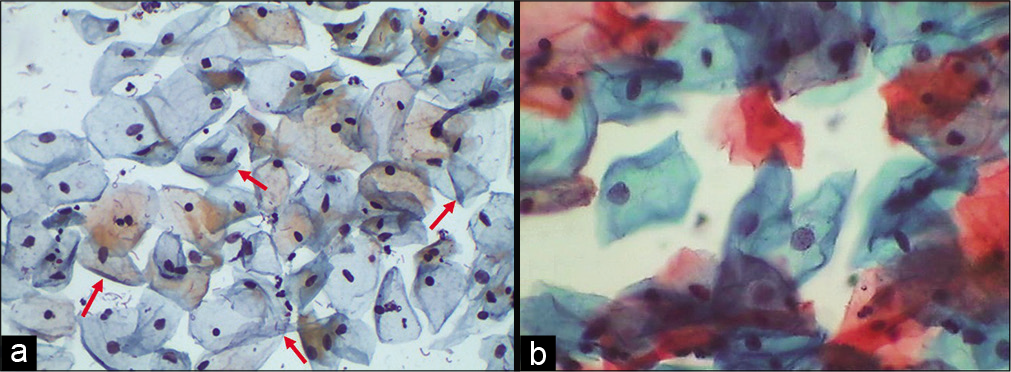
- (a and b) Conventional Pap smear showing intermediate squamous cells with folded cytoplasm (red arrows), feature commonly seen in smears taken during pregnancy (40×).
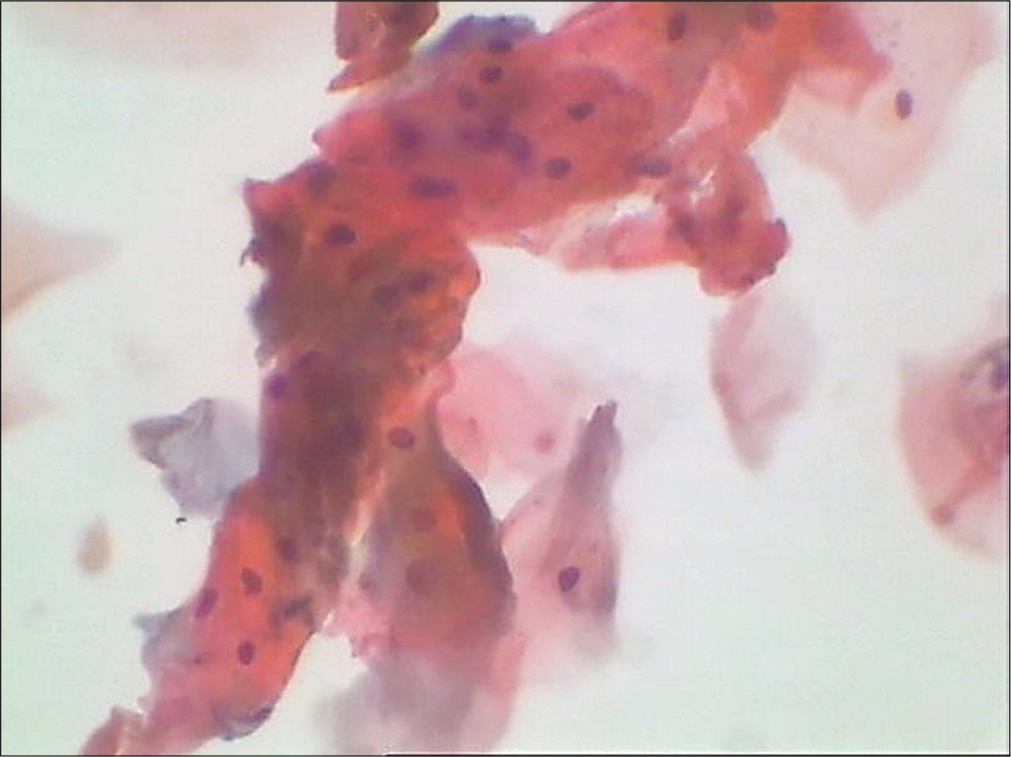
- Conventional smear showing mature squamous cells in clusters (40×).
Acknowledgment
We acknowledge the contributions of Dr. Prajakta Sathawne and Dr. Aishwarya Warke in the completion of this chapter.
LIST OF ABBREVIATIONS (In alphabetic order)
EI - Eosinophilic Index
HCGs - Hyperchromatic cell groups
KI- Karyopyknotic Index
LBC -Liquid based cytology
MI -Maturation Index
MV -Maturation Value
N/C - Nuclear / Cytoplasmic
OCPs - Oral Contraceptive Pills
References
- The pap test In: McDeMay R, ed. The Art and Science of Cytopathology (2nd ed). Hong Kong: American Society for Clinical Pathology; 2012. p. :2-156.
- [Google Scholar]
- Cytologic evaluation of menstrual disorders and Hormonal abnormalities In: Koss LG, Melamed MR, eds. Koss’ Diagnostic Cytology and its Histopathologic Bases (5th ed). Philadelphia, PA: Lippincott Williams and Wilkins; 2006. p. :227-40.
- [Google Scholar]








