Translate this page into:
Rhein-induced apoptosis in colorectal cancer cell lines: A mechanistic study of the myeloid differentiation primary response gene 88/toll-like receptor 4/nuclear factor kappa-B signaling pathway

*Corresponding author: Liangliang Mao, Department of Pathology, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, China. m63662697@163.com
-
Received: ,
Accepted: ,
How to cite this article: Zheng X, Zhang X, Hu L, Chen X, Zhao Z, Mao L. Rhein-induced apoptosis in colorectal cancer cell lines: A mechanistic study of the myeloid differentiation primary response gene 88/toll-like receptor 4/nuclear factor kappa-B signaling pathway. CytoJournal. 2025;22:39. doi: 10.25259/Cytojournal_257_2024
Abstract
Objective
Colorectal cancer (CRC) remains one of the leading causes of cancer-related mortality worldwide, and targeted therapies for CRC are urgently needed. This study aimed to investigate the mechanisms through which rhein induces apoptosis in CRC cells, focusing on its influence on the myeloid differentiation primary response gene 88 (MYD88)/toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) signaling pathway.
Material and Methods
Cell Counting Kit-8 assay was conducted, with three non-cytotoxic concentrations of rhein selected for further analysis. Cells were allocated into four groups: control, 10 μM rhein, 20 μM rhein, and 50 μM rhein. Migration ability was evaluated through wound healing assay, and invasive potential was assessed using Transwell invasion assay. Apoptotic rates were determined through terminal deoxynucleotidyl transferase dUTP nick-end labeling staining. The expression levels of apoptosis-related proteins and the key components of the MYD88/TLR4/NF-κB pathway were analyzed by quantitative reverse-transcription polymerase chain reaction and Western blotting after rhein treatment.
Results
The CRC HT-29 and SW480 cells’ capacity to migrate and invade was markedly reduced by rhein treatment. (P < 0.05) while markedly enhancing the apoptotic rates (P < 0.05). This finding was marked by a reduction in the expression levels of B-cell lymphoma 2 (BCL-2) protein and messenger RNA (mRNA, P < 0.05), along with a notable increase in the levels of Bcl-2-associated X and cysteinyl aspartate-specific protease 3 proteins and mRNAs (P < 0.05). The expression levels of MYD88, TLR4, and NF-κB proteins and mRNAs were substantially downregulated (P < 0.05). Adding the TLR4 agonist lipopolysaccharide partially reversed the inhibitory effects of rhein on this signaling pathway, thereby restoring some cellular functional behavior.
Conclusion
Rhein appears to promote apoptosis in CRC cells through the MYD88/TLR4/NF-κB signaling pathway, thus inhibiting tumor initiation and progression.
Keywords
Apoptosis
Colorectal cancer
Myeloid differentiation primary response gene 88/Toll-like receptor 4/Nuclear factor kappa-B signaling pathway
Rhein
INTRODUCTION
Within the digestive tract, colorectal cancer (CRC) is one of the most common and aggressive cancers. A complex interaction of lifestyle, environmental, and genetic variables influences its development, highlighting the need for more study into efficient treatment approaches. The incidence of CRC is on par with that of liver and kidney cancers.[1] At present, CRC holds the third-highest incidence rate among cancers worldwide, contributing to roughly 10% of cases, and ranks second in mortality, causing about 9.4% of cancer-related deaths.[2] Currently, clinical treatment for CRC primarily relies on a multifaceted approach that includes surgery, chemotherapy, biological therapies, and radiotherapy. However, these treatment strategies are associated with several limitations. They often lack high specificity, leading to significant adverse effects, and impose a substantial financial burden on patients. The side effects of these treatments can result in poor treatment adherence, premature discontinuation, or unsatisfactory therapeutic outcomes.[3] Consequently, creating safer and more potent anti-CRC drugs that specifically target the underlying mechanisms of CRC has become a critical and urgent challenge.
Traditional Chinese medicine (TCM) has shown considerable therapeutic potential in the management of CRC, offering advantages such as targeting multiple pathways, minimizing side effects, and enhancing therapeutic efficacy while reducing toxicity. Rhubarb (Rheum palmatum), with thousands of years of therapeutic history, this plant is well known in TCM. Respected for its many medicinal uses, rhubarb has been used in TCM to treat a wide range of ailments, from inflammatory illnesses to digestive issues. Rhein, a strong bioactive substance found in rhubarb, has drawn interest due to its wide range of medicinal applications. Rhein is a useful treatment for inflammatory diseases because of its well-known capacity to regulate inflammation. Furthermore, by preventing the development, invasion, and spread of several cancer types, rhein has demonstrated significant anticancer potential, indicating its promise as a cancer therapy adjuvant. It is also beneficial in treating diabetic nephropathy, which helps people with diabetes improve their renal function and lessen kidney damage. Rhein’s range of medicinal uses is further expanded by its potent antiviral and antibacterial qualities. Because of these several benefits, rhein, which is present in rhubarb, is a substance that may be used to treat a variety of illnesses.[4] Rhein has been shown to effectively inhibit the proliferation of CRC cells while simultaneously promoting apoptosis, functioning as an innovative blocker of signal transduction and transcriptional activation with significant potential in targeting CRC. A combination of in vitro and vivo investigations showed that rhein, in combination with an epidermal growth factor receptor inhibitor, exhibited enhanced efficacy in suppressing the proliferation and migration of CRC cells while promoting apoptosis, thus highlighting its therapeutic potential for treating CRC.[5] The present research investigated the apoptotic impact of rhein on human CRC cell lines HT-29 and SW480 and the underlying mechanisms involved.
The classical cell signaling pathway myeloid differentiation primary response gene 88 (MYD88)/toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) is crucial in controlling inflammatory reactions, oxidative stress and immune modulation in the body. Recent research has shown that this pathway contributes to the pathological progression of multiple cancers.[6,7] MYD88, an adaptor protein containing a TLR domain, interacts with the Toll domain to recruit interleukin-1 receptor-associated kinase (IRAK), leading to IRAK autophosphorylation. This process triggers the downstream activation of the NF-κB signaling cascade, it then sets off a chain of subsequent occurrences, the production and secretion of various cytokines, chemokines, and growth factors. These factors are essential in tumor initiation, progression, and metastasis by facilitating tumor cell growth, invasion, and survival, as well as promoting angiogenesis.[8] In CRC, TLR4 is notably overexpressed in tumor tissues and has been linked to various aggressive clinical features such as lymph node metastasis and advanced TNM staging. TLR4 expression has been correlated with tumor cell proliferation index and microvascular density, indicating that TLR4 could be involved in enhancing CRC cell growth and stromal angiogenesis, thereby influencing lymph node metastasis and clinical staging of CRC.[9,10] Despite the established involvement of TLR4/MYD88/NF-κB signaling in CRC, the exact role of rhein, a bioactive compound with potential anticancer properties, in modulating this pathway remains unclear. Therefore, the objective of this study was to investigate the anticancer properties of rhein, with a specific focus on its potential to induce apoptosis in CRC cells. Through a detailed exploration of the molecular mechanisms by which rhein modulates the TLR4/MyD88/NF-κB signaling pathway, this study sought to uncover the underlying processes that contribute to its anticancer effects. By investigating how rhein interacts with and regulates this critical immune and inflammatory pathway, the research aimed to provide valuable insights into the compound’s potential as a therapeutic agent for CRC.
MATERIAL AND METHODS
Cell culture
Human CRC HT-29 and SW480 cells (HT-29, batch number: CL-0118; SW480, batch number: CL-0223B) were obtained from Wuhan Pricella Biotechnology Co., Ltd. (Wuhan, China) and authenticated through short tandem repeat profiling and mycoplasma testing. Both cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) high-glucose medium (D6429, Sigma–Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (9014-81-7, Sigma–Aldrich, St. Louis, MO, USA) and 1% penicillin-streptomycin (V900929, Sigma–Aldrich, St. Louis, MO, USA) and incubated at 37°C with 5% carbon dioxide.
Cell counting kit-8 (CCK-8) assay for cell viability
Cells were divided into 14 groups: 0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 20, 50, 100, 200, 300, and 400 μM groups. Rhein (R7269, Sigma–Aldrich, St. Louis, MO, USA) was dissolved in a culture medium containing dimethyl sulfoxide (276855, Sigma–Aldrich, St. Louis, MO, USA). The cells were treated with different concentrations of rhein for 24 h. Then, 10 μL of CCK-8 solution (C0038, Beyotime, Shanghai, China) was added, and the cells were incubated for 2 h at 37°C. The absorbance (A) at 490 nm was measured with a microplate reader (1681130, Bio-Rad Laboratories, Hercules, USA).
Scratch assay
The HT-29 and SW480 cells digested with trypsin (15050057, GIBCO BRL, Grand Island, NY, USA) were seeded into a 24-well plate. Once the cells achieved approximately 90% confluence, a sterile 10 μL pipette tip was employed to make a vertical scratch through the monolayer. Following the removal of any leftover debris, the cells were incubated in a serum-free medium for 24 h. The width of the scratch was measured using an inverted microscope (DM3000, Leica, Wetzlar, Germany) at 0 and 24 h. The migration ratio was then calculated using ImageJ software (v1.48, National Institutes of Health, Bethesda, MD, USA).
Invasion assay
Matrix gel (354234, Corning, NY, USA) was diluted with serum-free medium and added to the upper chamber (355467, Corning, NY, USA) at a ratio of 1:40, with 200 μL placed in the upper chamber and dried in a sterile hood. The HT-29 and SW480 cells digested with trypsin were placed in the upper chamber. Subsequently, the lower chamber was filled with DMEM containing 15% FBS. After culturing was conducted under normal conditions for 24 h, the cells that invaded through the membrane were stained by crystal violet staining solution (C0121, Beyotime, Shanghai, China), and five random fields (×200 magnification) were selected to count and photograph the cells under an inverted microscope (DM3000, Leica, Wetzlar, Germany).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)
After treatment, the cells were fixed using formaldehyde and permeabilized with 0.1% Triton X-100 (BL934B, BioSharp, Hefei, China) to facilitate membrane penetration. The TUNEL reaction mixture was then added in accordance with the instructions of the TUNEL apoptosis detection kit (HY-K1091, MedChem Express, Monmouth Junction, NJ, USA) and incubated at 37°C for 60 min and 4’,6-Diamidino-2’-phenylindole (DAPI, BS097, Biosharp Life Science, Hefei, Anhui, China) for 15 min. DAPI is used to stain the nucleus. The apoptotic cells were identified by their reddish-colored nuclei, whereas the normal cell nuclei appeared blue. Each experiment was performed in triplicate under a fluorescence microscope (DM3000, Leica, Wetzlar, Germany) and analyzed by ImageJ software (version 1.48, National Institutes of Health, Bethesda, MD, USA). The apoptosis index (AI) was calculated by determining the percentage of apoptotic cells relative to the total number of cells in each field of view as follows: AI = (number of apoptotic cells/total number of cells) × 100.
Western blotting
After treatment, the logarithmic growth phase HT-29 and SW480 cells from each group were collected, and protein was extracted and quantified using bicinchoninic acid protein assay (P0012, Beyotime, Shanghai, China). Then, 30 μg of protein from each group was loaded onto a polyacrylamide gel for electrophoresis and transferred to a polyvinylidene fluoride membrane (IPFL00010, Merck Millipore, Billerica, MA, USA). The membrane was blocked with 2 mL of bovine serum albumin blocking solution (A1595, Sigma–Aldrich, St. Louis, MO, USA) for 1 h, followed by incubation with primary antibodies (MYD88: ab219413, TLR4: ab217274, p-NF-κB p65: ab247871, NF-κB p65: ab288751, B-cell lymphoma 2 [BCL-2]: ab182858, Bcl-2-associated X protein [BAX]: ab32503, cleaved-cysteinyl aspartate-specific protease 3 [CASPASE-3]: ab214430, and βeta-ACTIN (β-ACTIN): ab8226, Abcam, Cambridge, MA, USA, 1:1000 dilution) at 4°C overnight. Subsequently, the membrane was incubated with secondary antibodies (ab205718 and ab205719, 1:1000 dilution) at room temperature for 1 h. The protein bands were visualized using enhanced chemiluminescence chemiluminescent detection (34577, Thermo Fisher Scientific, Waltham, MA, USA). The membranes were captured using the ChemiDoc Imaging System (Bio-Rad, Hercules, CA, USA). Densitometry analysis was normalized to β-ACTIN, and gray values were analyzed using ImageJ software (version 1.48, National Institutes of Health, Rockville, Maryland, USA).
RNA isolation and quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
The TRIzol reagent (T9424, Sigma-Aldrich, St. Louis, MO, USA) was used to extract the total RNA, which was then incubated for 5 min at 65°C and then for 2 min at 4°C. The PrimeScript RT Reagent Kit (RR037A, TaKaRa, Tokyo, Japan) was used for reverse transcription. As directed by the manufacturer, a quantitative polymerase chain reaction was carried out on a LightCycler96 thermocycler (Roche, Shanghai, China) using the SYBR Premix ExTaq Kit (639503, TaKaRa, Tokyo, Japan). Table 1 contains the primer sequences. The 2−△△CT technique was used to evaluate gene expression, utilizing a control group sample as the reference and β-ACTIN as the internal control.
| Gene | Accession number | Primer sequences |
|---|---|---|
| MYD88 | NM_002468 | F:5'-GAGGCTGAGAAGCCTTTACAGG-3' R:5'-GCAGATGAAGGCATCGAAACGC-3' |
| TLR4 | NM_138554 | F:5'-CCCTGAGGCATTTAGGCAGCTA-3' R:5'-AGGTAGAGAGGTGGCTTAGGCT-3' |
| NF-κB p65 | NM_021975 | F:5'-TGAACCGAAACTCTGGCAGCTG-3' R:5'-CATCAGCTTGCGAAAAGGAGCC-3' |
| BCL-2 | NM_000633 | F:5'-ATCGCCCTGTGGATGACTGAGT-3' R:5'-GCCAGGAGAAATCAAACAGAGGC-3' |
| CASPASE-3 | NM_004346 | F:5'-GGAAGCGAATCAATGGACTCTGG-3' R:5'-GCATCGACATCTGTACCAGACC-3' |
| BAX | NM_004324 | F:5'-TCAGGATGCGTCCACCAAGAAG-3' R:5'-TGTGTCCACGGCGGCAATCATC-3' |
| β-ACTIN | NM_001101 | F:5'-CACCATTGGCAATGAGCGGTTC-3' R:5'-AGGTCTTTGCGGATGTCCACGT-3' |
MYD88: Myeloid differentiation primary response gene 88, TLR4: Toll-like receptor 4, NF-κB: Nuclear factor kappa-B, BCL-2: B-cell lymphoma 2, BAX: Bcl-2-associated X protein, CASPASE-3: Cysteinyl aspartate-specific protease 3, β-ACTIN: βeta-actin, A: Adenine, C: Cytosine, G: Guanine, T: Thymine
TLR4 pathway activation using lipopolysaccharides (LPSs)
LPS, a known activator of the TLR4 signaling pathway, was used to activate the MyD88/TLR4/NF-κB pathway in the experiments. LPS (L5293, Sigma–Aldrich, St. Louis, MO, USA) was dissolved in PBS and applied to the cells at a concentration of 500 ng/mL for 24 h to induce signaling activation.
Statistical analysis
Data were analyzed using GraphPad (GraphPad Software, San Diego, CA, USA). Normality testing was performed using the Shapiro–Wilk test. Normally distributed continuous variables are presented as mean ± standard deviation (x̄ ± s). Analysis of variance was used for comparison of multiple groups, and pairwise comparisons were performed using least significant difference t-test. A P < 0.05 was considered statistically significant.
RESULTS
Rhein inhibits the proliferative activity of CRC cells
Under treatment with different concentrations of rhein, the proliferative activity of HT-29 and SW480 cells was significantly inhibited (P < 0.05) [Figure 1a and b]. CCK-8 assays demonstrated a clear dose-dependent decrease in cell proliferation (P < 0.05), with higher concentrations of rhein leading to more significant inhibition (P < 0.05). When the rhein concentrations exceeded 50 μM, the cell viability of HT-29 and SW480 cells dropped below 80%, indicating significant cytotoxicity. On the basis of the findings, the concentrations of 10, 20, and 50 μM were chosen for further experiments because these doses did not exhibit significant cytotoxic effects.
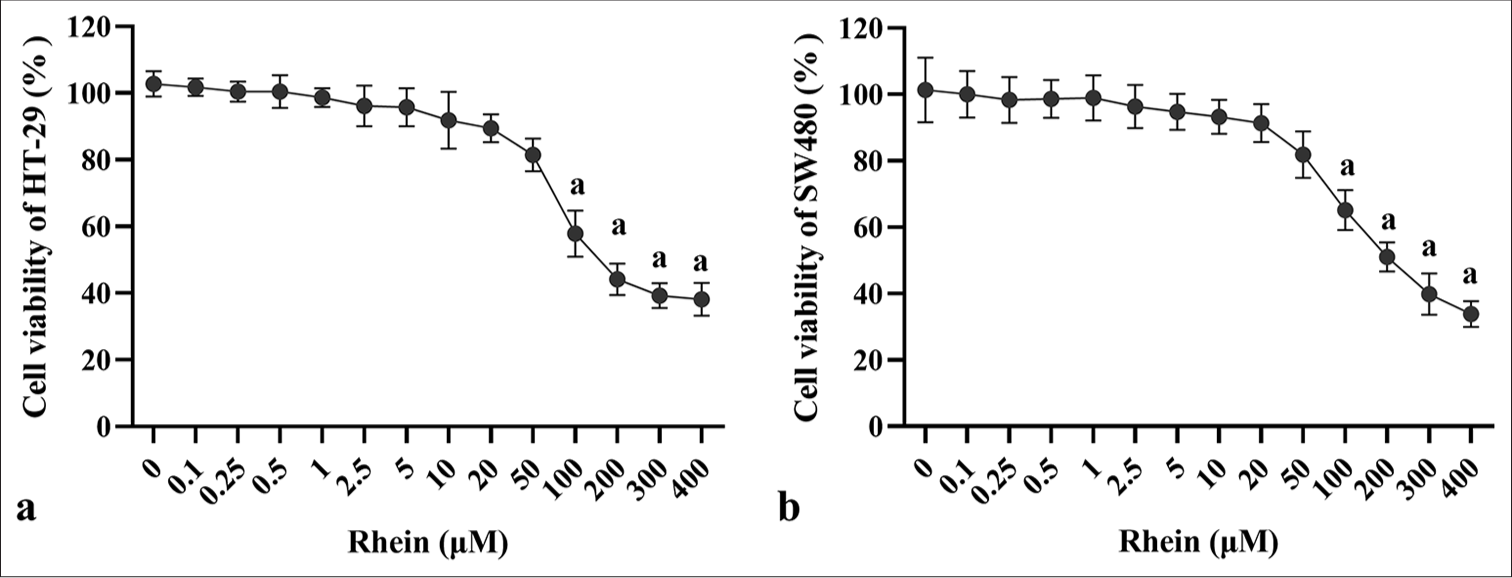
- Effects of rhein on the proliferative activity of CRC cells. (a) Cell viability of HT-29 and (b) SW480 cells after treatment with rhein (x̄ ± s, n = 3). Versus control group: a: P < 0.001., CRC: Colorectal cancer.
Rhein inhibits the migration and invasion of CRC cells
The impact of rhein on the growth inhibition of CRC cells was thoroughly investigated in this study. As shown in Figure 2, treatment with rhein resulted in a substantial decrease in the migration and invasion abilities of HT-29 and SW480 CRC cells (P < 0.05), indicating its potential to inhibit tumor progression and metastasis in CRC. In the scratch wound healing assay, the cells treated with rhein exhibited a significantly slower migration rate, and the extent of wound closure was considerably reduced (P < 0.05) [Figure 2a-d], suggesting that rhein impedes cell movement. Similarly, the rhein-treated groups showed a significantly decreased number of cells that passed the membrane in the transwell invasion experiment (P < 0.05) [Figure 2e and f], highlighting a substantial decrease in the invasive potential of the cells. These results collectively suggest that rhein effectively inhibits the migration and invasion abilities of CRC cells.
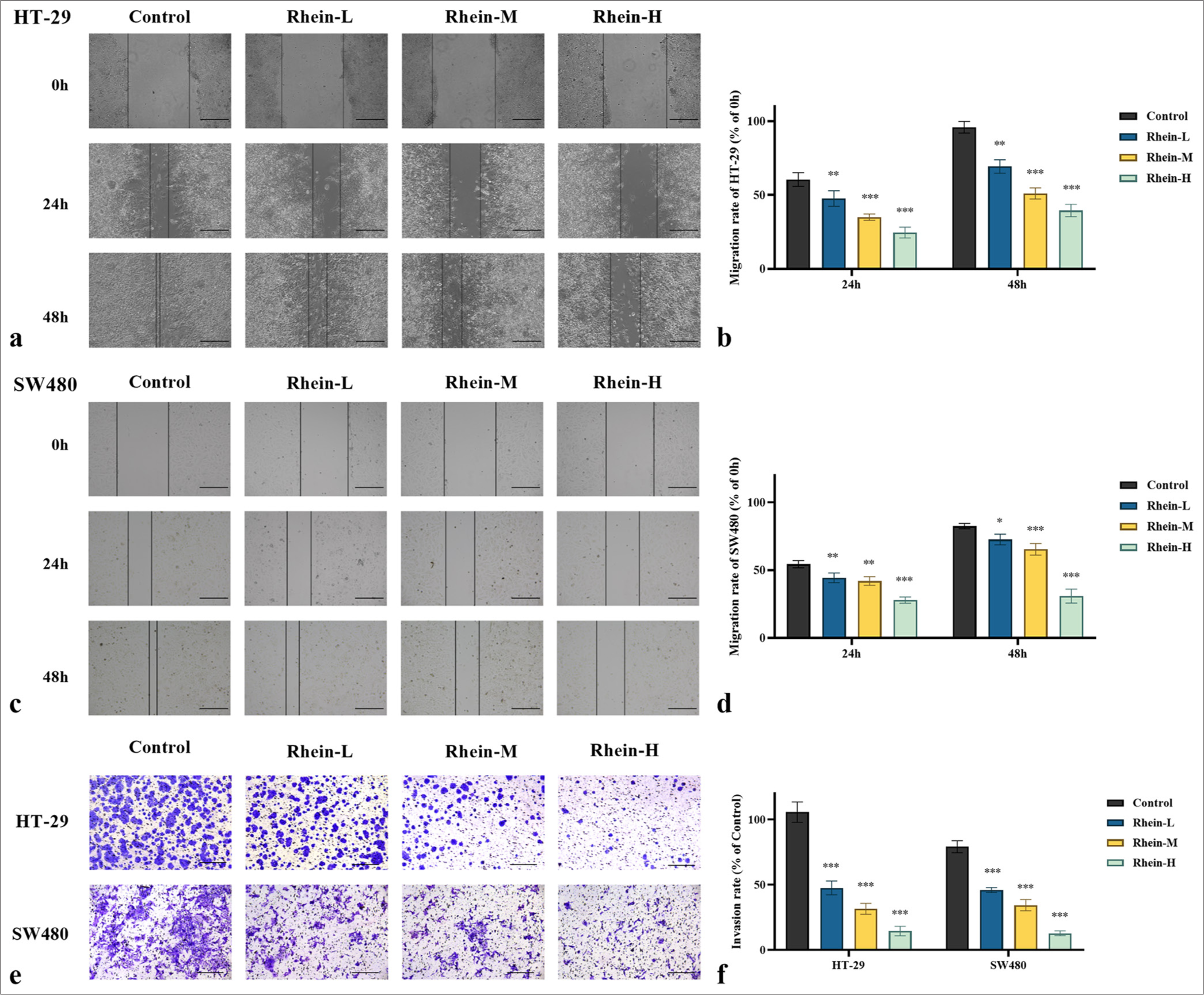
- Effect of rhein on the migration and invasion of CRC cells (x̄ ± s, n = 3). Migration ability of (a and b) HT-29 and (c and d) SW480 cells assessed by wound healing assay. (e and f) Invasion ability of HT-29 and SW480 cells assessed by Transwell assay (0.1% crystal violet staining). Scale bars: 100 μm (×200 magnification). Versus control group, ns: P > 0.05, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. CRC: Colorectal cancer.
Rhein induces apoptosis in CRC cells
The apoptotic levels and the expression levels of genes and proteins linked to apoptosis were assessed using the TUNEL test, qRT-PCR, and Western blotting. Rhein significantly promoted apoptosis in HT-29 and SW480 cells [Figures 3 and 4]. In the TUNEL assay, the rhein-treated cells exhibited increased DNA fragmentation (P < 0.05); however, there was no discernible difference between the rhein-L group and the control group in terms of the quantity of apoptotic cells (P > 0.05) [Figure 3a-d]. The qRT-PCR analysis revealed that after rhein treatment, the messenger RNA (mRNA) expression levels of BAX and CASPASE-3 were significantly upregulated, whereas that of BCL-2 was significantly downregulated (P < 0.05). There was no discernible difference in the BCL-2 mRNA expression between the rhein-L and control groups (P > 0.05) [Figure 4a and b]. Western blotting further confirmed these findings, showing an increase in the active form of CASPASE-3, an upregulation of BAX expression, and a downregulation of BCL-2 expression following rhein treatment (P < 0.05). In the meanwhile, there was no discernible difference in the BCL-2 protein expression between the rhein-L and control groups (P > 0.05) [Figure 4c-f]. The findings indicated that rhein induces apoptosis in CRC cells by modulating key genes and proteins in the apoptosis pathway, providing strong evidence for its potential as an anticancer agent.
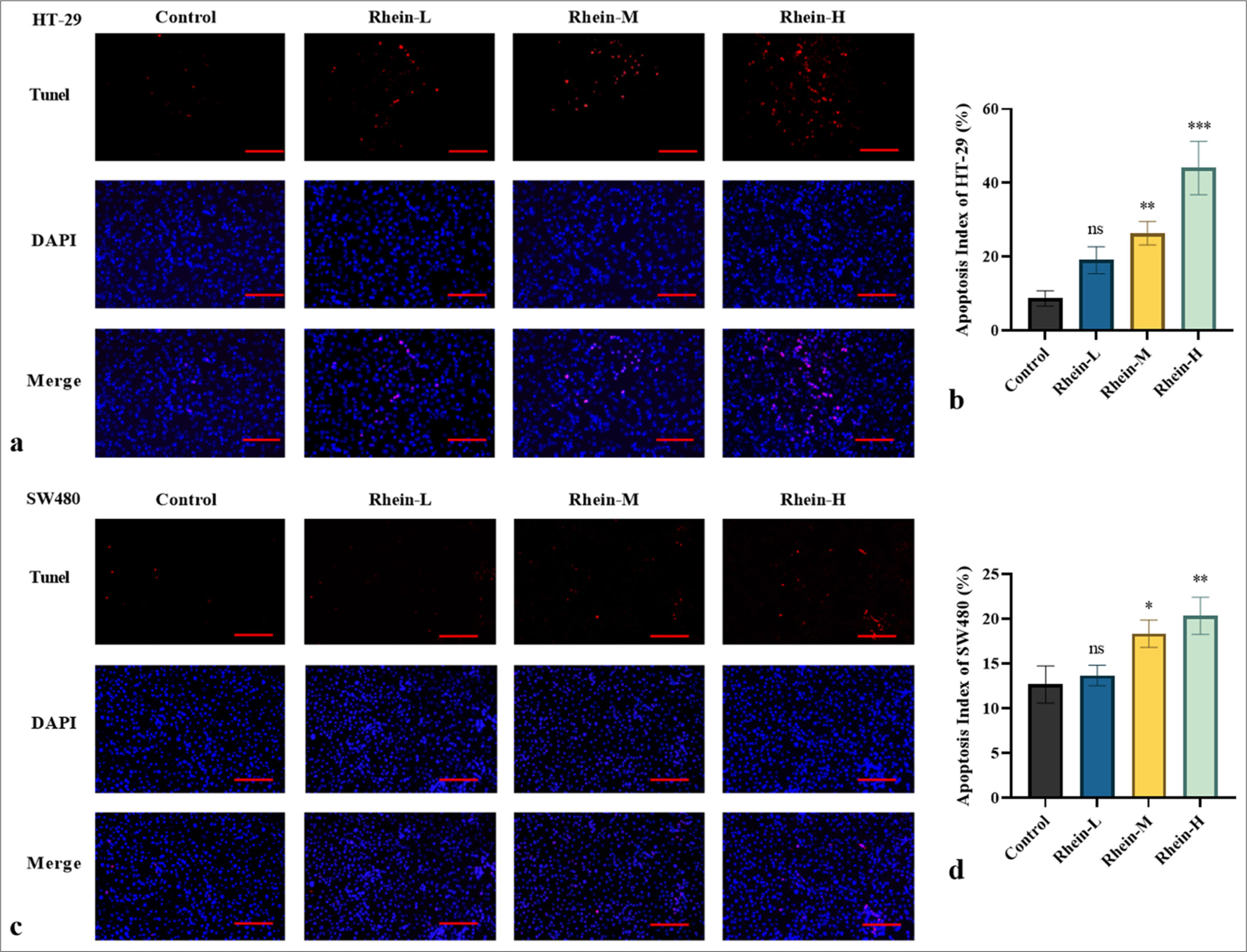
- Effect of Rhein on apoptotic levels in CRC cells (x̄ ± s, n = 3). Apoptotic levels in (a and b) HT-29 and (c and d) SW480 cells assessed by TUNEL assay. Scale bars: 100 μm (×200 magnification). Versus control group, ns: P > 0.05, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. CRC: Colorectal cancer.
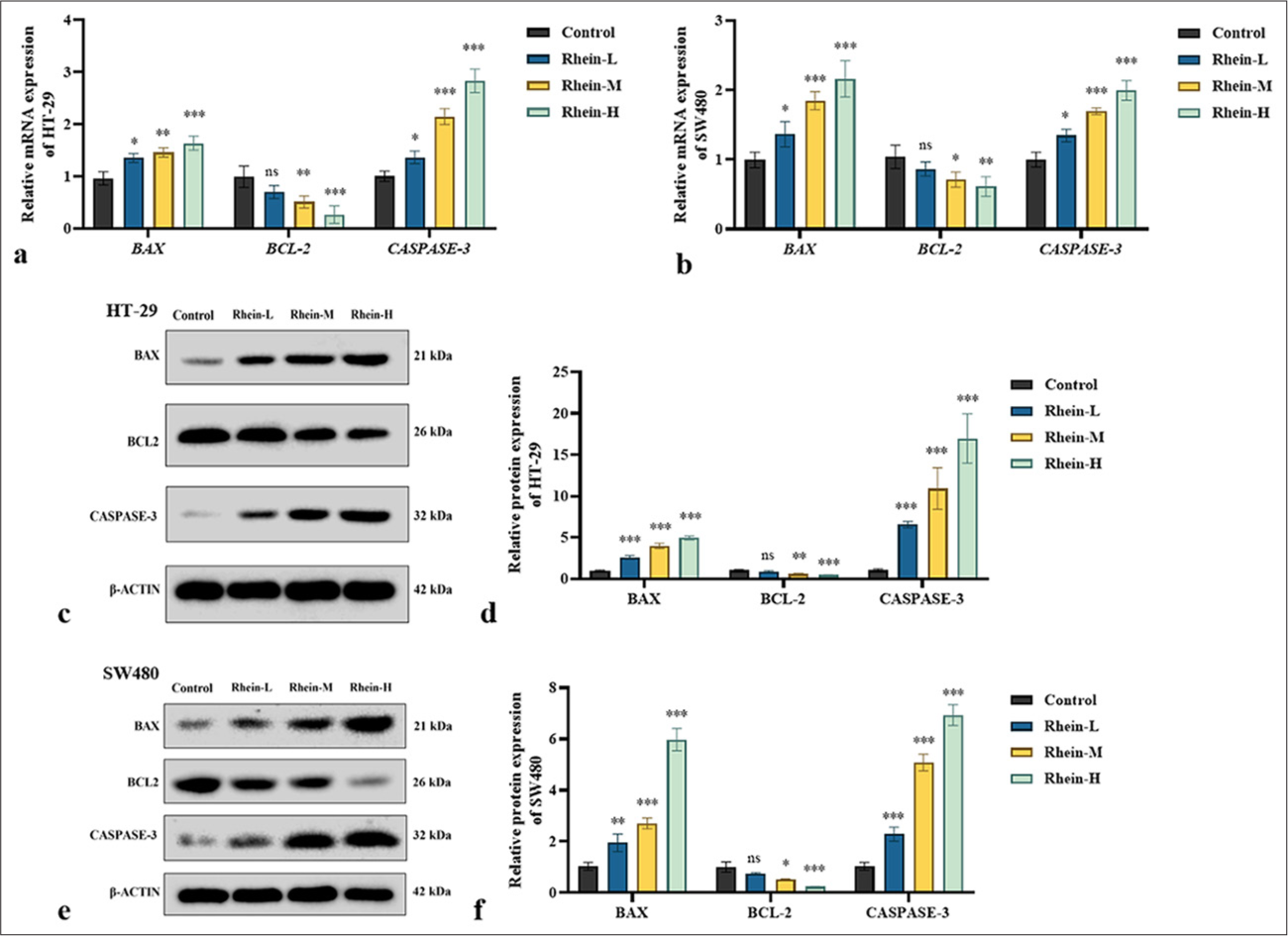
- Effect of Rhein on the levels of apoptosis-related proteins in CRC Cells (x̄ ± s, n = 3). (a and b) mRNA expression levels of BAX, BCL-2, and CASPASE-3 in HT-29 and SW480 cells. (c-f) Protein expression levels of BAX, BCL-2, and CASPASE-3 in HT-29 and SW480 cells. Versus control group, ns: P > 0.05, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. BCL-2: B-cell lymphoma 2. BAX: Bcl-2-associated X protein. CASPASE-3: Cysteinyl aspartate-specific protease 3, CRC: Colorectal cancer, mRNA: messenger RNA.
Rhein inhibits TLR4/MYD88/NF-κB signaling pathway
The impact of rhein on the activity of the TLR4/MYD88/NF-κB signaling pathway in CRC cells was investigated using qRT-PCR and Western blotting. Rhein treatment dramatically decreased the mRNA expression levels of TLR4 and MYD88, as well as NF-κB mRNA levels, according to the qRT-PCR study (P < 0.05) [Figure 5a and b]. Similarly, Western blotting confirmed a decrease in the corresponding protein expression levels (P < 0.05) [Figure 5c-f]. However, in the SW480 cells, the MYD88 expression in the rhein-L group and the control group did not differ significantly (P > 0.05). These findings indicated that rhein exerts its anticancer effects by targeting and suppressing the TLR4/MYD88/NF-κB signaling pathway, which is essential in CRC progression. By inhibiting this pathway, rhein promotes apoptosis, offering potential therapeutic value in CRC management.
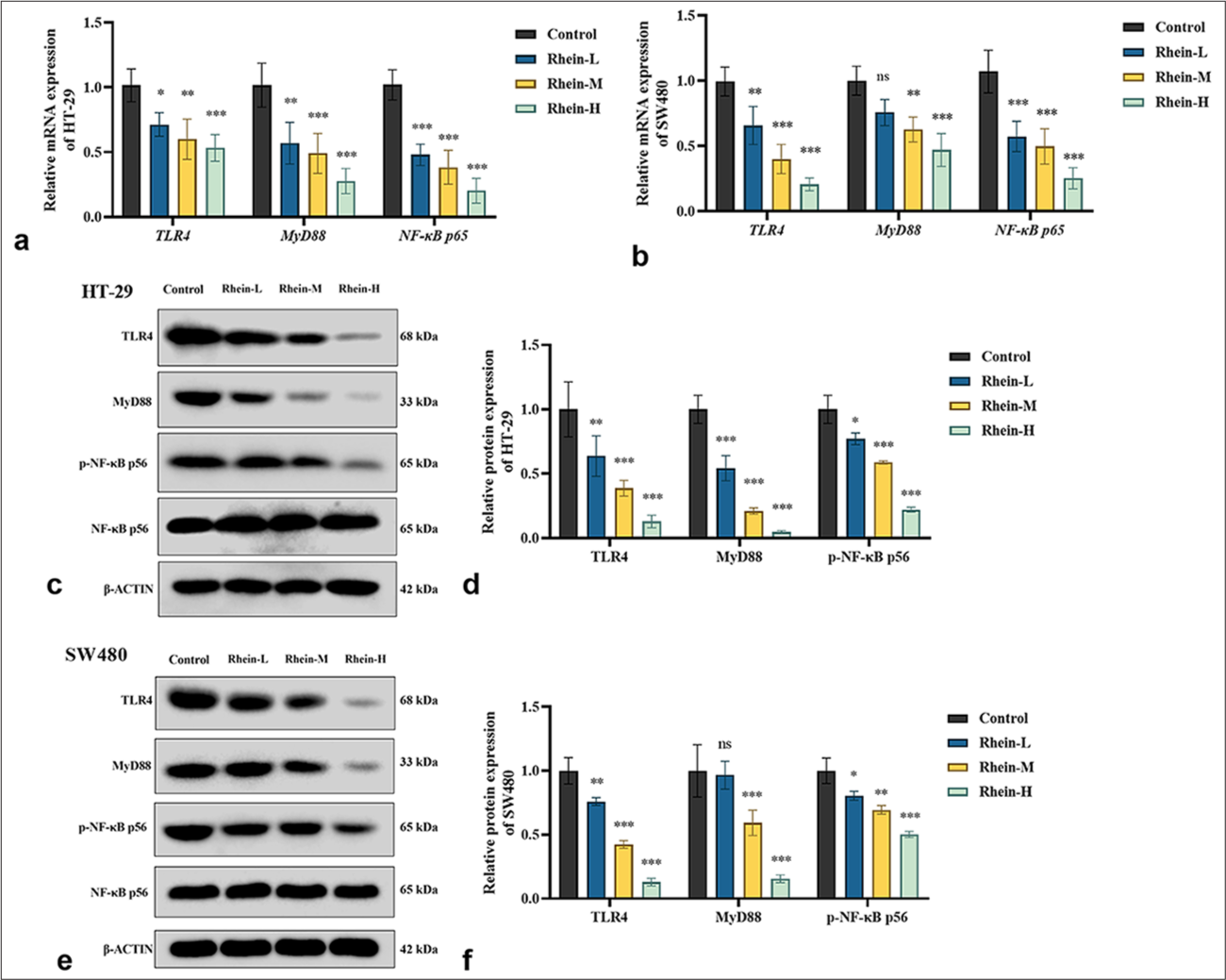
- Effect of Rhein on TLR4/MYD88/NF-κB signaling pathway in CRC cells (x̄ ± s, n = 3). (a and b) mRNA expression levels of TLR4, MYD88, and NF-κB in HT-29 and SW480 cells. (c-f) Protein expression levels of TLR4, MYD88, and NF-κB in HT-29 and SW480 cells. Versus control group, ns: P > 0.05, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. MYD88: Myeloid differentiation primary response gene 88, TLR4: Toll-like receptor 4, NF-κB: Nuclear factor kappa-B, CRC: Colorectal cancer, mRNA: messenger RNA.
Rhein modulates TLR4/MYD88/NF-κB signaling pathway to influence CRC cell apoptosis
Validation experiments were conducted using LPS, a specific activator of the TLR4 pathway, to explore the effect of rhein on the TLR4/MYD88/NF-κB signaling pathway and its involvement in apoptosis in HT-29 and SW480 cells. The TUNEL assay indicated that rhein-induced apoptosis was significantly reduced under TLR4 activation (P < 0.05) [Figure 6a-d]. The findings also showed that the TLR4 activator significantly reduced the expression levels of the pro-apoptotic markers BAX and CASPASE-3, whereas the antiapoptotic BCL-2 was upregulated (P < 0.05) [Figure 6e-j]. The TLR4 activator partially restored the activity of the TLR4/MYD88/NF-κB signaling pathway. Figure 7a-f demonstrates a significant increase in the expression levels of TLR4, MYD88, and NF-κB in the presence of TLR4 activators (P < 0.05). The results indicated that rhein induces apoptosis in HT-29 and SW480 cells primarily by regulating the TLR4/MYD88/NF-κB pathway. The partial reversal of this effect by TLR4 activation provides further evidence of the critical role of this pathway in the apoptotic mechanisms modulated by rhein, underscoring its therapeutic potential in CRC treatment.
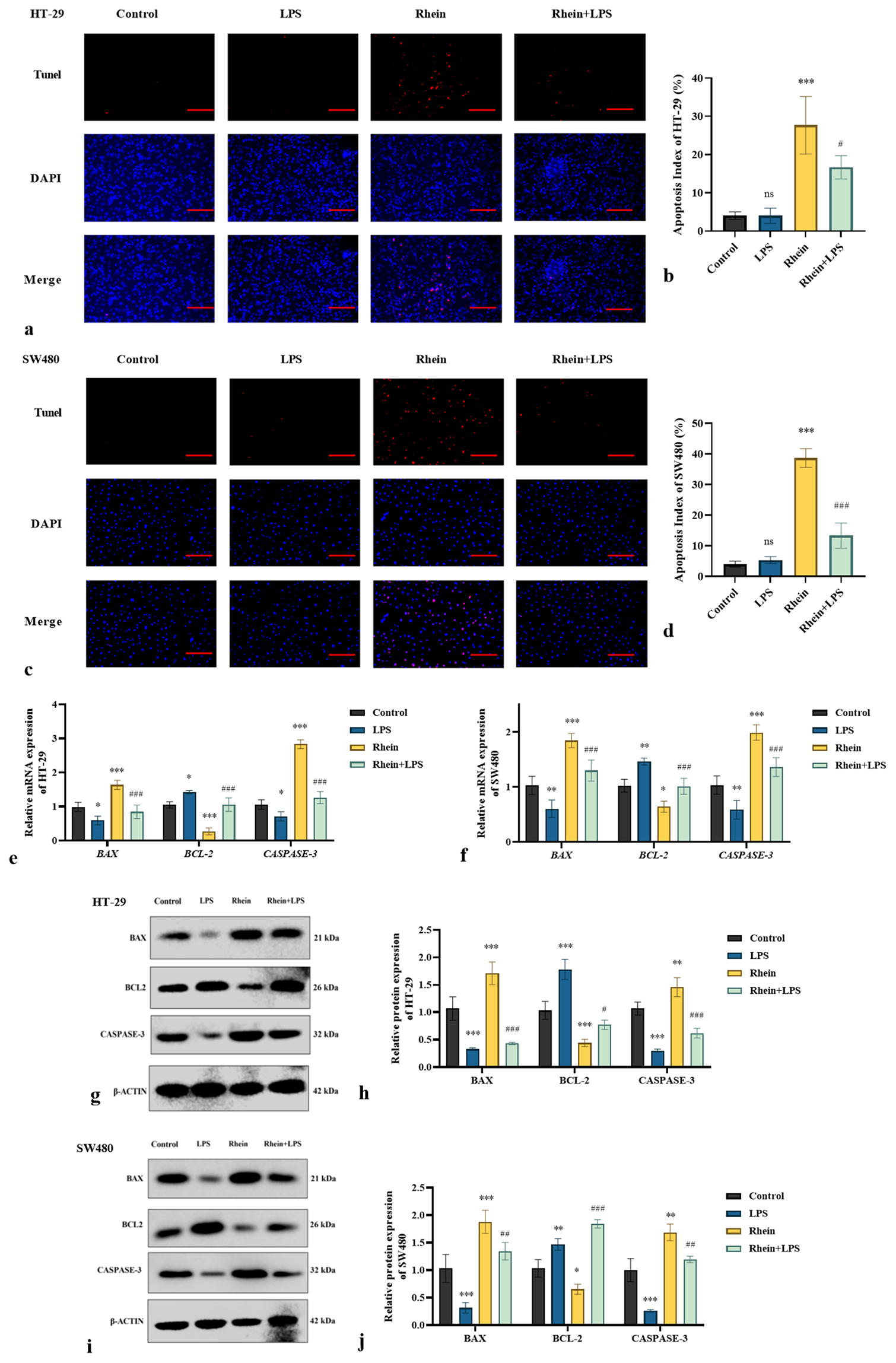
- LPS reverses the pro-apoptotic effects of rhein on CRC cells (x̄ ± s, n = 3). Levels of apoptosis in (a and b) HT-29 and (c and d) SW480 cells assessed by TUNEL assay. (e and f) mRNA and (g-j) protein expression levels of BAX, BCL-2, and CASPASE-3 in HT-29 and SW480 cells. Scale bars: 100 μm (×200 magnification). Versus control group, ns: P > 0.05, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. Versus rhein group, #P < 0.05, ##P < 0.01, ###P < 0.001. BCL-2: B-cell lymphoma 2, BAX: Bcl-2-associated X protein, CASPASE-3: Cysteinyl aspartate-specific protease 3, LPS: Lipopolysaccharides, CRC: Colorectal cancer, TUNEL: Terminal deoxynucleotidyl transferase dUTP nick-end labeling, mRNA: messenger RNA.
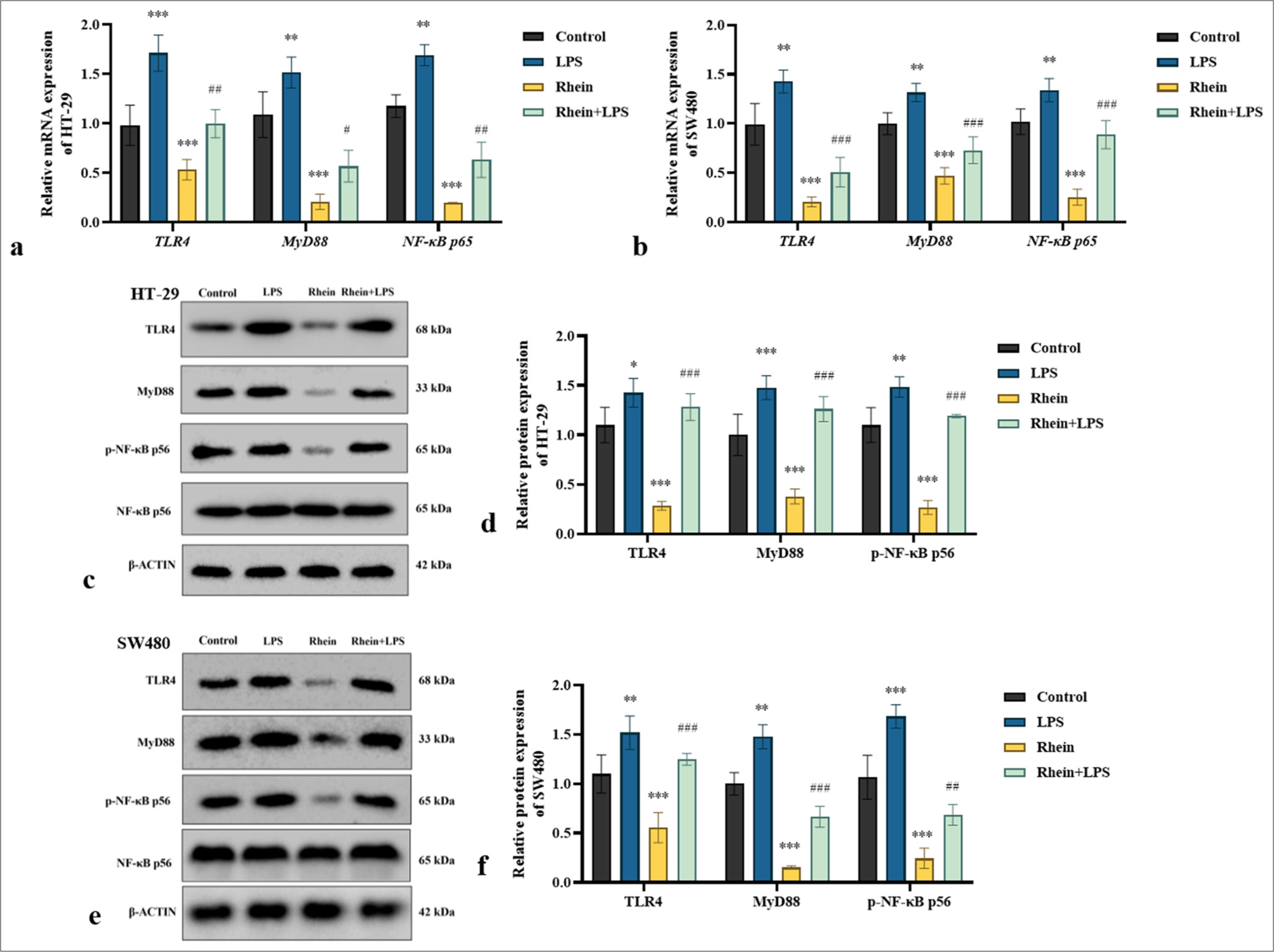
- LPS reverses the effect of rhein on the activation of TLR4/MYD88/NF-κB pathway in CRC cells (x̄ ± s, n = 3). (a and b) mRNA and (c-f) protein expression levels of TLR4, MYD88, and NF-κB in HT-29 and SW480 cells. Versus control group, ns: P > 0.05, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. Versus rhein group, #P < 0.05, ##P < 0.01, ###P < 0.001. MYD88: Myeloid differentiation primary response gene 88, TLR4: Toll-like receptor 4, NF-κB: Nuclear factor kappa-B, LPS: Lipopolysaccharides, CRC: Colorectal cancer, mRNA: messenger RNA.
DISCUSSION
CRC, a prevalent malignancy of the digestive system, is typically managed through surgery, chemotherapy, and targeted therapies. However, these approaches face challenges such as high postoperative recurrence rates, severe chemotherapy-associated toxicity, poor prognosis, and low survival rates among patients. These challenges highlight how urgently we need to comprehend the molecular mechanisms underlying the origin and progression of CRC as well as the development of more efficient and focused therapy approaches. The complexity and heterogeneity of CRC remain major barriers to attaining the best possible patient outcomes, even with advancements in treatment choices. Thus, it is essential to look into the specific molecular processes that lead to CRC development and to discover novel, efficient treatment approaches.[11] Rhein, a natural compound derived from the herb R. palmatum, has demonstrated promising antitumor properties in various cancer types, including CRC. It has shown efficacy in inhibiting tumor growth while exhibiting a relatively high safety profile. Despite these promising characteristics, the precise molecular mechanisms through which rhein exerts its anticancer effects remain poorly understood.[12] Further investigation into the pathways affected by rhein could provide crucial insights into its therapeutic potential as an effective treatment for CRC.
During tumorigenesis, the loss of key regulatory checkpoints in the cell cycle results in the uncontrolled proliferation of cells, a hallmark of cancer development. Prior studies have repeatedly demonstrated that rhein has strong anticancer effects, successfully preventing the development and multiplication of different kinds of cancer cells. These include gliomas, lung cancer, liver cancer, cervical cancer, and ovarian cancer,[13,14] suggesting its potential as a broad-spectrum anticancer agent targeting multiple malignancies. The findings of the present study clearly showed that rhein treatment significantly reduces cell proliferation activity in human CRC HT-29 and SW480 cells, supporting its potential as a therapeutic option for CRC.
Invasion and migration are crucial characteristics of many malignant tumors, contributing to poor prognosis and therapeutic challenges in many types of cancer. The results of this study demonstrated that rhein treatment substantially reduces the scratch closure rate and the number of invasive cells in CRC HT-29 and SW480 cells, indicating that rhein effectively inhibits these critical processes. Similar anti-invasive and anti-migratory effects have been reported for rhein in liver cancer cells,[15] further supporting its broad anticancer properties.
The induction of apoptosis is a fundamental mechanism through which anticancer drugs effectively target and eliminate tumor cells. Rhein has been demonstrated to effectively trigger apoptosis in multiple cancer cell types, including glioma, liver cancer, and breast cancer cells.[16-18] In the present study, rhein treatment led to a notable rise in apoptotic cell count in human CRC HT-29 and SW480 cell lines. Previous research indicated that rhein induces apoptosis in tumor cells through intrinsic pathways. Specifically, in SGC-7901 cells, it has been demonstrated that rhein raises the BAX/BCL-2 ratio, a critical factor in apoptosis regulation. In addition, it increased the expression of cytochrome C, further supporting its pro-apoptotic effects in these cancer cells.[19] Studies suggest that the ability of rhein to trigger apoptosis in tumor cells is strongly linked to mitochondrial dysfunction. In HepG2 cells, rhein enhanced mitochondrial permeability, which led to the release of pro-apoptotic markers.[15] This disruption in mitochondrial integrity contributes significantly to apoptosis induction, promoting cell death in liver cancer cells.
The caspase family is crucial for the process of apoptosis, with CASPASE-3 recognized as a key executioner protein that irreversibly drives cells toward apoptosis. In this study, rhein significantly increased the BAX/BCL-2 ratio in CRC cells, promoting a pro-apoptotic environment. It also increased the expression of activated CASPASE-3 in HT-29 and SW480 cells, indicating its caspase-dependent apoptotic activity. Relevant research has shown that rhein induces apoptosis in human primary hepatocytes (HL-7702) through a caspase-dependent mechanism involving endoplasmic reticulum stress, elevated amounts of intracellular calcium, and cleaved CASPASE-3 expression.[20] The results of the present study align with those of earlier research, suggesting that rhein induces apoptosis in CRC HT-29 and SW480 cells. However, the precise mechanisms responsible for this apoptotic effect remain to be explored in more detail.
The TLR family is widely present in mammalian cells and is a critical group of pattern-recognition receptors essential for detecting pathogen-associated molecular patterns.[21] TLR4 plays a vital role in initiating immune responses by specifically recognizing and binding LPS. Upon activation, TLR4 facilitates the functional maturation of antigen-presenting cells, essential components in enabling the adaptive immune system to identify and combat pathogens. This activation triggers the secretion of various pro-inflammatory mediators, which help orchestrate the body’s defense mechanisms against infections and promote immune responses.[22,23] Once NF-κB is translocated into the nucleus, it can regulate the synthesis and secretion of early inflammatory mediators.[24,25]
The TLR4/MYD88/NF-κB signaling pathway often exhibits abnormal regulation in numerous malignant tumors. Disruption of this pathway not only promotes apoptosis in cancer cells but also influences the immune response in the tumor microenvironment. This interaction significantly enhances the body’s capacity to mount an effective antitumor response. Targeting this pathway provides a promising strategy to suppress tumor growth and improve the therapeutic efficacy of cancer treatments. For instance, adamantane-linked isothiocyanate derivatives have demonstrated the ability to inhibit experimental liver cancer growth by suppressing this signaling pathway, further confirming the essential function of this pathway in cancer progression.[26] These studies highlight the significant role of the TLR4/MYD88/NF-κB signaling pathway in driving the initiation and progression of malignant tumors. The current investigation focused on exploring the impact of rhein on the modulation of this pathway, employing Western blotting to assess changes in protein expression and signaling activation. By examining these molecular alterations, this research aims to provide deeper insights into how rhein may influence key signaling mechanisms involved in tumorigenesis. The results demonstrated a clear dose-dependent decline in the expression levels of TLR4, MYD88, and NF-κB proteins as the concentration of rhein was increased. The observed trend highlights the relationship between rhein concentration and its ability to suppress the activation of the TLR4/MYD88/NF-κB pathway. These results suggest that rhein effectively suppresses the activation of the TLR4/MYD88/NF-κB pathway, potentially contributing to its anticancer properties.
We thoroughly explored how rhein influences CRC cells by examining its mechanisms of action through the use of the TLR4 agonist LPS. LPS is one of the most classical activators of TLR4. After TLR4 on the cell membrane recognizes and binds LPS, the LPS-TLR4 complex initiates the MYD88-dependent signaling pathway at the cell membrane, transmitting the inflammatory signal cascade into the cell, which sustains the cascade reaction. After LPS was added, the inhibitory effect of rhein on this signaling pathway was partially reversed, and certain functional characteristics of the cells were restored. This observation provides further evidence to support the hypothesis that rhein exerts its antitumor activity through the disruption of the TLR4/MYD88/NF-κB signaling pathway. By modulating this critical pathway, rhein may alter the expression of downstream targets involved in tumor progression, thereby inhibiting key processes. It confirms the pivotal role of this pathway in rhein-mediated cellular effects and reveals the possible involvement of the TLR4 signaling pathway in the progression of CRC. The results offer compelling mechanistic evidence supporting rhein as a potential therapeutic agent for CRC while suggesting that TLR4 may serve as a viable target for future targeted therapies, carrying important clinical implications.
The limitation of this study lies in the fact that we have only conducted in vitro experiments using cell lines. Further in vivo experiments with mice will be conducted to address these limitations and explore the mechanisms in more detail.
SUMMARY
This study comprehensively investigated the antitumor activity of rhein on CRC cells, focusing on its molecular mechanisms of action. The findings revealed that rhein significantly suppressed the proliferation, migration, and invasion of CRC cells while effectively inducing apoptosis. These effects were closely linked to the suppression of the TLR4/MYD88/NF-κB pathway. Further validation using the TLR4 activator LPS partially reversed rhein’s inhibitory effects on this pathway, highlighting the crucial role of the TLR4/MYD88/NF-κB pathway in mediating rhein-induced apoptosis. The findings suggest that rhein exerts its anti-CRC effects by disrupting the TLR4/MYD88/NF-κB pathway and provide substantial theoretical support for considering rhein as a potential therapeutic agent for CRC. The TLR4 signaling pathway is proposed to be an important target for future treatment strategies in CRC therapy.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
A: Adenine
AI: Apoptosis index
BAX: Bcl-2-associated X protein
BCL-2: B-cell lymphoma 2
C: Cytosine
CASPASE-3: Cleaved-cysteinyl aspartate-specific protease 3
CRC: Colorectal cancer
DAPI: 4’,6-Diamidino-2’-phenylindole
DMEM: Dulbecco’s Modified Eagle Medium
FBS: Fetal bovine serum
G: Guanine
IRAK: Interleukin-1 receptor-associated kinase
mRNA: Messenger RNA
MYD88: Myeloid differentiation primary response gene 88
NF-κB: Nuclear factor kappa-B
qRT-PCR: Quantitative reverse-transcription polymerase chain reaction
T: Thymine
TCM: Traditional Chinese medicine
TLR4: Toll-like receptor 4
TUNEL: Terminal deoxynucleotidyl transferase dUTP nick-end labeling
β-ACTIN: βeta-ACTIN
AUTHOR CONTRIBUTIONS
XLZ (Xinglu Zheng): Concepts, design, experimental studies, data analysis, manuscript preparation, manuscript editing, and review; XLZ (Xiaolan Zhang): Experimental studies, data acquisition, data analysis, statistical analysis; LFH: Data acquisition, data analysis, XXC: Data acquisition, data analysis, statistical analysis; ZSZ: Data acquisition, data analysis, statistical analysis; LLM: Concepts, design, literature search, experimental studies, manuscript preparation, manuscript editing, and review. All authors meet ICMJE authorship requirements.
ACKNOWLEDGMENT
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval and consent to participate are not required as this study does not involve animal or human experiments.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: This research was funded by the Science and Technology Program of Wenzhou (NO. Y20240295).
References
- Colon and rectal cancer: An emergent public health problem. World J Gastroenterol. 2024;30:644-51.
- [CrossRef] [PubMed] [Google Scholar]
- Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel). 2022;14:1732.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of efficacy, safety and underlying mechanism on traditional Chinese medicine as synergistic agents for cancer immunotherapy: A preclinical systematic review and meta-analysis. J Ethnopharmacol. 2024;338:119035.
- [CrossRef] [PubMed] [Google Scholar]
- Rhein for treating diabetes mellitus: A pharmacological and mechanistic overview. Front Pharmacol. 2022;13:1106260.
- [CrossRef] [PubMed] [Google Scholar]
- Rhein enhances the cytotoxicity of effector lymphocytes in colon cancer under hypoxic conditions. Exp Ther Med. 2018;16:5350-8.
- [CrossRef] [PubMed] [Google Scholar]
- Xiaochai-hu-tang ameliorates tumor growth in cancer comorbid depressive symptoms via modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling pathway. Phytomedicine. 2021;88:153606.
- [CrossRef] [PubMed] [Google Scholar]
- Evolutionary and functional conservation of myeloid differentiation factor 88 (MyD88) in amphibian Xenopus tropicalis. Gene. 2023;865:147332.
- [CrossRef] [PubMed] [Google Scholar]
- Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome P450/epoxyoctadecenoic acid axis via TLR4/Keap1/NRF2 signaling. Cancer Res. 2021;81:4485-98.
- [CrossRef] [PubMed] [Google Scholar]
- Tocotrienol suppresses colitis-associated cancer progression through TLR4 signaling in a mouse model of colorectal cancer. Curr Res Toxicol. 2024;7:100196.
- [CrossRef] [PubMed] [Google Scholar]
- Angelica sinensis aboveground part polysaccharide and its metabolite 5-MT ameliorate colitis via modulating gut microbiota and TLR4/MyD88/NF-κB pathway. Int J Biol Macromol. 2023;242:124689.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of timely cancer diagnosis on age disparities in colon cancer survival. J Geriatr Oncol. 2021;12:1044-51.
- [CrossRef] [PubMed] [Google Scholar]
- Rhein promotes the proliferation of keratinocytes by targeting oestrogen receptors for skin ulcer treatment. BMC Complement Med Ther. 2022;22:209.
- [CrossRef] [PubMed] [Google Scholar]
- The natural agent rhein induces β-catenin degradation and tumour growth arrest. J Cell Mol Med. 2018;22:589-9.
- [CrossRef] [PubMed] [Google Scholar]
- Rhein inhibits the migration of ovarian cancer cells through down-regulation of matrix metalloproteinases. Biol Pharm Bull. 2019;42:568-72.
- [CrossRef] [PubMed] [Google Scholar]
- Rhein induces liver cancer cells apoptosis via activating ROS-dependent JNK/Jun/caspase-3 signaling pathway. J Cancer. 2020;11:500-7.
- [CrossRef] [PubMed] [Google Scholar]
- Rhein induces cell death in hepaRG cells through cell cycle arrest and apoptotic pathway. Int J Mol Sci. 2018;19:1060.
- [CrossRef] [PubMed] [Google Scholar]
- Design and synthesis of novel anti-proliferative emodin derivatives and studies on their cell cycle arrest, apoptosis pathway and migration. Molecules. 2019;24:884.
- [CrossRef] [PubMed] [Google Scholar]
- Rhein induces apoptosis and autophagy in human and rat glioma cells and mediates cell differentiation by ERK inhibition. Microb Pathog. 2017;113:168-75.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of rhein on gastric cancer cells HGC-27 apoptosis and its mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022;38:584-9.
- [Google Scholar]
- Rhein triggers apoptosis via induction of endoplasmic reticulum stress, caspase-4 and intracellular calcium in primary human hepatic HL-7702 cells. Biochem Biophys Res Commun. 2016;473:230-6.
- [CrossRef] [PubMed] [Google Scholar]
- Angelica polysaccharide ameliorates memory impairment in Alzheimer's disease rat through activating BDNF/TrkB/CREB pathway. Exp Biol Med (Maywood). 2020;245:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Taxus chinensis (Pilg.) Rehder fruit attenuates aging behaviors and neuroinflammation by inhibiting microglia activation via TLR4/NF-κB/NLRP3 pathway. J Ethnopharmacol. 2025;337:118943.
- [CrossRef] [PubMed] [Google Scholar]
- Lipopolysaccharide recognition in the crossroads of TLR4 and caspase-4/11 mediated inflammatory pathways. Front Immunol. 2020;11:585146.
- [CrossRef] [PubMed] [Google Scholar]
- Calycosin stimulates the osteogenic differentiation of rat calvarial osteoblasts by activating the IGF1R/PI3K/Akt signaling pathway. Cell Biol Int. 2019;43:323-32.
- [CrossRef] [PubMed] [Google Scholar]
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility. Cancer Biol Med. 2019;16:38-54.
- [CrossRef] [PubMed] [Google Scholar]
- PI3K-AKT pathway protects cardiomyocytes against hypoxia-induced apoptosis by mitoKATP-mediated mitochondrial translocation of pAKT. Cell Physiol Biochem. 2018;49:717-2.
- [CrossRef] [PubMed] [Google Scholar]








