Translate this page into:
Oncocytic variant of poorly differentiated thyroid carcinoma: “Is diagnosis possible by fine-needle aspiration?”
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Poorly differentiated thyroid carcinoma (PDTC) is a very rare entity, and the diagnosis can be made on histopathology specimens. However, recognition of characteristic features of PDTC is significant on fine-needle aspirations (FNAs) to differentiate this entity from well-differentiated and anaplastic thyroid carcinomas. Here, we present an FNA case concordant with “oncocytic variant of PDTC” and discuss whether definitive diagnosis can be given on FNAs to assess the prognosis in clinically inoperable patients.
Keywords
Fine-needle aspiration
oncocytic variant
poorly differentiated thyroid carcinoma
INTRODUCTION
Poorly differentiated thyroid carcinoma (PDTC) is a very rare entity and constitutes 2–4% of all thyroid carcinomas which mostly seen between 40 and 60 years of age. PDTC derives from follicular cells, and its immunohistochemical features are intermediate to well-differentiated and anaplastic thyroid carcinomas, as is its prognosis.[1] It was described by Sakamoto et al. and Carcangiu et al., in the early 1980s and recognized as a distinct entity in 2004 WHO classification.[234] In 2006, diagnostic criteria defined by the Turin proposal: Solid/trabecular/insular growth pattern, presence of at least one of the following features: (a) Convoluted nuclei, (b) mitotic activity (3 or more mitosis per 10 HPF), (c) necrosis and absence of nuclear features of papillary thyroid carcinoma.[5]
The oncocytic variant of PDTC is even a more controversial topic. In 1996, Papotti et al. investigated the trabecular/insular pattern, cellular features, p53 and Bcl2 expression in a series of oncocytic thyroid carcinomas, and reported 20 well-documented cases of oncocytic variant of PDTC.[6] Although it was excluded from the Turin consensus, the literature mostly concurs on the existence and importance of the oncocytic variant based on large series and case reports.[5789] Moreover, the WHO classification recognizes this entity as a variant of PDTC.[4]
The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) implicates PDTC as a specific tumor type in the malignant category.[10] However, according to WHO, definitive diagnosis cannot be given solely by fine-needle aspiration (FNA) without a surgical pathology follow-up.[4]
PDTC can present as a large mass with extensive extrathyroidal extension as well as a nodule confined to thyroid.[58] Therefore, in relatively smaller nodules (< T4), recognizing the specific features of PDTC and discriminating this aggressive tumor from well-differentiated thyroid carcinomas (WDTC) becomes important.[8] Similarly, when PDTC presents with extrathyroidal extension or metastasis, it becomes noteworthy to distinguish PDTC from anaplastic thyroid carcinoma, since the prognosis and treatment of these entities could differ.[11] However, in some of the clinically advanced cases, where thyroidectomy is not the treatment of choice, FNA specimens appear as the only source rendering definite diagnosis for further clinical management.[4]
In this report, we present findings of a large thyroid mass which we diagnosed as “malignant; suggesting a high suspicion for oncocytic variant of poorly differentiated thyroid carcinoma” based on FNA and aim to discuss if it is appropriate to give PDTC diagnosis in this unique situation.
CASE REPORT
A 63-year-old man presented with a tumor localized on the right side of the neck which has gradually enlarged during the last 2 years. Computed tomography (CT) revealed a 76 mm solid mass in the right lobe of the thyroid gland, displacing the trachea medially, common carotid artery posteriorly, and sternocleidomastoid muscle laterally. The lesion had heterogeneous contrast enhancement with lobulated contour, and internal macrocalcifications with necrotic areas were obvious [Figure 1]. There were metastatic lymph nodes in cervical lymph node stations of 2-3-4-5 and the supraclavicular region. Thoracic CT showed mediastinal lymph nodes and lung metastases.
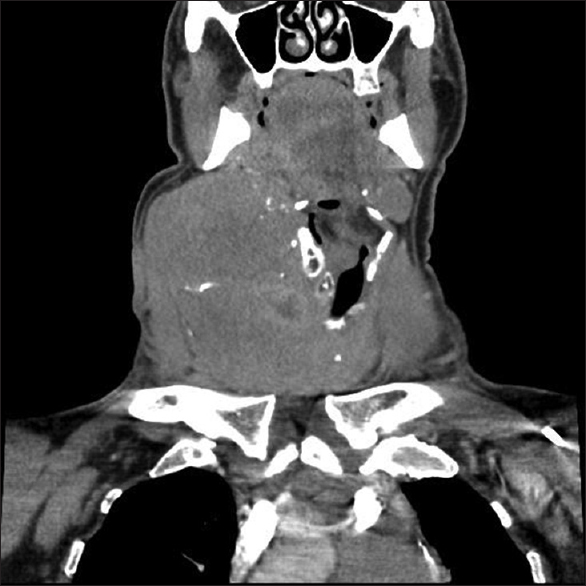
- Large mass in the right lobe of the thyroid displacing the trachea medially, common carotid artery posteriorly, and sternocleidomastoid muscle laterally
The FNA was hypercellular not only as single cells but also insular and trabecular arrangements were noticed [Figures 2 and 3]. Colloid was scarce and necrosis with accompanying polymorphonuclear leukocytes was seen in the background. Cells were uniform and had large granular cytoplasm with an increased nuclear/cytoplasmic ratio. Nuclei were round, slightly hyperchromatic, and macronucleoli were prominent [Figure 4]. Nuclear pleomorphism was not striking. Except for nuclear membrane irregularity as rasinoid, the rest of the characteristic nuclear features of papillary thyroid carcinoma and medullary thyroid carcinoma were absent.
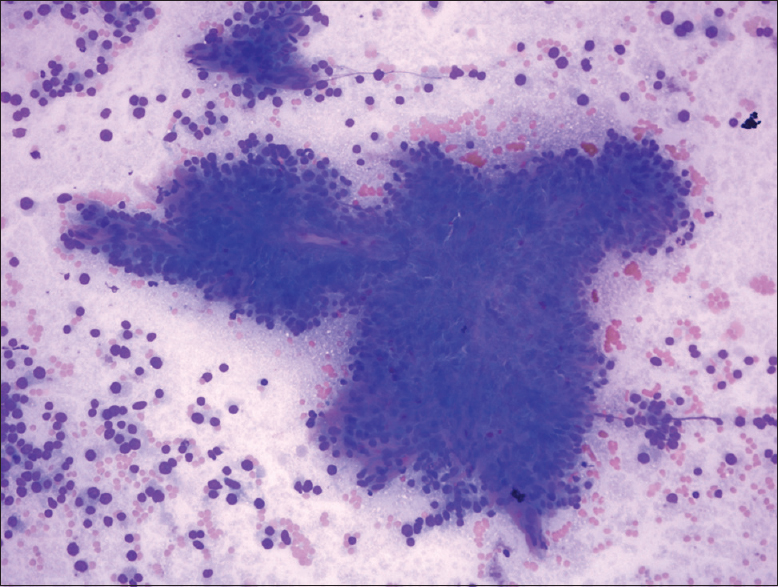
- Hypercellular smear with prominent single cells, insular pattern, and transgressing vessels (Diff-Quik, ×40)
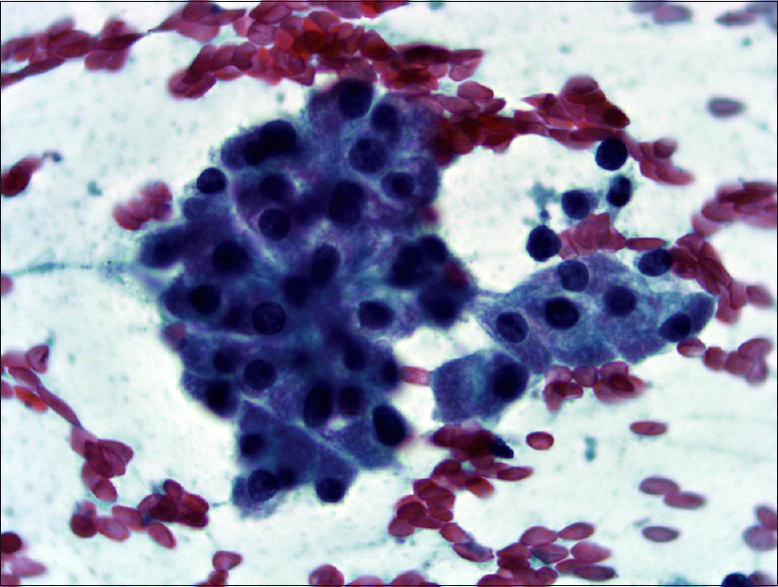
- Cells have delicate eosinophilic cytoplasm (Pap, ×400)
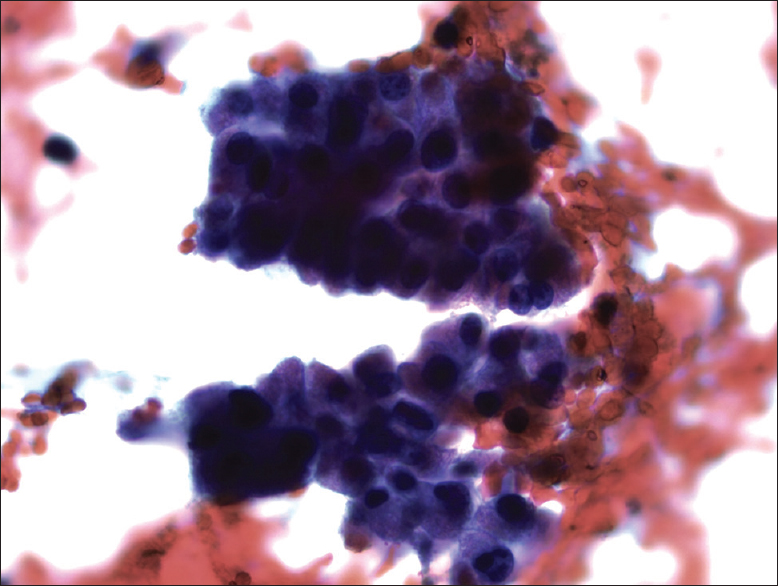
- Cells have a high nuclear/cytoplasmic ratio, nuclei are round, slightly hyperchromatic and relatively uniform with macronucleoli (Pap, ×400)
Immunohistochemical analyses were performed on the cell-block [Figure 5]. TTF-1 and PAX-8 revealed diffuse nuclear positivity, whereas p53 was positive in 9.2% of cell nuclei [Figures 6 and 7]. Pan-CK showed extensive and thyroglobulin (TG) weak focal cytoplasmic staining. Ki-67 proliferation index was 10%. Carcinoembryonic antigen (CEA), calcitonin, and Bcl-2 were negative. All immunostains were correlated with convenient positive controls.
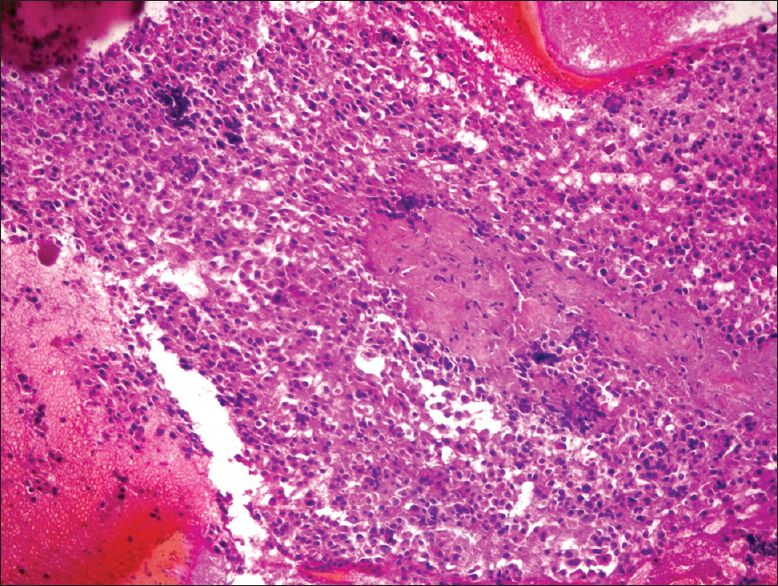
- Cell block (H and E, ×40)
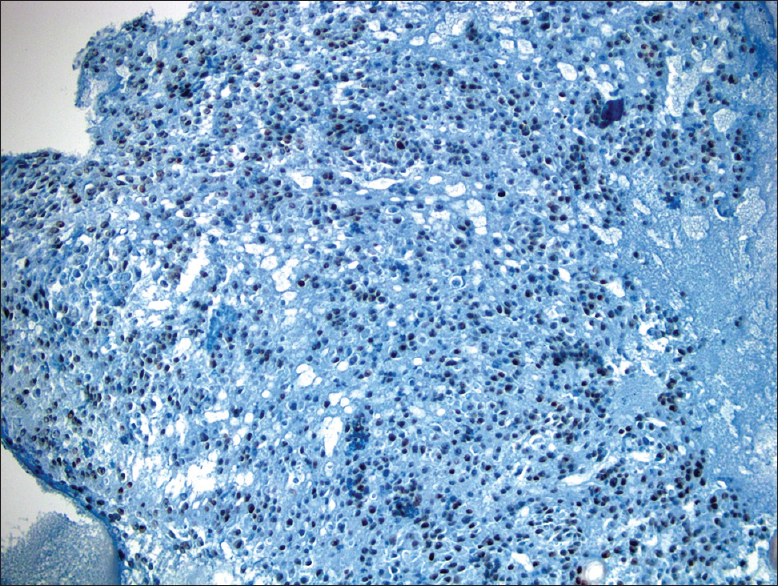
- Diffuse nuclear TTF-1 positivity (×40)
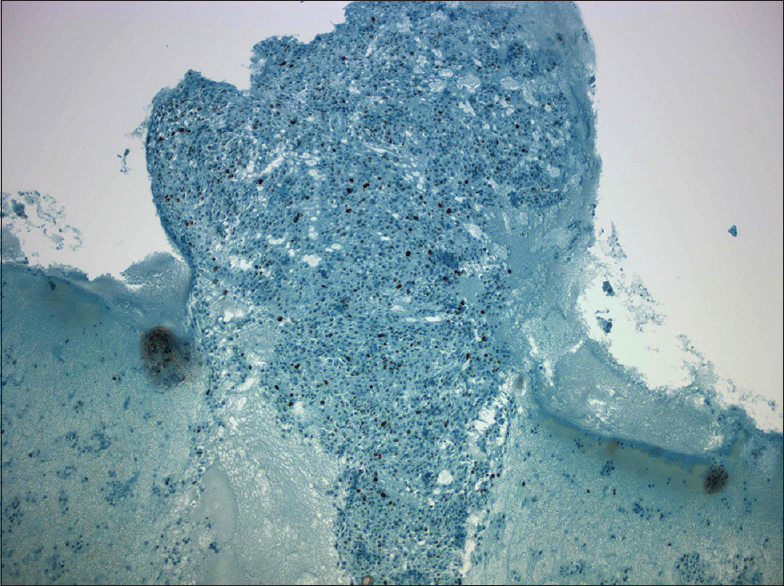
- Nuclear p53 positivity (×40)
Based on the monomorphic oncocytic morphology, the presence of necrosis, immunohistochemical profile, and clinicoradiologic findings; FNA diagnosis was given as “malignant; highly suggesting oncocytic variant of poorly differentiated thyroid carcinoma” according to TBSRTC. The patient was considered inoperable due to the radiologic findings and received chemo/radiotherapy. No further metastases were detected with positron emission tomography in the clinical follow-up of 18 months.
DISCUSSION
Contrary to well-established diagnostic criteria in the histopathologic evaluation, cytological characteristics of PDTC were defined in a limited number of case reports.[1213] The largest FNA cohort includes 40 proven cases of PDTCs analyzed for 32 cytomorphologic features by Bongiovanni et al.[9] The study underlines the importance of four major cytomorphological characteristics; insular/solid/trabecular pattern, high nuclear/cytoplasmic ratio, severe crowding, and single cells. Bare nuclei due to delicate oncocytic cytoplasm, polymorphonuclear leukocytes, and endothelial wrapping were defined as other distinctive features.[9] The presented case met these criteria to be regarded as PDTC. WDTC was excluded based on the cytoarchitectural pattern and lack of conventional nuclear features of papillary thyroid carcinoma. The literature does not suggest any particular immunostain panel for PDTC; however, p53 positivity favors PDTC more than WDTC.[711] Similarly, medullary thyroid carcinoma was excluded using cytomorphologic features and immunohistochemical calcitonin and CEA negativity.
Anaplastic carcinoma was also in the differential diagnosis. Nevertheless, FNAs consisted of a uniform, large, oncocytic cell population in all three passes of the FNA procedure (six slides) and no cytomorphologic features concordant with anaplastic carcinoma (i.e., large pleomorphic giant cells, spindle or squamoid cells) were revealed. It is important to note that tumor cells in anaplastic carcinoma almost never present uniformly with a particular pattern.[11] Due to loss of the capability for follicular differentiation, TTF-1 and TG are also expected to be negative in anaplastic carcinoma, whereas focal TG, persistent TTF-1, and keratin expressions should be observed in PDTC.[11] Unlike the current case, necrosis is generally more extensive in anaplastic carcinoma, with a high p53 (> 39–100%) and Ki67 (50%) staining in tumor cells.[11] Anaplastic carcinoma also shows rapid growth, which is defined as weeks and most of the patients die 3–6 months after the diagnosis, contrary of the presented case.[11]
CONCLUSION
Although according to WHO, the definitive diagnosis of PDTC can only be given in histopathologic specimens, we believe that in clinically advanced tumors, use of the Turin criteria as emphasized by Bongiovanni et al. in FNA specimens can aid in differentiating PDTC from anaplastic carcinoma and thus provide a better prognostication for the patient.[9]
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this letter declare that we qualify for authorship as defined by ICMJE. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. Mine Onenerk and Sule Canberk drafted the manuscript; Pembegul Gunes, and Murat Erkan contributed to conception of thestudy. Gamze Z. Kilicoglu interpretated the radiological findings, and criticized the article. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This letter does not require approval from Institutional Review Board.
LIST OF ABBREVIATIONS (In alphabetic order)
CEA - Carcinoembryonic Antigen
CT - Computed Tomography
FNA - Fine-Needle Aspiration
PDTC - Poorly Differentiated Thyroid Carcinoma
TBSRTC - The Bethesda System for Reporting Thyroid Cytopathology
TG - Thyroglobulin
WDTC - Well-Differentiated Thyroid Carcinomas
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Poorly differentiated thyroid carcinoma: Diagnostic features and controversial issues. Endocr Pathol. 2008;19:150-5.
- [Google Scholar]
- Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983;52:1849-55.
- [Google Scholar]
- Poorly differentiated (“insular”) thyroid carcinoma. A reinterpretation of Langhans’ “wuchernde Struma”. Am J Surg Pathol. 1984;8:655-68.
- [Google Scholar]
- DeLellis RA, Lloyd RV, Heitz PU, Eng C, eds. Tumors of the thyroid. In Pathology and Genetics of Tumours of Endocrine Organs. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2004. p. :73-6.
- Poorly differentiated thyroid carcinoma: The Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31:1256-64.
- [Google Scholar]
- Poorly differentiated oxyphilic (Hurthle cell) carcinomas of the thyroid. Am J Surg Pathol. 1996;20:686-94.
- [Google Scholar]
- Poorly differentiated carcinoma of the thyroid: Validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol. 2010;23:1269-78.
- [Google Scholar]
- Poorly differentiated oncocytic thyroid carcinoma - Diagnostic implications and outcome. Histopathology. 2012;60:1045-51.
- [Google Scholar]
- Cytomorphologic features of poorly differentiated thyroid carcinoma: A multi-institutional analysis of 40 cases. Cancer. 2009;117:185-94.
- [Google Scholar]
- NCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658-65.
- [Google Scholar]
- Diagnostic Pathology and Molecular Genetics of the Thyroid: A Comprehensive Guide for Practicing Thyroid Pathology (2nd ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2012. p. :242-62.
- Poorly differentiated insular thyroid carcinoma. A case report with identification of intact insulae with fine needle aspiration biopsy. Acta Cytol. 1998;42:991-7.
- [Google Scholar]
- Cytologic features of poorly differentiated ‘insular’ carcinoma of the thyroid, as revealed by fine-needle aspiration biopsy. Am J Clin Pathol. 1990;94:687-92.
- [Google Scholar]








