Translate this page into:
Prognostic markers in smear preparations for pancreatic endocrine neoplasms: A cytomorphologic study and statistical analysis of 20 potential prognostic features
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Papanicolaou Society of Cytopathology guidelines place low- and intermediate-grade pancreatic endocrine tumors into the “neoplastic, other” category whereas high-grade pancreatic endocrine tumors are placed in the “malignant” category. No attempt was made to stratify pancreatic endocrine tumors in the “neoplastic, other” category by likelihood for metastases. Histologically, pancreatic endocrine tumors are divided into well, intermediate, and poorly differentiated examples based on mitotic count and Ki-67 proliferation index (PI). PI has been used in the evaluation of cytologic specimens utilizing cell block material. Unfortunately, cell block material may not always be available for analysis, and little data exists as to cytomorphologic features in smear preparations which might distinguish between low- and intermediate-grade endocrine neoplasms and predict metastases.
Methods:
We studied 36 cases of Diff-Quik stained smear preparations for 20 morphologic features to determine which best-classified cases into poor and not poor outcome categories. Hierarchical logistic regression analysis was used to determine associations between the morphologic features and outcomes.
Results:
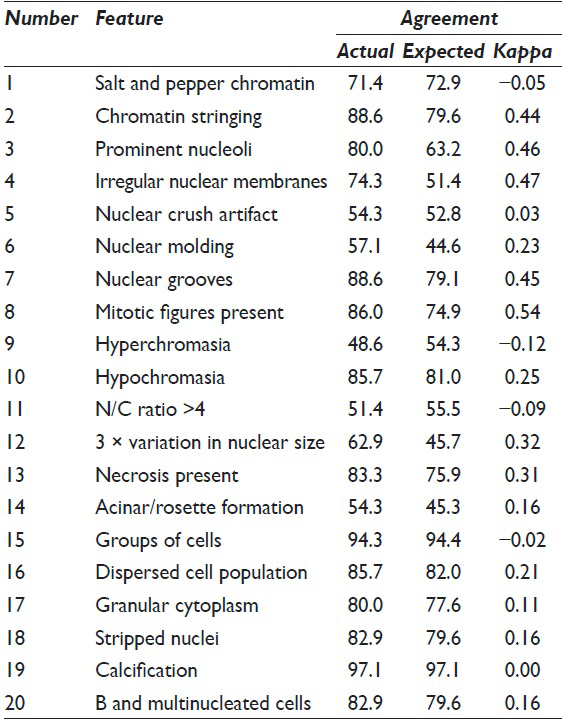
Absolute agreement between raters ranged from 51% to 97% across the 20 morphologic features. About 12 of the 20 morphologic features showed statistically significant associations with poor outcome. Mitoses, irregular nuclear membranes, and 3-fold variation in nuclear size are the best discriminators between poor and not poor outcomes.
Conclusions:
A scoring system was developed utilizing mitoses, irregular nuclear membranes, and 3-fold variation in nuclear size to divide smears of pancreatic endocrine tumors into poor and not poor outcome groups. The scoring system achieved 84% accuracy in separating cases into poor and not poor outcomes.
Keywords
Classification
cytology
grading
pancreatic endocrine tumors
INTRODUCTION
Histopathologic classification of pancreatic endocrine neoplasms is complex and the recent World Health Organization (WHO) classification of tumors of the digestive system divides pancreatic endocrine tumors into three grades based on mitotic rate and proliferation index (PI) as determined by Ki-67 immunohistochemical staining.[123] The WHO classification is formulated for the evaluation of histologic specimens which are paraffin embedded and formalin fixed. Specific recommendations are not given for the evaluation of cytologic specimens. The Papanicolaou Society of Cytopathology (PSC) has developed a set of guidelines for the investigation and diagnosis of pancreatic and biliary tract lesions.[4] These guidelines recommend a categorization scheme and diagnostic criteria. The scheme includes six categories composed of “nondiagnostic,” “negative,” “atypical,” “neoplastic,” “suspicious for malignancy,” and “malignant.”[4] The “neoplastic” category is further sub-divided into neoplasm, benign, and “neoplasm, other”. Following the PSC criteria, pancreatic endocrine neoplasms are placed in either the “neoplasm, other” or “malignant” categories depending on cytologic features.
Several authors have applied the WHO recommendations for classification to formalin fixed paraffin-embedded cell block specimens obtained by fine needle aspiration (FNA).[5678] These studies have shown good correlation between results obtained from cell block specimens and subsequent surgical specimens.[678] Analysis of histologic specimens using the WHO criteria appears to have clinical relevance.[9] Endoscopic ultrasound (EUS) guided FNA biopsies of pancreatic lesions do not invariably result in cell block material and may produce only smear preparations. Such preparations may be stained by either the Diff-Quik or the Papanicolaou methods, and if rapid on-site evaluation is utilized, a significant proportion of the resultant smears are Diff-Quik stained. This may result in the only material available for evaluation being air-dried Diff-Quik stained smears.
Little information exists as to criteria for separating cytologic specimens of pancreatic endocrine neoplasms into low-, intermediate-, and high-grade lesions corresponding to the WHO system. The PSC guidelines recommend categorizing pancreatic endocrine neoplasms as high-grade lesions (malignant, pancreatic endocrine carcinoma) and low- or intermediate-grade lesions (“neoplasm, other”, pancreatic endocrine tumor).[4] While the high-grade malignant lesions are invariably aggressive neoplasms and require an aggressive therapeutic approach, neoplasms cytologically characterized as “neoplasm, other (pancreatic endocrine tumor)” have a variable biological behavior with some showing aggressive features including metastases while others have a more indolent behavior. Malignant neuroendocrine carcinomas in the PSC classification are histologically small cell and large cell neuroendocrine carcinomas with aggressive behavior. The PSC guidelines do no offer criteria for separating pancreatic endocrine neoplasms categorized as “neoplasm, other” into those likely to behave aggressively and those with a more indolent clinical course. Prior studies have demonstrated the utility of cell block material for such separation but criteria for the analysis of smear preparations have not been published. We utilized a series of 36 pancreatic endocrine tumors including low-, intermediate-, and high-grade examples to develop a set of cytomorphologic criteria and a related scoring system to identify pancreatic endocrine neoplasms with a poor outcome in smear preparations. Herein, we report the results of that study.
METHODS
The present study was reviewed by the Institutional Review Board and determined to be exempt. The study follows the tenets of the Helsinki agreement. The case files of the Department of Pathology and Anatomical Sciences at the University of Missouri and the Department of Pathology and Laboratory Medicine at the University of Utah/ARUP Laboratories were searched for all FNA biopsies of pancreatic endocrine neoplasms performed between January 2008 and January 2014. Cases had a clinical follow-up of between 1 and 7 years. Thirty-two cases classified as “neoplasm, other, pancreatic endocrine neoplasm” and four classified as “malignant, pancreatic endocrine carcinoma” were identified and the corresponding slides obtained. The malignant cases included two small cell carcinomas and two large cell neuroendocrine carcinomas. Cases with biopsy or resection specimen were graded using the WHO recommendations and classified as neuroendocrine neoplasm Grade 1, neuroendocrine neoplasm; Grade 2 or neuroendocrine neoplasm; Grade 3. The histologic grading was based on Ki-67 index, mitotic counts, the presence or absence of necrosis, and the presence or absence of angioinvasion. Histology, in some cases, was performed on only biopsy specimens while others were resection specimens. All cases had Diff-Quik stained smears, and these smears formed the basis of the present study. For each case, twenty morphologic features [Table 1] were independently evaluated by two raters. Evaluations were made only on the basis of morphology as seen in Diff-Quik stained slide material. Diff-Quik material was used as this is the preparation type used for rapid on-site evaluation. Raters were blinded to the original diagnoses and did not have access to clinical information. Both raters were board-certified cytopathologists with 8 and 30 years of experience, respectively. The morphologic features scored are listed in Table 1. Chromatin stringing is defined as artifactual pulling of nuclear chromatinic material into long chords due to the smearing process. For cytologic evaluation, mitotic activity was graded as absent (no mitotic figures seen) or present (one or more mitotic figures seen in the smear). Morphologic features were classified as absent or present (either focal or diffuse). Outcomes were classified as poor or not poor. Poor outcomes included metastases, death, or high-grade lesions (pancreatic endocrine carcinoma) as determined by histologic evaluation of surgical specimens.
Associations between morphologic features (absent vs. present) and outcomes (poor vs. not poor) were evaluated using hierarchical logistic regression. Features were included as fixed effects and raters were included as random effects. Inter-rater agreement was assessed with absolute agreement and chance-corrected agreement (Kappa). Discriminatory power was assessed using the area under the receiver operating characteristic (AUROC) curve. Statistical calculations (Kappa, logistic regression, ROC analysis) were performed using the Stata 13 (Stata Corporation, College Station, TX, USA). Recursive partitioning was conducted using the JPM 11.0 (SAS Corporation, Cary, NC, USA).
RESULTS
Absolute inter-rater agreement ranged from 51.4% to 97.1% across the 20 morphologic features [Table 1]. Table 2 shows the corresponding histologic diagnoses and clinical outcome with the cytologic category as poor or not poor outcome. The absolute agreement exceeded 70% for 14 of the 20 features. The chance-corrected agreement (Kappa) ranged from 0.12 to 0.54. Raters achieved Kappa scores of at least 0.3 for seven morphologic features (chromatin stringing, prominent nucleoli, irregular nuclear membranes, nuclear grooves, mitotic figures, necrosis, and 3-fold variation in nuclear size).

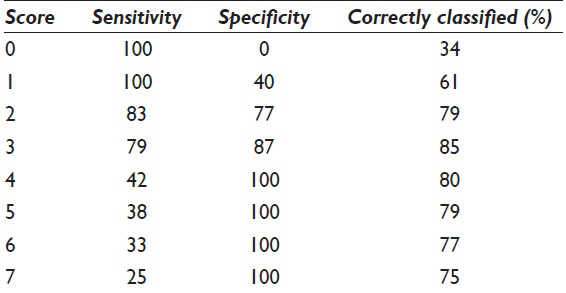
Sensitivity and specificity both range from 4% to 100% [Table 3]. Twelve of the twenty morphologic features showed statistically significant associations with poor outcomes [Table 4]. Overall discriminatory power was highest for irregular nuclear membranes (AUROC = 0.76), mitoses (AUROC = 0.71), 3-fold variation in nuclear size (AUROC = 0.67), and necrosis (AUROC = 0.65).
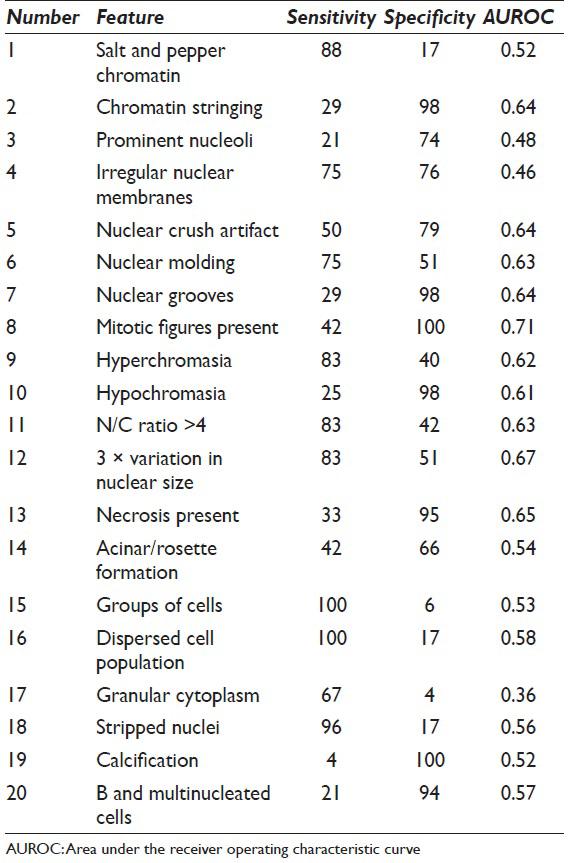

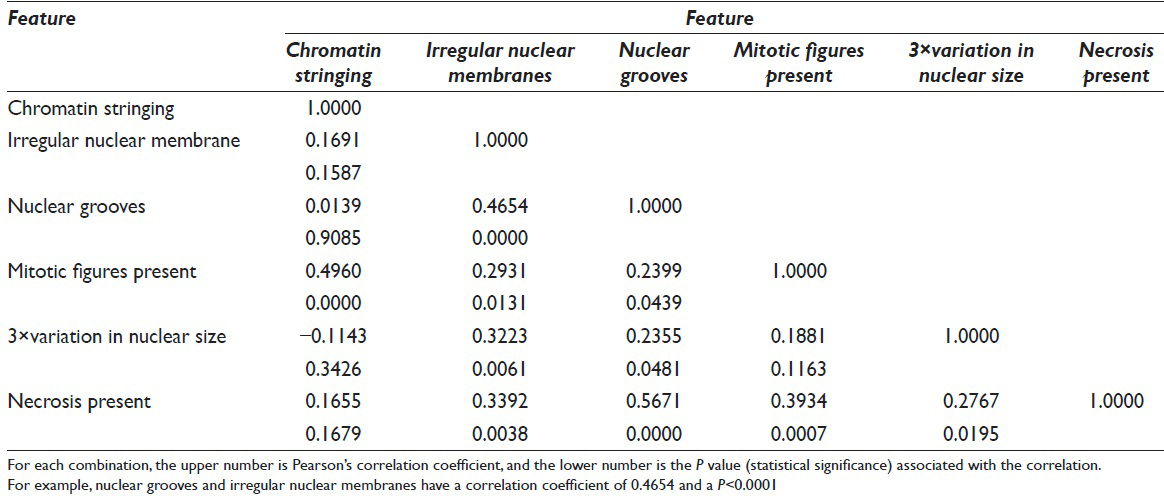
Many of the morphologic features had statistically significant associations [Table 5]. For example, chromatin stringing had a statistically significant correlation with mitotic figures (r = 0.50, P < 0.001). Irregular nuclear membranes had statistically significant correlations with nuclear grooves, mitotic figures, 3-fold variation in nuclear size, and the presence of necrosis.
Recursive partitioning analysis showed that mitotic figures had the highest discriminatory power [Figure 1]. All cases with mitotic figures were associated with poor outcomes (specificity of mitoses = 100%). Twenty-two percent of cases without mitotic figures had poor outcomes. Thus, lack of mitoses could not be used to exclude a poor outcome. Cases without mitoses could be further classified on the basis of irregular nuclear membranes and 3-fold variation in the nuclear size. There were no poor outcomes in cases, which lacked mitoses, irregular nuclear membranes, and a 3-fold or greater variation in nuclear size. Thus, the absence of these three markers is specific for nonpoor outcome. We developed two different scoring systems based on odds ratios [Table 6] and recursive partitioning [Table 7 and Figure 1]. For the odds ratio method, we use the relative odds ratio of three factors:
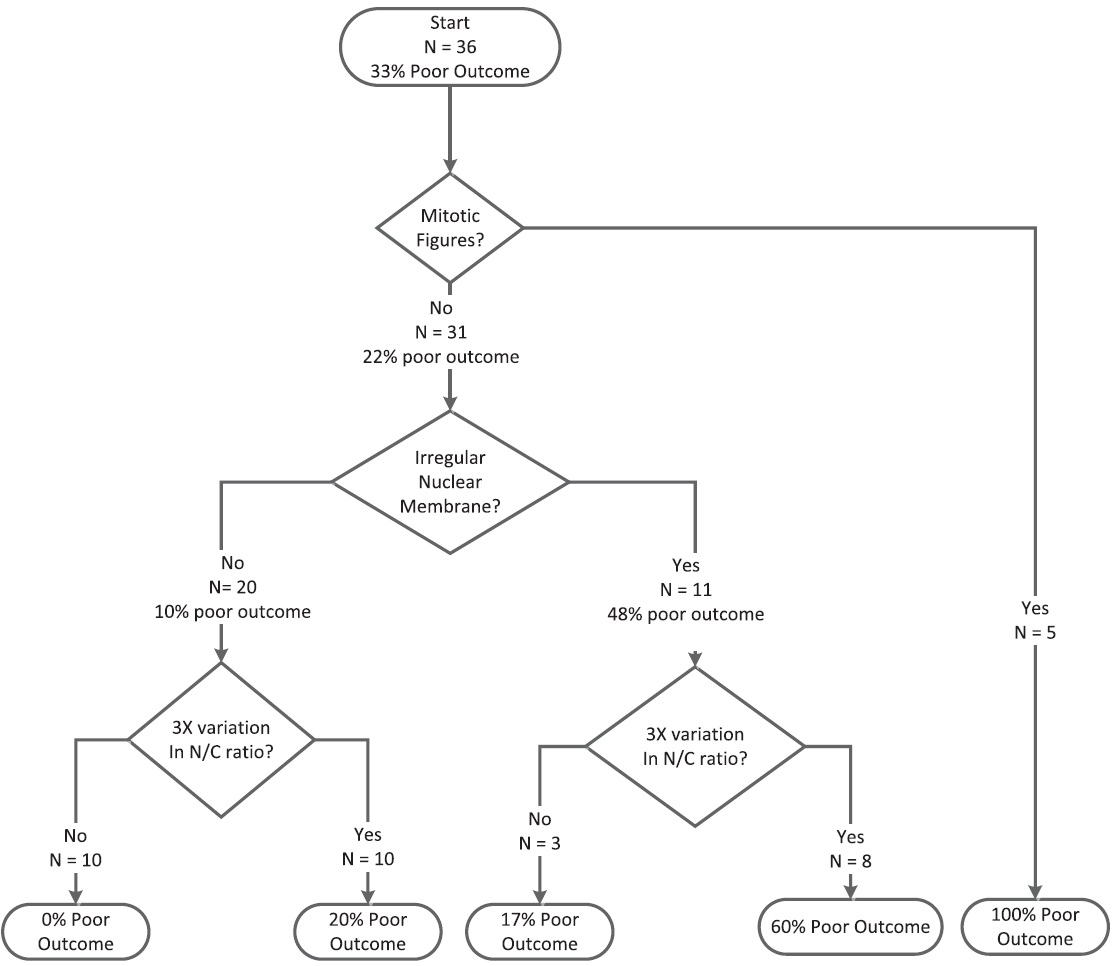
- Classification and Regression Tree for Morphological Features. The tree splits the cases based on the features with the highest discriminatory power (mitotic figures). Then for each branch, the feature with the highest discriminatory power is selected
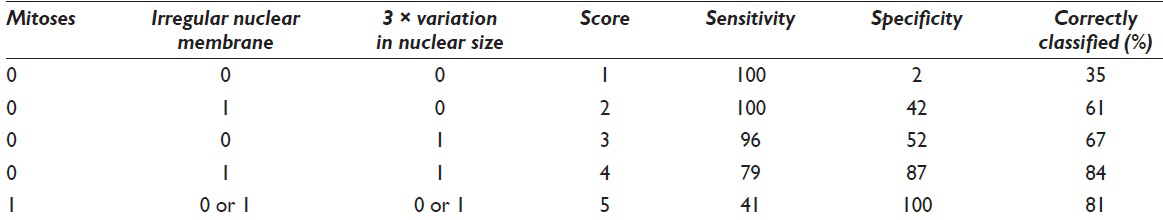
Score = 4*l (mitoses) + 4*l (irregular nuclear membranes) + l (3-fold variation in nuclear size)
where l (X) is the indicator function and l (X) = 1 if feature X is present and l (X) = 0 if the feature X is absent [Table 5]. For the partitioning method, scores for the five categories are shown in Figure 1 [Table 7]. ROC analysis showed that there was no statistically significant difference between the two scoring systems. The area under the ROC curve was 0.90 for both methods. Both methods were able to achieve an accuracy rate of 84%. An ROC curve for the score based on odds ratios is presented in Figure 2. Using the regression tree classification shown in Figure 1, cytologic features stratified malignancy risk between 0% and 100% [Table 1]. Using a risk of malignancy of 60% for assigning a specimen to the poor risk category, cytology correctly assigned specimens to the poor outcome category in 9 of 12 cases (75%) and when high-grade lesions were excluded, correct assignment to the poor outcome category occurred in 5 of 8 cases (62.5%). The scoring system correctly assigned a case to the favorable category (risk 20% or below) in 20 of 24 cases (83%). Histologic grading using the WHO system correctly predicted aggressive behavior in 14 of 13 cases (37.7%) and when high-grade lesions were excluded histologic grading failed to predict aggressive behavior in any case. Figures 3–5 illustrate Grade 1 (risk of poor outcome 20% or less), Grade 2 (risk of poor outcome >20 and ≤60%), and Grade 3 (risk of poor outcome 100%).

- Receiver operating characteristic curved for scoring scheme based on odds ratio

- Photomicrograph of low-grade neuroendocrine tumor. Cells have smooth nuclear contours and <3-fold variation in nuclear size. No mitotic figures were present (Diff-Quik, ×1000)
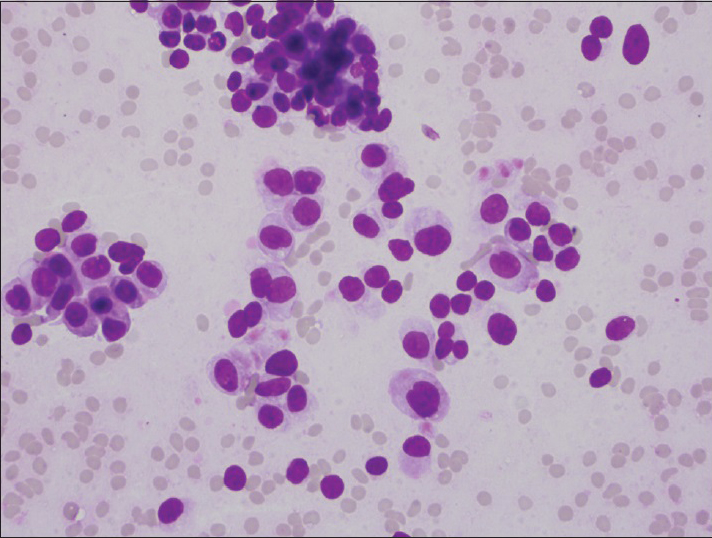
- Photomicrograph of intermediate-grade neuroendocrine tumor. Cells have smooth nuclear contours, but greater than a 3-fold variation in nuclear size (Diff-Quik, ×400)

- Photomicrograph of high-grade (small cell carcinoma) neuroendocrine tumor. Cells show >3-fold variation in nuclear size and irregularities of nuclear membranes. Rare mitotic figures were present in smear (Diff-Quik, ×1000)
DISCUSSION
In 2010, the WHO published a classification for pancreatic endocrine neoplasms addressing both staging and grading issues.[1] They proposed a three-tiered categorization system based on mitotic rate and PI as determined by Ki-67 staining.[1] This classification system has been shown to have clinical utility.[9] The WHO grading scheme is based on histopathologic material, and specific guidance was not given for the classification's application to cytologic material. The PSC developed guidelines for pancreatic and biliary tract cytology which include a categorization scheme with diagnostic criteria.[4] In the PSC guidelines, pancreatic endocrine tumors are placed into one of two categories depending on cytomorphologic appearance. Grade 1 and 2 neoplasms of the WHO recommendations are placed in the “neoplasm, other” category and further categorized as pancreatic endocrine neoplasms. WHO high-grade lesions are placed in the malignant category with further categorization as pancreatic neuroendocrine carcinoma, either small or large cell variants.[4] The WHO grading system for pancreatic endocrine neoplasms is most appropriately performed on resection specimens and not small biopsies as the small biopsy may not be representative of the final resection specimen grade. This limitation on histologic grading may be a reason for the superior predictive value of the cytologic grade over the histologic grade obtained from small biopsy specimens in the present study. Assignment of WHO low- and intermediate-grade pancreatic endocrine tumors to the “neoplasm, other” category of the PSC guidelines is based on the desire to maximize therapeutic options for treating clinicians and their patients. Thus, patients with histologically low- and intermediate-grade pancreatic neuroendocrine tumors could be followed if the patient's clinical status made operative intervention undesirable. These lower grade neoplasms were not assigned to the malignant category because it was believed such an assignment would make it difficult to clinically follow patients with low- and intermediate-grade pancreatic endocrine neoplasms if such neoplasms were designated cytologically malignant. The PSC guidelines made no attempt to distinguish between low- and intermediate-grade pancreatic endocrine tumors of the WHO classification and assigned both to a single category. A number of authors have recommended performing Ki-67 immunohistochemical staining on formalin fixed paraffin-embedded cell block preparations to prognostically stratify pancreatic endocrine neoplasms and have shown good correlation between such cytologic stratification and subsequent histologic grading of surgical resections.[5678] This approach does require cell block preparations which may not always be obtained during EUS-FNA sampling. In some cases, only smear preparations are available for analysis and little data exists as to the possibility of and the methodology for stratifying pancreatic endocrine tumors in smear preparations.
We analyzed a series of 36 pancreatic endocrine neoplasms of which 32 were designated as “neoplasm, other” (pancreatic endocrine tumor) and four were diagnosed as malignant: Pancreatic endocrine carcinoma. We rated the Diff-Quik stained smear preparations for the presence or absence of 20 morphologic features and correlated these features with poor outcome (development of metastatic disease, death or high-grade history morphology on resection). While several of the features analyzed are nuclear features and might be better evaluated on Papanicolaou-stained preparations, the staining preparation generally used for rapid on-site assessment is air-dried Diff-Quik stained material. This preparation appears suitable for assessment of the features utilized in this study. Using these correlations, we developed two different scoring schemes based on odds ratios and recursive partitioning.
The statistical analyses revealed that mitotic count is highly specific for poor outcome. Poor outcomes were unlikely (<20%) if mitoses were not present and the cells demonstrated no nuclear membrane irregularity or 3-fold variation in nuclear size. Cases that lacked mitotic figures on smear preparations but demonstrated nuclear membrane irregularity and a 3-fold variation in nuclear size had a 60% chance of a poor outcome. Morphologic features in our study were highly correlated [Table 3], so that it is difficult to build a predictive model. Once a feature is selected, correlating other features adds relatively little information to the model.
The scoring system developed by the odds ratio method utilized three morphologic factors as shown below:
Score = 4*l (mitoses) + 4*l (irregular nuclear membranes) + l (3-fold variation in nuclear size)
where l (X) is the indicator function and l (X) = 1 if the feature is present and 0 if the feature is absent [Table 6].
For the partitioning method, scores were based on the five categories shown in Figure 1 [Table 7]. Using recursive partitioning analysis, mitotic figures were shown to have the highest discriminatory power [Figure 1]. All cases which had mitotic figures were associated with a poor outcome and specificity for mitoses was 100%. Twenty-two percent of cases without mitotic activity also had poor outcomes. Lack of mitoses could not be used to exclude a poor outcome. Cases lacking mitoses were further classified on the basis of irregular nuclear membranes and 3-fold variation in nuclear size. When mitotic figures, irregular nuclear membranes and 3-fold variation in nuclear size were absent, no poor outcomes were present. The evaluation of specimens for these three markers is a sensitive measure for poor outcome.
ROC analysis demonstrated that there was no statistically significant difference between the two scoring systems.
CONCLUSION
The present study demonstrated that a simple scoring system utilizing the presence of mitotic figures, irregular nuclear membranes, and 3-fold or greater variation in nuclear size can subdivide pancreatic endocrine neoplasms into those with poor and those with a more favorable outcome. Given the prominence of mitotic rate and PI (Ki-67) in the WHO classification, it is not surprising that the presence of any mitotic figures is a strong predictor of poor outcome in smear preparations. The proposed scoring system is applicable to Diff-Quik stained smear preparations and is useful for evaluating both of the PSC categories “neoplasm, other (pancreatic endocrine neoplasm)” and malignant (pancreatic endocrine carcinoma). The scoring system is able to achieve an accuracy rate of 84%. The cytologic system had superior predictive value for poor outcome than did the WHO grading system which had an accuracy of classification of 69% (24 of 36 cases). The cytologic system is most useful when cell block preparations are not available for analysis. From the previously published data, cell block immunohistochemical staining for Ki-67 appears to represent the optimal technique to evaluate grade in cytologic specimens obtained from pancreatic endocrine neoplasms. Because our proposed cytologic scoring system yielded higher accuracy than histologic evaluation of small biopsy specimens, it might be considered as superior to evaluation by the WHO system for small samples. However, several studies have demonstrated the high reliability of cell blocks for grading, so we recommend their use when such material is available. When only smears are available for analysis, our scoring system appears to be a helpful alternative.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they do not have competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Lester J. Layfield, M.D. developed the study concept and design, evaluated the specimens for data collection and wrote the draft article.
Robert L. Schmidt M.D., Ph.D. performed the data analysis and helped prepare the draft article.
Jack Campbell, BA collected the cases for analysis and prepared the data for analysis.
Magda Esebua, M.D. analyzed the cases for data collection.
All authors acknowledge reading and approving the final draft version.
ETHICS STATEMENT BY ALL AUTHORS
The present study was reviewed by the Institutional Review Board and determined to be exempt. The study follows the tenets of the Helsinki agreement.
LIST OF ABBREVIATIONS (In alphabetic order)
EUS - Endoscopic Ultrasound
FNA - Fine Needle Aspiration
PI - Proliferation Index
PSC - Papanicolaou Society of Cytopathology
WHO - World Health Organization
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Neuroendocrine neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System (4th ed). Lyon, France: IARC Press; 2010. p. :322-6.
- [Google Scholar]
- Poorly differentiated neuroendocrine carcinomas of the pancreas: A clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437-47.
- [Google Scholar]
- Determining prognosis in patients with pancreatic endocrine neoplasms: Can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609-15.
- [Google Scholar]
- Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol. 2014;42:338-50.
- [Google Scholar]
- Grading of neuroendocrine tumors with Ki-67 requires high-quality assessment practices. Am J Surg Pathol. 2012;36:1359-63.
- [Google Scholar]
- Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32-8.
- [Google Scholar]
- Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest Endosc. 2012;76:570-7.
- [Google Scholar]
- Cytological Ki-67 in pancreatic endocrine tumours: An opportunity for pre-operative grading. Endocr Relat Cancer. 2008;15:175-81.
- [Google Scholar]
- Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824-33.
- [Google Scholar]








