Translate this page into:
Diagnostic utility of double immunostaining of a urine cytology preparation for cytokeratin 20/p53 expression in a young woman with micropapillary urothelial carcinoma of the renal pelvis presenting as an unknown primary malignancy
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Atypical urine cytology (CYT) triggers a cystoscopic or another ancillary investigation that targets urothelial neoplasms. We report a case presenting as an unknown primary malignancy, which illustrated the diagnostic utility of direct double immunostaining for cytokeratin 20 (CK20)/p53 expression in a urine CYT specimen. A 42-year-old woman visited the emergency room for pain in her right lower abdominal quadrant. Computed tomography revealed postrenal obstructive hydronephrosis, and her urine CYT showed malignancy, type undetermined. Atypical cells that are positive for cytoplasmic expression of CK20 and nuclear expression of p53 could facilitate the decision to perform a nephroureterectomy for urothelial carcinoma.
Keywords
Cytokeratin 20
double immunostaining
p53
unknown primary
urothelial carcinoma
INTRODUCTION
Atypical urine cytology (CYT) triggers a cystoscopic or ancillary investigation that targets urothelial neoplasms. Several investigations have aimed to improve the diagnosis of these neoplasms in urine specimens, however, cytological material from urine specimens is too limited to allow multiple immunocytochemical analyses. Double immunostaining of cytokeratin 20 (CK20) expression in the cytoplasm and p53 in the nuclei of cells in the urinary sediment is a promising method for diagnosing urothelial carcinoma (UC). We report a case presenting as an unknown primary malignancy, which illustrates the diagnostic utility of CK20/p53 double immunostaining directly performed on a urine CYT specimen.
CASE REPORT
A 42-year-old woman visited the emergency room for pain in her right lower abdominal quadrant. Abdominopelvic computerized tomography (CT) revealed postrenal obstructive hydronephrosis without a definitive mass in the upper urinary tract [Figure 1a], which suggested renal/ureteral tuberculosis or an unknown masked metastatic carcinoma. Positron emission tomography/CT revealed multiple high-intensity signals in the C7 to T4 spinal vertebrae and intra-abdominal para-aortic lymph nodes, suggesting metastatic malignancy of unknown origin (MUO) [Figure 1b]. Examination of a cytological preparation from a urine specimen found clusters of large atypical cells with abundant cytoplasm and prominent nucleoli, suggesting malignancy, type undetermined [Figure 2a]. Subsequent double immunostaining for CK20/P53 expression of a cytological preparation from the same urine sample found CK20-positive umbrella cells [Figure 2b] and CK20-positive/p53-positive atypical cells, suggestive of UC [Figure 2c]. Systemic review was unable to identify definitive evidence of a metastatic primary malignancy that caused ureteral obstruction. The patient underwent right nephroureterectomy for the definitive diagnosis and treatment. The operative findings included an ill-defined infiltrating mass (2.5 cm) in the lower right renal pelvis [Figure 3a]. Histopathological examination found tumor cells that were arranged in micropapillary clusters with frequent lymphovascular invasion [Figure 3b]. The tumor cells infiltrated through the renal parenchyma into perinephric fat tissue, indicating advanced disease (pT4) [Figure 3c]. Diffuse in situ UC was found in the associated ureter. The patient has been stable over 3-month observation period after two cycles of adjuvant chemotherapy.
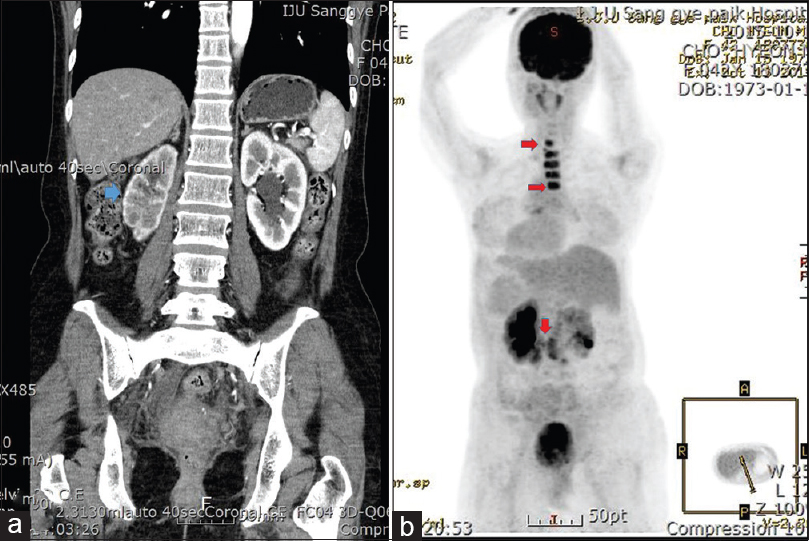
- (a) Abdominopelvic computed tomography image shows marked hydronephrosis (blue arrow) affecting the right kidney without an obvious mass in the upper urinary tract. (b) Positron emission tomography/computed tomography revealed multiple high signal intensities (C7-T4 spinal vertebrae, para-aortic lymph nodes, red arrows) suggesting metastatic carcinoma from an unknown primary
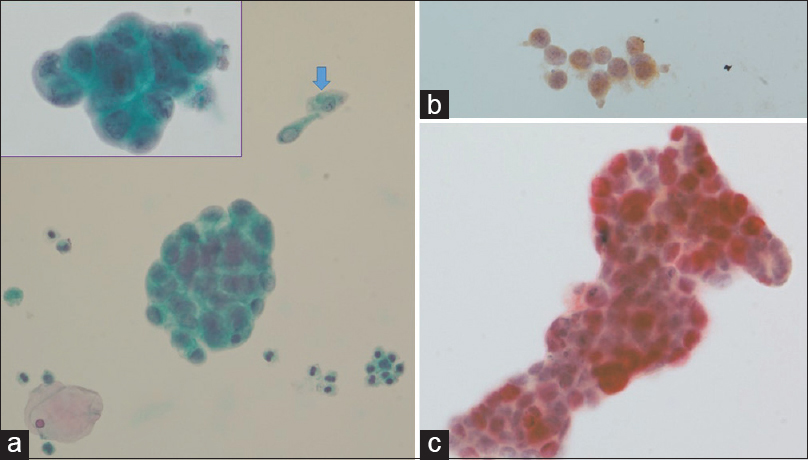
- (a) Urine cytology preparation containing clusters of atypical epithelial cells in a background of umbrella cells (blue arrow) and inflammatory cells. The atypical cells are arranged in three-dimensional clusters, resembling an adenocarcinoma (Inset) (Pap, ×400). (b) The umbrella cells only express cytokeratin 20 antigen (p53/CK20 double immunostaining, ×400). (c) The atypical cells demonstrate a distinct pattern of double staining: red nuclei (p53) and brown cytoplasm (cytokeratin 20) (p53/CK20 double immunostaining, ×400)
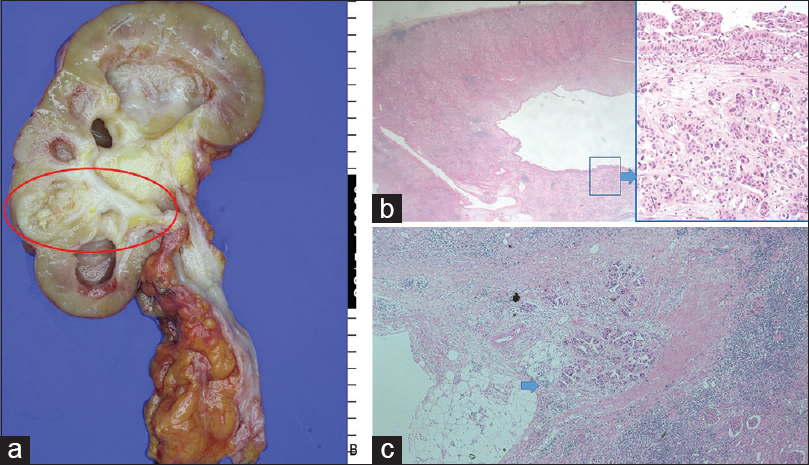
- (a) Nephroureterectomy specimen showing cystically dilated renal pelvis and ill-defined mass (2.5 cm) in the lower renal pelvis (red circle). (b) The scanning power view shows relatively intact urothelium and underlying atrophic renal parenchyma (H and E, ×10) Papillary proliferation of urothelium and underlying micropapillary nests in lacunar spaces suggest micropapillary urothelial carcinoma (Inset) (H and E, ×100). (c) Tumor cells are seen infiltrating the perirenal fat tissue (blue arrow) (pT4 disease) (H and E, ×10)
DISCUSSION
UC accounts for >70,000 new cancer cases and approximately 14,000 deaths annually in the United States.[1] Approximately 70% of patients initially present with Ta/T1 tumors. Depending on risk stratification, up to 15% of these tumors progress to a muscle-invasive stage. Therefore, early diagnosis is essential for preventing progression to an advanced stage, which is associated with a remarkable increase in mortality.[2] Cystoscopy is still the gold standard for the primary diagnosis and follow-up of patients with UC.[3] However, the cystoscopy procedure is associated with patient discomfort and stress.
Current guidelines recommend performing urine CYT as an adjunctive tool in patients with suspected UC.[3] Although urine CYT has relatively high specificity, its diagnostic value is impaired because of its low sensitivity.[4] Therefore, several additional urinary cytological assessments have been developed over the last 20 years to improve the detection rate of UC. These include fluorescence in situ hybridization (FISH)[5] for the detection of chromosomal aberrations (loss of the 9p21 locus, which is the site of the p16 tumor suppressor gene, has been identified as a common chromosomal aberration in patients with UC) and an immunocytology test (uCyt+ test),[6] which combines conventional urine cytology and an immunofluorescence assay using a cocktail of antibodies against UC-associated surface antigens such as carcinoembryonic antigen and mucin-like glycoprotein. Furthermore, protein-based tests such as the quantitative nuclear matrix protein 22-enzyme-linked immunoadsorbent assay (NMP22-ELISA)[7] have become widely available and are frequently used by specialized centers and practitioners. The common limitation of some of these markers is a relatively high false-positive rate because of endogenous or exogenous factors that include mechanical manipulations, urinary tract infection, hematuria, and impaired renal excretory function. Some investigators have studied using a combination of the four available urine tests (CYT, FISH, uCyt+, and NMP22-ELISA) for a large cohort of 808 patients suspected of having UC.[8]
Another group of investigators designed a study to investigate the use of a triple cocktail of antibodies against CK20, p53, and CD44 in urine CYT samples for identifying and differentiating UC from benign mimics.[9] This triple cocktail method had been tried on tissue specimens. Triple immunostaining of sections of urinary cytological specimens imbedded in paraffin yielded 94% sensitivity and 93% specificity for UC.[10] They reported that CK20 and p53 were useful for identifying cases with subsequent carcinoma. CD44 was very reliable, being present only in a minority of cases.
For our patient, we tried direct double immunostaining of cells from a urine sample, which were not embedded in a paraffin block. The staining method clearly demonstrated the double-positive features of carcinoma cells, which were similar to those of cells in a tissue section.
The patient was a relatively young woman who presented with an unknown primary metastatic malignancy. The only positive finding was ‘malignancy, type undetermined’ on urine CYT. The tumor cells on the CYT preparation were arranged in three-dimensional clusters, mimicking adenocarcinoma and were later found to represent the micropapillary component of conventional UC. The micropapillary cytomorphology of an unidentified intra-abdominal malignancy of women is frequently confused with an ovarian/primary peritoneal serous tumor. Micropapillary UC (MPUC) has an aggressive clinical course with early nodal/distant metastasis, which may alert the clinician.[11] Some investigators have suggested using p63, p40, and GATA-binding protein 3 immunohistochemistry for differentiating MPUC from typical UC.[12]
SUMMARY
Double immunostaining of a liquid-based urine CYT specimen from a 42-year-old woman with an MUO appeared to be a useful method for confirming UC. The use of an antibody combination against both CK20 and P53 to test urine samples with atypical CYT might allow differentiation between reactive atypia and carcinoma.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Hyun-Jung Kim designs this case report and first author. Lucky Sung contributes a urine sample and tissue specimen with informed consent collaborate ancillary studies using liquid-based smear (urine). Jung-Yeon Kim collaborates the cytologic diagnosis and interpretation of double staining. Kyeongmee Park collaborates the cytologic diagnosis and interpretation of double staining.
ETHICS STATEMENT BY ALL AUTHORS
This report was approved by the Institute Review Board of Inje Univ Sanggye Paik Hospital (approval number 2016-02-013).
LIST OF ABBREVIATIONS (In alphabetic order)
CK - Cytokeratin
CT - Computerized tomography
CYT - Cytology
FISH - Fluorescence in situ hybridization
MPUC - Micropapillary urothelial carcinoma
MUO - Malignancy of unknown origin
UC - Urothelial carcinoma.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714-36.
- [Google Scholar]
- Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164(3 Pt 1):680-4.
- [Google Scholar]
- EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997-1008.
- [Google Scholar]
- Biomarkers for detection and surveillance of bladder cancer. Can Urol Assoc J. 2008;2:212-21.
- [Google Scholar]
- Multiprobe fluorescence in situ hybridization (UroVysion) for the detection of urothelial carcinoma – Fishing for the right catch. Acta Cytol. 2011;55:113-9.
- [Google Scholar]
- Immunocyt: A new tool for detecting transitional cell cancer of the urinary tract. J Urol. 1999;161:1486-9.
- [Google Scholar]
- Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14:1-331.
- [Google Scholar]
- Combined application of cytology and molecular urine markers to improve the detection of urothelial carcinoma. Cancer Cytopathol. 2013;121:252-60.
- [Google Scholar]
- Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: An analysis of cytokeratin 20, p53, and CD44 antigens. Am J Surg Pathol. 2001;25:1074-8.
- [Google Scholar]
- Evaluation of a triple combination of cytokeratin 20, p53 and CD44 for improving detection of urothelial carcinoma in urine cytology specimens. Cytojournal. 2013;10:25.
- [Google Scholar]
- cytological findings of the micropapillary variant of urothelial carcinoma: A comparison with typical High grade Urothelial carcinoma. J Pathol Transl Med. 2013;47:365-71.
- [Google Scholar]
- The utility of p63, p40, and GATA-binding protein 3 immunohistochemistry in diagnosing micropapillary urothelial carcinoma. Hum Pathol. 2014;45:1824-9.
- [Google Scholar]








