Translate this page into:
Pleural fluid metastases of myoepithelial carcinoma: A case report and review of the literature
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Myoepithelial carcinoma (MECA) is one of the rarest salivary gland neoplasms, which may either arise de novo or develop within a preexisting pleomorphic adenoma or benign myoepithelioma. The tumor occurs mainly in the parotid gland followed by minor salivary glands and other body sites. As a result of their morphologic heterogeneity, they can be confused easily with many tumors. Awareness of their unique cytoarchitectural patterns and immunohistochemical profile is crucial for accurate identification. Herein, we report a rare case of a 51-year-old female patient with MECA of the maxillary sinus that metastasized to the pleural fluid. To the best of our knowledge, this is the first case of pleural fluid involvement by MECA reported in the literature.
Keywords
Cytology
metastases
myoepithelial carcinoma
pleural
INTRODUCTION
According to the World Health Organization (WHO) classification, myoepithelial carcinoma (MECA) is referred to those lesions which are composed almost exclusively of tumor cells that exhibit a dual epithelial and smooth muscle differentiation.[1] Up to 75% of MECAs occur mainly in the parotid gland followed by minor salivary glands (include the palate, cheek, gum, nasal cavity, maxillary sinus, nasopharynx, base of tongue, and supraglottic larynx) and the submandibular gland.[2345] MECAs have been described in other sites such as lungs, skin, breast, stomach, and soft tissues.[6789] The mean age at presentation is in the fifth to sixth decade and in general, MECAs exhibit no gender predilection, with the exception of primary lung MECAs that affect males more than females. The majority of patients with head and neck tumors present with the primary complaints of a painless mass with duration of symptoms ranging from 2 months to 7 years.[2] MECAs may arise de novo, but a half or more develop in preexisting pleomorphic adenomas or benign myoepitheliomas. Histologically, they exhibit a wide morphologic and cytologic diversity. Their diverse morphology is a potential diagnostic pitfall that has historically led to their misdiagnosis and underrepresentation in the literature. We herein report a unique case of a 51-year-old female with MECA arising in the maxillary sinus that metastasized to the pleural fluid. To the best of our knowledge, this is the first reported case in the literature.
CASE REPORT
The case we present here is about a 51-year-old female patient presented with a facial mass along with diplopia, watery eyes, rhinorrhea, and epistaxis. The patient was nonsmoker and had no past history of cancer or surgery. Computed tomography (CT) scan showed a maxillary mass invading into the adjacent tissue. A magnetic resonance imaging (MRI) of the face and neck was performed and demonstrated an 8 cm solid mass centered in the left maxillary sinus with extension to the orbit, nose, left ethmoid sinus, and left pterygopalatine fossa [Figure 1].
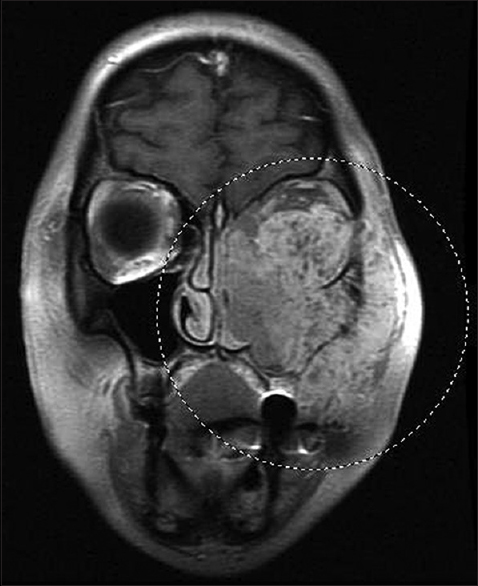
- Coronal plane magnetic resonance imaging of the face shows a large tumor that fills the left maxillary sinus and invades the nasal cavity, face, and orbit, displacing the left globe
The patient underwent fine needle aspiration (FNA) of the mass under CT-guidance using coaxial technique and a 22-gauge needle. Air-dried and alcohol-fixed smears were prepared and stained with Diff-Quick and Papanicolaou stains, respectively. A cell block was prepared from the sample rinsed in alcohol fixative, using the agar method. Evaluation of the aspirate smears showed a cellular specimen consisting of numerous cells arranged singly or in small clusters. Some fragments appeared three-dimensional (3D) with high cellularity and nuclear overlapping. The cells were round-to-oval and exhibited eccentrically located nuclei and scant-to-moderate amount of cytoplasm. The nuclei showed fine chromatin and some with nucleoli and mild nuclear pleomorphism [Figure 2]. Background showed numerous naked nuclei and stromal fragments. Immunocytochemical stains were performed on the cell block preparation slides in an automated immunostainer in the presence of appropriate positive and negative controls. The malignant cells were positive for pan-cytokeratin (AE1/AE3) and p63 and negative for CD45, CD30, and HMB45. The diagnosis of malignant neoplasm with myoepithelial differentiation was rendered. The differential diagnosis at this time included a polymorphous low-grade adenocarcinoma, an adenoid cystic carcinoma, a basal cell carcinoma of salivary gland, and MECAs. The patient subsequently underwent a biopsy of the mass that showed a proliferation of monotonous epithelial cells with a plasmacytoid appearance within a myxoid stroma. Although the cells were uniform, cytologic atypia, frequent mitotic activity, and areas of necrosis were identified [Figure 3]. Immunohistochemical stains were performed and showed the lesional cells to be strongly positive for AE1/AE3, epithelial membrane antigen (EMA) and p63 with focal smooth muscle actin staining, and a 20% ki-67 proliferation index. They were negative for S-100. Given these findings, the biopsy was diagnosed as a high-grade MECA.
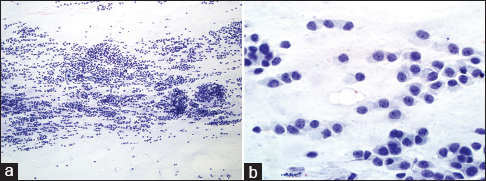
- Fine needle aspiration smears of the maxillary sinus mass showing (a) cellular specimen consists of numerous cells arranging singly and in small clusters (Papanicolaou, ×10). (b) The malignant cells are round-to-oval and exhibit eccentrically located nuclei with fine chromatin and nucleoli (Papanicolaou, ×40)
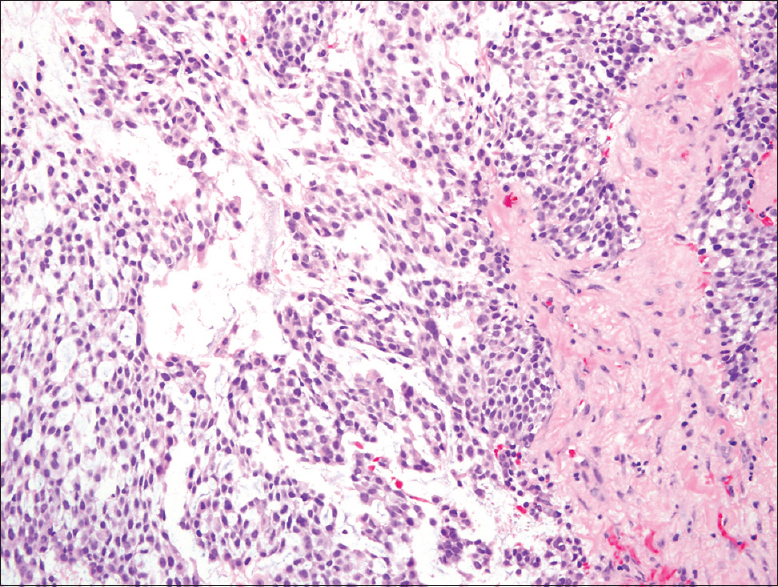
- Tissue biopsy of the maxillary sinus mass showing sheets of plasmacytoid malignant cells with thick fibrous intervening myxoid stroma (H and E, ×20)
Follow-up MRI 1 month later revealed further progression of the mass with invasion to the left orbit and displacement of the left eye and orbital cavity. At this time, the patient reported complete loss of vision on the left eye. In addition, there was near occlusion of the left nasal cavity, increasing tumor invasion into the left ethmoid and sphenoid sinuses, and bony invasion into the maxilla and mandible. While strong recommendations for surgery were provided by the clinical team, the patient opted for radiotherapy and chemotherapy. Eight months after completing chemoradiation, the patient presented with shortness of breath and chest pain. A chest X-ray showed bilateral pulmonary metastases range from 0.6 to 1.6 cm, pleural implants, and a pleural effusion. Consequently, ultrasound-guided thoracentesis of a left pleural effusion yielded 60 cc of bloody, opaque fluid. Cytological evaluation revealed malignant cells in 3D tight clusters with high cellularity and nuclear overlapping. Many single cells and naked nuclei were noted in the background [Figure 4a]. These cells were plasmacytoid in appearance and exhibited round-to-oval nuclei with slight nuclear membrane irregularity and nucleoli noted in the majority of cells [Figure 4b, c]. Metachromatic stromal fragments were seen intermixed with neoplastic cells.
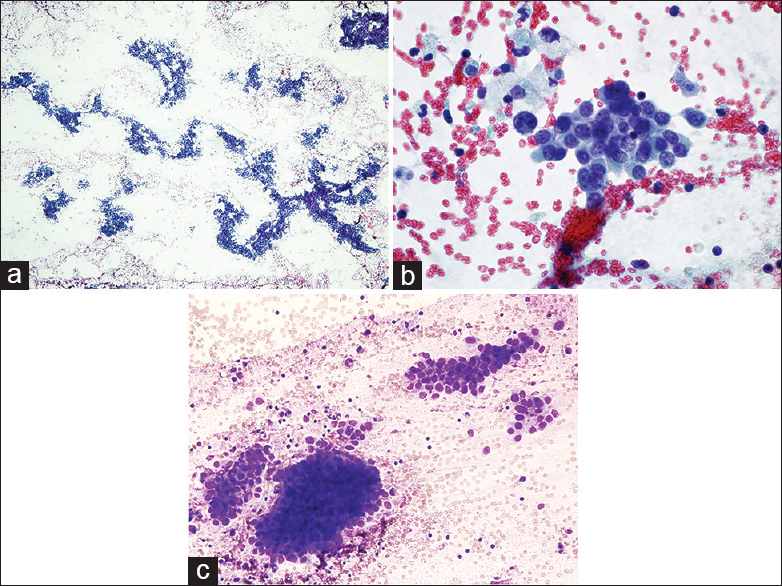
- Pleural fluid smears showing (a) numerous malignant cells arranging singly and in small aggregates (Papanicolaou, ×4). (b) The malignant cells exhibit eccentrically located nuclei with fine chromatin and nucleoli (Papanicolaou, ×40) and (c) (Diff-Quick, ×20)
Immunohistochemical stains performed on the cell block tissue sections showed the cells to be positive for pancytokeratin (AE1/AE3), 34βE12 (high molecular weight cytokeratin), vimentin, EMA, p63, and were negative for actin, S-100, calponin, and TTF1 [Figure 5]. The morphologic and immunohistochemical profile was similar to the original maxillary mass pathology, and a diagnosis of metastatic MECAs was rendered. Unfortunately, a month later, our patient died secondary to the respiratory failure.
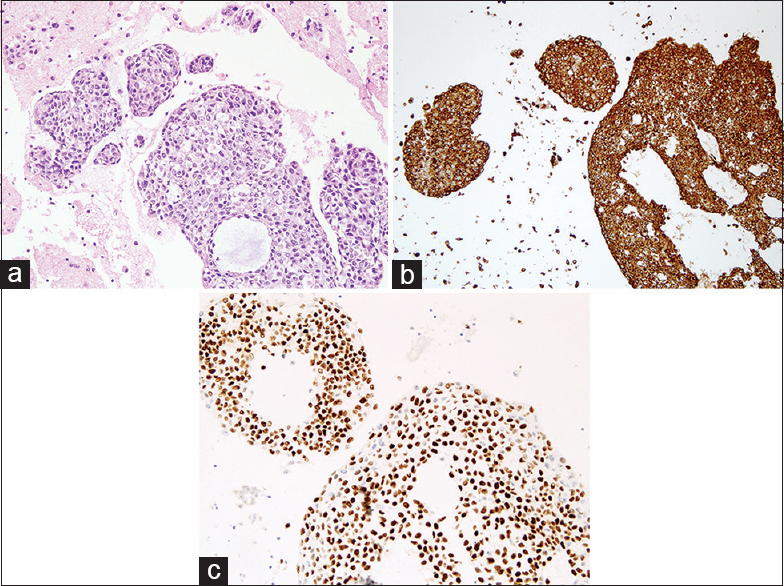
- Cell block tissue section showing (a) clusters of malignant cells (H and E, ×20), (b) positive for p63, and (c) pancytokeratin (AE1/AE3)
DISCUSSION
In 1943, Sheldon was the first to identify myoepithelial tumors as a distinct salivary gland tumor entity.[10] Thefirst reported case of a MECA was described in 1975 by Stromeyer et al. in a case study of a 14-year-old boy with a gingival mass that invaded the anterior maxilla.[11] The 25 and 27 cases of myoepithelial salivary gland tumors reported by Savera et al. in 2000 and Yu et al. in 2003, respectively, are among the largest series reported in English literature.[1213] This category of tumor was added to the classification of salivary gland tumor as a distinct clinicopathological entity by the WHO in 1991.[14]
FNA of MECAs usually yields highly cellular smears with the neoplastic cells arranged singly, small groups, and as large cohesive fragments. The tumor shows a wide variety of cytomorphology that can be divided into 4 main subtypes including plasmacytoid (also known as hyaline cells), epithelioid, spindle, or clear.[1516] Many MECAs exhibit more than one cell type, but even in these, one cell type usually predominates. In plasmacytoid cell type, as in our case, the cells tend to be discohesive and occur mainly in small aggregates of cells separated by a loose, myxoid stroma. The cells are round-to-ovoid with abundant eosinophilic nongranular cytoplasm and eccentrically located nuclei. In epithelioid cell types of MECAs, the neoplastic cells are large polygonal with a moderate amount of dense cytoplasm sometimes focally clear, central ovoid or round nuclei with mild nuclear pleomorphism. Spindle cell types of MECAs consist of a proliferation of spindled cells arranged in interlacing fascicles with central fusiform or cigar-shaped nuclei, eosinophilic cytoplasm with tapered ends. Tumors are hypercellular and have limited myxoid or mucoid stroma. Clear cells, the rarest cell type of MECAs, are polygonal cells with clear cytoplasm. The nuclei are small with wrinkled nuclear membranes and the cells can exhibit a signet-ring or lipoblast-like appearance in some cases.[17] De-differentiated MECA, in which nuclei exhibit clear high-grade undifferentiated features, including bizarre or giant cell forms with loss of myoepithelial marker expression, have also been described.[12] Two main extracellular types of matrix are noted in MECA that include myxoid and hyalinized. The myxoid matrix is considered to be an important clue to myoepithelial differentiation. Needle-shaped, eosinophilic, nonrefractile fibers (collagenous crystalloids) have been described. In some cases, metaplastic changes have been noted including squamous, chondroid, and sebaceous metaplasia. In the current case report, both the FNA of the primary tumor and the metastatic tumor in the fluid show predominant population of plasmacytoid cells with nuclear crowding and overlapping, but other features of malignancy including pleomorphism, prominent nucleoli, mitotic figures, or necrosis were not seen.
Many investigators also observed no discernible histologic features that correlate with biologic behavior, as some very bland tumors had a fatal clinical course, whereas other tumors with marked atypical histologic features did not. The absence of obvious malignant features in most reported cases of MECAs make the diagnosis of malignancy difficult on cytological smears.[18] Savera et al. classify MECA into low-grade lesions and high-grade lesions based on nuclear parameters. The high-grade MECAs displayed nuclear enlargement, nuclear pleomorphism, chromatin clumping, abnormal chromatin distribution, prominent nucleoli, and nuclear membrane irregularities.[1215] There was a relative correlation between cytologic atypia and poor outcome in their series. In another study, Darvishian and Lin reported that cytologic atypia, including pleomorphism, coarse chromatin, and prominent nucleoli, although variably expressed, are seen most frequently in malignant lesions in contrast to benign.[18] None of the benign lesions in his study exhibited pleomorphism, coarse chromatin pattern, or prominent nucleoli. These findings are similar to the results obtained by Chhieng and Paulino, DiPalma et al.[1920] Necrosis, a finding usually associated with malignancy, was noted in MECAs studied by Savera et al. and Nagao et al.[1221] Mitotic figures may be scanty to plentiful (range 3–51 per 10 high power fields) and include atypical forms.[1718] Hornick and Fletcher reported that recurrent/metastatic myoepithelial neoplasms had a higher mitotic rate than the other lesions.[9] Kane and Bagwan also reported that the proliferation index, using ki-67, above 10% is considered diagnostic of malignancy in a myoepithelial neoplasm.[2] However, some authors warn that while this cutoff is useful, it may not be helpful in every case.[22]
Histologically malignant features of myoepithelial neoplasm include infiltrative and destructive into the surrounding structures, multinodular architecture and zonal cellular arrangement, perineural and vascular invasion.[5823] Unfortunately, these features cannot be assessed on cytology sample. Hence, histopathology is mandatory to reach the correct diagnosis.
Determination of myoepithelial differentiation on the sole basis of routine morphology could be difficult given the wide morphologic heterogeneity of the tumor. Reactivity for a cytokeratin including CAM 5.2, AE1/AE3, 34βE12, vimentin, and at least one of the myoepithelial markers including smooth muscle actin, calponin, p63, or S-100 is required.[23] In our present case, most tumor cells show immunoreactivity for AE1/AE3, 34βE12, vimentin, p63, and focal SMA that support myoepithelial phenotype. While S-100 is consistently expressed in many MECAs, the negative reactivity for S-100 in our case does not preclude its diagnosis. In the most recent study of MECA by Kong et al., 11% of the tumors examined did not stain for S-100. In one of the largest case studies of MECA by Kane and Bagwan, 18% of the reported cases did not stain for S-100.[2] Even among the studies that showed positive staining for S-100, the tumors show variability of staining.
Due to its diverse cytological presentation, the differential diagnoses of MECAs are broad and depend on the predominant cell type of the tumor. MECAs that consist mainly of plasmacytoid type could mimic various neoplasms such as cellular pleomorphic adenoma, oncocytic adenoma, melanoma, metastatic carcinoma with plasmacytoid morphology (such as lobular carcinoma of the breast), plasmacytoma, or malignant lymphoma. Pleomorphic adenoma should be included in the differential diagnosis of MECAs, particularly when yielding very little matrix and highly cellular smears.[24] Diligent search for epithelial element and chondromyxoid matrix is recommended before excluding the diagnosis of pleomorphic adenoma. DiPalma et al., Chhieng and Paulino described 2 cases of MECA with abundant metachromatic substances intermixed with small clusters of plasmacytoid neoplastic cells.[1920] Both cases were diagnosed cytologically as a pleomorphic adenoma. The distinction between the cells of oncocytic adenomas and myoepithelial lesions lies in their cytoplasmic features. Oncocytes present with abundant granular cytoplasm, whereas myoepithelial cells display dense nongranular cytoplasm. Melanoma might also be confused with a myoepithelial neoplasm as both may share similar cytologic features such as loosely cohesive polygonal or plasmacytoid tumor cells, prominent nucleoli, and binucleation. Intranuclear pseudoinclusion, a feature associated with melanoma, has also been reported in MECA.[19] Melanomas can be distinguished from myoepithelial cells by the identification of intracytoplasmic pigments and immunoreactivity for melanoma markers, such as HMB-45, MART-1, and tyrosinase. Lobular carcinomas of the breast can be distinguished from myoepithelial lesions by the presence of occasional signet-ring cells, intracytoplasmic canaliculi, and their immunoreactivity for breast cancer markers, include mammaglobin, GCDFP-15, or GATA3. MECAs that consist of largely or exclusively of spindled myoepithelial cells also give rise to a variety of incorrect interpretations and is difficult to differentiate from mesenchymal lesion. In cases where MECAs have spindle cell morphology, the important differentials are sarcomatoid squamous carcinoma, spindle cell melanoma, and mesenchymal neoplasms, such as tumors of smooth muscle, fibroblasts, or Schwann cells. The differential diagnosis with schwannoma may be particularly difficult, since either tumor may have palisading, spindly nuclei. Immunocytochemical studies may be helpful to determine a myoepithelial origin. Myoepithelial cells are immunoreactive for cytokeratin, S-100, smooth muscle actin, and calponin. No other lesion in the differential diagnosis of a spindle cell lesion is immunoreactive for the combination of these markers. When clear cells predominate, a definitive diagnosis may be problematic because many other tumors will share common histologic features. The differential diagnosis encompasses a broad range of possibilities, including clear cell variants of primary tumors such as clear cell acinic cell carcinoma, hyalinizing clear cell carcinoma, clear cell adenocarcinoma of salivary glands, clear cell mucoepidermoid carcinoma, clear cell oncocytoma, and clear cell odontogenic tumors.[525] Metastatic tumors such as carcinomas from the kidney, liver, balloon cell melanoma, and thyroid have the potential for clear cell differentiation and all of them are able to metastasize to the maxillofacial area, with renal cell carcinoma doing so most frequently.[26] Immunohistochemical evaluation and clinical/radiologic correlations are essential in distinguishing these tumors. For instance, clear-cell variant of acinic cell carcinoma is usually periodic acid-Schiff positive and diastase-resistant, and mucoepidermoid carcinomas show positivity for mucin. Metastatic renal cell carcinomas are immunohistochemically positive for vimentin, PAX8, RCC, and CD10.
Complete excision with tumor-free margin remains the first choice of treatment for MECAs. Different studies regarding the recurrence and prognosis of MECA have provided variable data.[12] MECAs are recognized to be a low-grade malignant tumor by the WHO salivary gland classification, due to their low metastatic potential and potential for local recurrence. However, some studies show that the behavior of MECAs is more infiltrative and metastatic than that expected of a low-grade malignancy.[32728]
SUMMARY
Our case is unusual for two reasons: The rarity of its primary location and the metastasis identified in the pleural fluid. Our diagnosis of the pleural fluid specimen was ultimately based on the combination of the patient's clinical history and pathologic findings. The clinical presentation of a large, unresected maxillary sinus tumor and multiple lung and pleural lesions was suspect for a metastatic origin. The similar cytomorphology between the current pleural fluid specimen and the prior surgical FNA and biopsy along with immunohistochemical studies supporting a myoepithelial origin and excluding a lung origin ultimately established the diagnosis of a metastatic MECA to the pleural fluid.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. Each author participated sufficiently in the work and took public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from the Institutional Review Board.
LIST OF ABBREVIATIONS (In alphabetic order)
CT - Computed Tomography
EMA - Epithelial Membrane Antigen
FNA - Fine Needle Aspiration
MECA - Myoepithelial Carcinoma
MRI - Magnetic Resonance Imaging
WHO - World Health Organization.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- World Health Organization Classification of Tumors. Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005.
- [Google Scholar]
- Myoepithelial carcinoma of the salivary glands: A clinicopathologic study of 51 cases in a tertiary cancer center. Arch Otolaryngol Head Neck Surg. 2010;136:702-12.
- [Google Scholar]
- Malignant myoepithelioma of the salivary glands: Clinicopathological and immunohistochemical features. Br J Oral Maxillofac Surg. 1999;37:64-6.
- [Google Scholar]
- Myoepithelial carcinoma (malignant myoepithelioma): First report of an occurrence in the maxillary sinus. Histopathology. 1998;32:239-41.
- [Google Scholar]
- Myoepithelioma of the lung: Report of two cases and review of the literature. Lung Cancer. 1998;20:47-56.
- [Google Scholar]
- A cutaneous myoepithelial carcinoma arising in a papillary eccrine adenoma. Korean J Pathol. 2011;45:644-9.
- [Google Scholar]
- Myoepithelial carcinoma of the stomach: A diagnostic pitfall. World J Gastroenterol. 2015;21:4391-6.
- [Google Scholar]
- Myoepithelial tumors of soft tissue: A clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-96.
- [Google Scholar]
- Myoepithelioma of minor salivary gland origin. Light and electron microscopical study. Arch Pathol. 1975;99:242-5.
- [Google Scholar]
- Myoepithelial carcinoma of the salivary glands: A clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-74.
- [Google Scholar]
- Myoepithelial carcinoma of the salivary glands: Behavior and management. Chin Med J (Engl). 2003;116:163-5.
- [Google Scholar]
- Histological Typing of Salivary Gland Tumors (World Health Organization) (2nd ed). Berlin, Heidelberg, New York: Springer-Verlag; 1991. p. :23-4.
- [Google Scholar]
- Defining the role of myoepithelium in salivary gland neoplasia. Adv Anat Pathol. 2004;11:69-85.
- [Google Scholar]
- Tumors of the Salivary Glands. AFIP Atlas of Tumor Pathology. 4th Series, Fascicle 9 Silver. Spring, Maryland: ARP Press; 2008. p. :341-9.
- [Google Scholar]
- Clear cell neoplasms in salivary glands: Clearly a diagnostic challenge. Ann Diagn Pathol. 1998;2:61-78.
- [Google Scholar]
- Myoepithelial cell-rich neoplasms cytologic features of benign and malignant lesions. Cancer Cytopathol. 2004;102:355-61.
- [Google Scholar]
- Fine needle aspiration (FNA) appearances of malignant myoepithelioma of the parotid gland. Cytopathology. 1996;7:357-65.
- [Google Scholar]
- Salivary gland malignant myoepithelioma: A clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83:1292-9.
- [Google Scholar]
- The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-6.
- [Google Scholar]
- Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med. 2015;139:55-66.
- [Google Scholar]
- Myoepithelial carcinoma (malignant myoepithelioma) of the parotid gland arising in a pleomorphic adenoma. J Clin Pathol. 1998;51:552-6.
- [Google Scholar]
- Primary salivary clear cell tumors – A diagnostic approach: A clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial-myoepithelial carcinoma. Arch Pathol Lab Med. 2002;126:676-85.
- [Google Scholar]
- Metastatic renal cell carcinoma presenting as a parotid tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:554-7.
- [Google Scholar]
- Prognostic factors in myoepithelial carcinoma of salivary glands: A clinicopathologic study of 48 cases. Am J Surg Pathol. 2015;39:931-8.
- [Google Scholar]
- Salivary and lacrimal glands. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. Philadelphia, PA: WB Saunders Company; 2001.
- [Google Scholar]








