Translate this page into:
Screening technologies for cervical cancer: Overview

*Corresponding author: Dipanwita Banerjee, Department of Gynaecological Oncology, Chittaranjan National Cancer Institute, Kolkata, West Bengal, India. dr.dipanwita@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Banerjee D, Mittal S, Mandal R, Basu P. Screening technologies for cervical cancer: Overview. CytoJournal 2022;19:23.
Abstract
Ever since the introduction of the Papanicolaou (PAP) smear test was published in 1941 in American Journal of Obstetrics and Gynecology, PAP test linked with definitive treatment has prevented millions of women from cervical cancer in the developed countries. Due to limited availability of resources, a lack of infrastructure and difficulty in getting highly trained professionals, widespread implementation of PAP test dependent cervical cancer screening program has not been established in low and middle income countries such as India. Therefore, after availability of non-cytological tests such as visual inspection on acetic acid (VIA) and human papillomavirus (HPV) DNA test, there is a paradigm shift in cervical cancer screening methods. In past two decades, various research work has convincingly established the utility of VIA and HPV test in developing countries. The evidences were evaluated by the World Health Organization (WHO) and recommendations have been recently published for comprehensive cervical cancer control strategies for the low and middle income countries. For any successful screening program, achieving high coverage (>70%) of the target population rather than frequent screening is the most important determinant. It is also equally important to ensure appropriate investigations of the screen positive women to establish the disease and treatment of the screen detected cases of cervical intra epithelial neoplasia (CIN) and cancer. HPV testing is the WHO recommended test for cervical cancer screening especially in view of widespread HPV vaccination in young population leading to lower prevalence of CIN and other HPV related diseases.
Keywords
HPV DNA in cervix cancer
VIA
PAP
HPV in cervical screening
Cervical pre cancers

GUIDING PRINCIPLES OF CERVICAL CANCER SCREENING
Screening target age and screening interval
Cancer screening aims to detect preclinical disease in an apparently healthy population through systematic administration of a simple and safe test to all men/women belonging to a specified target age group. Since the primary objective of cervical cancer screening is to detect the premalignant lesions (known as cervical intraepithelial neoplasia [CIN]), World Health Organization (WHO) recommended screening of women between 30 and 49 years of age.[1] At this age, the women have the highest possibility of harboring the CIN 2 and CIN 3 lesions that are considered as true cervical cancer precursors or high grade lesions. Screening at younger age detects large number of low grade lesions that regress of their own and the women are subjected to unnecessary biopsies and treatment. However, screening of HIV positive women should be initiated whenever they are sexually active. The upper age range can be extended to 55–60 years age if resources permit. The frequency of screening in the screen negative women depends on the screening test being used and the resources available within the program. Too frequent screening should be avoided and the minimum interval between two rounds of screening should be 5 years. Programs with limited resources can extend the interval to 10 years, if a highly sensitive screening test (such as HPV DNA test) with very low possibility of missing cases can be used. Even once a lifetime screening around the age of 40 years can reduce cervical cancer incidence by 30%.[2] Achieving high coverage (>70%) of the target population rather than frequent screening is the most important determinant of success of the screening program.
Confirmation of diagnosis of screen positive women
Administering the screening test is only a component of cervical cancer screening that involves community mobilization to motivate large number of women to participate, training of all levels of service providers, ensuring further assessment of screen positive women and ensuring appropriate treatment, and follow-up of the screen detected abnormalities. It is extremely important to ensure appropriate investigations of the screen positive women to establish the disease and treatment of the screen detected cases of CIN and cancer. Traditionally, colposcopy followed by directed biopsies have been used to confirm diagnosis in the screen positive women and subsequent treatment decisions were made on the basis of histology report. However, this multiple visit approach (at least three) is very inconvenient for the women and the compliance is often poor. Several alternate strategies have been recommended and adopted to reduce the number of visits and improve compliance of the women. The positive predictive value of colposcopy is reasonably high to detect CIN 2/CIN 3 disease and treatment can be done on the basis of colposcopy diagnosis alone. Such strategy of “colposcopy and treat, also known as see and treat” improves compliance to treatment and is convenient to the women though some over-treatment is unavoidable. Over-treatment is acceptable as the treatment methods are simple and safe and the treated women with negative histology will require less intensive follow-up. In more basic settings where organizing colposcopy and histopathology is challenging, a more simpler approach like direct treatment of the screen positive women (“screen and treat”) is also recommended.[1] This will be further discussed in the subsequent sections.
A second screening test is sometimes recommended for the women positive on the primary screening test. The second test is called triaging test and the women positive on both the tests are only referred for colposcopy. Such triaging strategy is used if the primary screening test is less specific and/or if the colposcopy services are insufficient and expensive.
Treatment of women with premalignant disease
To achieve desired effectiveness of program to reduce cervical cancer burden, the screen detected CIN 2/CIN 3 lesions must be treated appropriately. CIN lesions can be treated by ablation or by excision. Ablative techniques are simpler, less expensive, can be performed by trained nonclinicians and have very low complication rates. Two different ablative techniques are available – Cryotherapy and Thermocoagulation; both of them are equally effective and safe even when performed by nurses.[3] A major advantage of ablative therapies is that the treatment can be done in primary care or secondary care settings. The excision methods of treatment include large loop excision of transformation zone (LLETZ) and cold knife conization (CKC). In both the techniques, the entire transformation zone (TZ) is excised and submitted for histopathological evaluation. While LLETZ is much simpler, requires local anesthesia only and has low complication rate, CKC has to be done under regional or general anesthesia, requires higher level of technical expertise and has higher complication rates.
All the screen positive women need to be assessed for suitability of treatment by ablative techniques using the following criteria (with colposcopy or with naked eye after application of 3–5% acetic acid):
Squamocolumnar junction (SCJ) is fully visible
Type 1 TZ
Lesion occupying <75% of cervix
Lesion not extending to endocervix or vagina
There is no suspicion of invasive or glandular disease.
If suitable, the lesions may be treated by Cryotherapy or Thermocoagulation without waiting for histopathological verification. Only the women with lesions not suitable for Cryotherapy should be referred for treatment by exciscion.[4]
Follow-up of the women after treatment is also important. Women treated for CIN 2 or CIN 3 have much higher risk of developing cervical cancer even after 10 years of treatment compared to the normal women.[5] Post-treatment initial follow-up should be done after 1 year either by the screening test or by colposcopy. The protocol for subsequent checkups varies from program to program. Usually, the women are sent back to routine screening if they are normal on 2–3 consecutive rounds of yearly screening.
CERVICAL CANCER SCREENING TESTS
Cervical screening tests such as conventional cytology (PAP smear), liquid based cytology (LBC), human papillomavirus (HPV) testing, and visual inspection on acetic acid (VIA) can detect cervical precancerous lesions in apparently healthy, asymptomatic women.
Cytology
The most widely used cervical screening test is cytology. High-income countries have integrated cytology screening services in medical and public health services and have achieved high coverage through better program organization. This has resulted in substantial declines in cervical cancer incidence and mortality over time.[6] Some of the middle-income countries in South and Central America and in Asia also implemented population-based cervical cancer screening using cytology for a few decades. However, these programs were largely ineffective in reducing cervical cancer burden due to poor coverage with screening, treatment and follow-up care and lack of quality assurance.[7] Majority of the low middle income countries (LMICs) have neither initiated nor have the capacity to initiate and sustain quality assured cytology screening programs in their underdeveloped and fragmented health services with several competing priorities, lack of resources, and trained manpower. Cytology needs good quality laboratory infrastructure, rigorous monitoring and supervision, highly skilled technicians or pathologists and a good system of recalling the screen positives. All these are very challenging to implement in the low resourced countries.
Cytology results are most widely reported using The Bethesda system (TBS); the major categories of 2014 TBS is given in [Table 1].[8] The threshold for positive cytology for referral for triaging/diagnostic investigations such as colposcopy may be at atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) in various settings. All women with atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion (ASC-H), LSIL or high-grade squamous intraepithelial lesion (HSIL) report on cytology must have further evaluation with colposcopy. The management options for ASCUS smears are either repeat smear after 6 months or HPV test (triaging) or direct colposcopy.
| Specimen adequacy |
| • Satisfactory for evaluation |
| • Unsatisfactory for evaluation |
| Interpretation/results |
| Negative for intraepithelial lesion or malignancy |
| Epithelial cell abnormalities: Squamous cells |
| • ASCUS or ASC-H |
| • LSIL, encompassing HPV/mild dysplasia/CIN 1 |
| • (HSIL, encompassing moderate and severe dysplasia, CIS; CIN 2 and CIN 3); (if invasion is suspected), with features suspicious for invasion |
| • Squamous cell carcinoma |
| Epithelial cell abnormalities: Glandular cells |
| • Atypica |
| • Endocervical adenocarcinoma in-situ |
| • Adenocarcinoma |
| Other malignant neoplasms (specify) |
| Adjunctive testing (brief description of the test method and result) |
| Computer-assisted interpretation of cervical cytology (if case examined by an automated device, specify device and result) |
| Educational notes and comments |
ASCUS: Atypical squamous cells of undetermined significance, LSIL: Low-grade squamous intraepithelial lesion, ASC-H: Atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion, HSIL: High-grade squamous intraepithelial lesion, TBS: The Bethesda System, CIN: Cervical intra epithelial neoplasia, HPV: Human papillomavirus
Cytology smears are prepared by spreading the cervical cell specimen collected using a spatula and cervical brush on to a glass slide which is then fixed and stained using Papanicolaou (PAP) staining. Cytology is a highly subjective and provider dependent test with varying performance between laboratories and cytologists reading the smears.
High frequency of cervical inflammation in developing countries contributes to significant amount of inflammatory debris in PAP smear, posing major challenges in interpretation and reporting. The high false negative rate (14–33%) is largely due to limitations of sampling and smear preparation. Quality assurance in preparing, fixing, staining, reading and reporting the smears is critical for accurate results. The sensitivity and specificity of a single, quality assured PAP smear to detect CIN 2+ lesions is around 60– 95%, respectively.[9,10]
LBC
LBC offers an improved test specimen collection with lower frequency of unsatisfactory smears, lower debris, and shorter time needed for interpretation compared to conventional cytology. It is the first technical advancement in cervical cytology in more than 50 years. For LBC, the cervical cell specimen is washed into a vial of liquid transport medium and filtered and a random sample is presented as a thin layer on a slide avoiding overlapping of cells. LBC samples may be used for reflex HPV and other molecular testing (for triaging in case of ASCUS report). However, LBC has more or less equivalent sensitivity and specificity as compared to cytology for the detection of CIN 2 or worse lesions.[11]
HPV testing
HPV testing involves detecting HPV DNA or mRNA of two oncoproteins (E6 and E7) in cervical cell samples collected by pelvic examination or by self-sampling. HPV testing is the most accurate, reproducible and provider independent cervical screening test. Its sensitivity to detect CIN2+ lesions exceeds 90% and CIN3+ exceeds 95%; it is more sensitive but less specific than cytology.[12]
If available, HPV testing is the most suitable test to screen women above the age of 30 years, since HPV negative women can be left alone for at least 7–10 years in view of the high Negative Predictive Value of a negative HPV test for CIN3 and cervical cancer.[13,14] Characteristics of some selected clinical HPV tests are given in the [Table 2].
| Assay technology | HPV types detected | Advantages and disadvantages |
|---|---|---|
| Hybrid capture 2 (HC2) HR HPV | HPV DNA of types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 | • Robust and relatively simple technology • Semi-quantitative viral load estimation possible • Cross-react with non-targeted nononcogenic types • Genotype information not obtained |
| CareHPV | HPV DNA of types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 | • Does not require elaborate laboratory infrastructure • Flexible temperature ranges • Minimal training needs • Run time 2.5 h • Less expensive • Genotype information not obtained |
| GeneExpert | HPV DNA of types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 (+ concurrent genotype information for HPV 16, 18 and 45) | • Results are available in 1–2 h • The test platform is available in many low resourced countries (used to detect MDR TB) • Sample preparation very simple • Still expensive |
| Cervista HPV | HPV DNA of types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 | • Less cross-reactivity to low risk types • Internal control to assess DNA content and integrity • Genotype information not obtained |
| Abbott | Concurrent individual genotyping for types 16/18 and pooled detection of types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 | • Simultaneous detection of types 16/18 allows triaging |
| Cobas HPV HR | Concurrent individual genotyping for types 16/18 and pooled detection of types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 | • Simultaneous detection of types 16/18 allows triaging |
| PreTect HPV | mRNA of E6/E7 of types 16, 18, 31, 33, 45 | • More predictive of lesions with potential for progression • Less sensitive due limited genotype detection • Sample storage conditions more stringent |
| Aptima HPV | mRNA of E6/E7 of types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 | • More predictive of lesions with potential for progression • Less cross reaction with low risk types • Sample storage conditions more stringent |
HPV: Human papillomavirus, MDR-TB: Multidrug-resistant tuberculosis
HPV tests have been widely evaluated and compared with cytology in several randomized controlled trials. A cluster randomized trial in India involving around 135,000 women aged 30–59 years demonstrated that following a single round of HPV testing, a significant 53% reduction in the incidence of advanced cancer (stage II+) and a 48% reduction in cervical cancer mortality could be achieved.[14] In four European randomized trials involving 176,464 women aged 20–64 years, HPV testing was compared with cytology screening. These women were followed up for a median 6.5 years and the relative efficacy of HPV- versus cytology-screening for prevention of invasive cancer was compared. HPV testing provided 60–70% greater protection against invasive cervical carcinomas compared with cytology.[15] Data from the randomized trials in Europe and India[14,15] support initiation of HPV based screening and extension of screening intervals to at least 5 years. The WHO recommends repeat screening after 10 years in HPV negative women in resource limited countries.[16]
Cervical cell samples are collected with a brush in a liquid medium for HPV test. The test positive women need further evaluation by colposcopy. In some settings, HPV positivity may be high and sending all HPV positive women to colposcopy may overburden the services. Reflex cytology testing or genotyping for HPV 16 or 18 may be used to triage HPV positive women before referral for colposcopy; In LMICs, HPV positive women may be triaged by VIA screening in a two-visit “screen and treat” approach.
HPV testing is the most promising test for cervical screening in the future including LMICs, in view of the inevitability of widespread HPV vaccination leading to lower prevalence of CIN. In such a scenario, an objective test such as HPV testing will be the most suitable screening test. Already many European countries, Australia and some Latin American countries have introduced HPV test as the primary screening test. Collection of high vaginal samples by the women themselves (self-collection) is feasible and has almost same accuracy as that of provider collected cervical samples.[17] Such a strategy can significantly improve the compliance of the women and improve the logistics of the program.
Visual screening tests
VIA involves naked eye visualization of the cervix 1 min after the application of 3–5% dilute acetic acid under bright light. Test results are reported as negative, positive or suspicious of invasive cancer and their criteria[18] are given in the [Table 3].
| VIA is reported Negative when any of the following features are seen |
| • No acetowhite lesions on the cervix |
| • Thin transparent acetowhite lesions or faint patchy lesions or lesions without definite margins |
| • Polyp protruding from the external os taking up acetowhite |
| • Nabothian cysts taking up acetowhite and appearing as whitish acne |
| • Faint line-like acetowhitening at the junction of columnar and squamous epithelium |
| • Acetowhite lesions away from the transformation zone |
| • Streak-like acetowhitening |
| • Dot like areas in the endocervix, which are due to grape-like columnar epithelium transiently staining with acetic acid |
| VIA is reported Positive when any of the following features are seen: |
| • Distinct, well defined, dense, opaque or dull white or oyster white acetowhite areas touching the SCJ or touching the external os (if SCJ not seen) |
| • The lesion should have a well-defined margin may or may not be raised from the surface |
| VIA is reported Suspicious of Invasive Cancer when any of the following features are seen: |
| • Visible growth or ulcer on the cervix that bleeds on touch |
| • The growth or ulcer may or may not be acetowhite after acetic acid application |
SCJ: Squamocolumnar junction, VIA: Visual inspection on acetic acid
A positive test is defined by the appearance of well-delineated, dense acetowhite area abutting the SCJ in the TZ of the cervix. It is a suitable test in premenopausal women under 50 years of age when the TZ is fully visualized on the ectocervix. Interpretation of test is difficult in postmenopausal and older women. VIA is not ideal for women above 50 years of age or when the SCJ is not fully visible. [Figure 1] shows images of cervix for different VIA outcome categories.
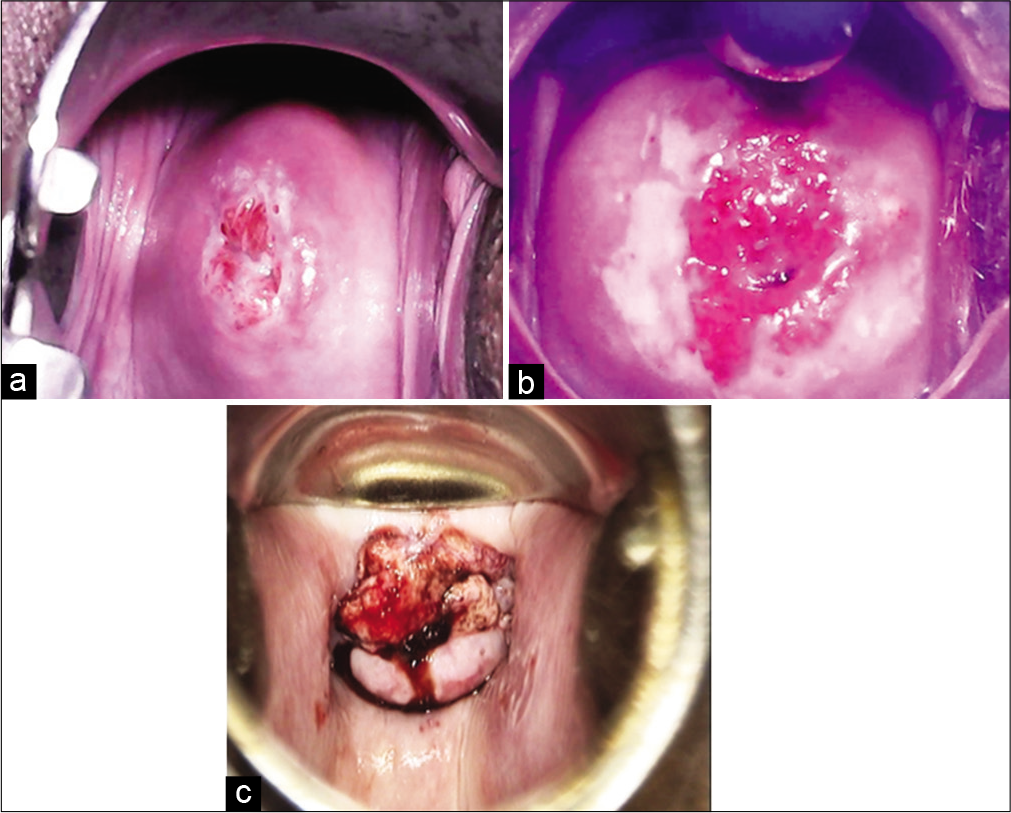
- Visual inspection on acetic acid outcome categories. (a) VIA – Negative, (b) VIA – Positive, (c) VIA - Suspicious of invasive cancer.
VIA is a simple, feasible and affordable point of care test, providing immediate results enabling diagnosis and/or treatment to be carried out in the same visit for screen positive women. Wide range of health professionals including doctors, nurses, midwives and primary health care workers can be trained to perform VIA after a short period of training. The infrastructural need is minimal and the consumables are universally available.
VIA has been extensively evaluated in a variety of settings. Although the pooled sensitivity and specificity of VIA to detect CIN 2 + lesions were 80–92%, respectively, in a pooled analysis,[19] these values varied widely in different settings due to the subjective and provider dependent nature of the test, varying quality of reference standard investigations and differing age groups included in the studies. VIA also suffers from high false positive rates.
VIA screening was followed by a 31% reduction in cervical cancer mortality in a randomized trial in Mumbai[5] In a randomized trial in South India, VIA screening was associated with 25% decline in cervical cancer incidence and 35% reduction in mortality.[20] This evidence culminated in the launching of a population based VIA screening program in the state of Tamil Nadu in India. Large number of nurses have been trained in providing VIA throughout the primary health centers in the state and 55% of targeted 15 million have been screened through the first round of a VIA screening program during 2012–2014.
The visual tests seem to perform better in HIV-infected women than in the general population due to the increased prevalence of large high-grade lesions.[21,22]
The safety, acceptability and effectiveness of a single visit “screen and treat” approach (SVA) in which VIA positive women eligible for cryotherapy, are treated in the same sitting has been well established.[23-25] Women with precancerous lesions not eligible for Cryotherapy may be referred for loop electrosurgical excision procedure for diagnosis and treatment. The SVA paradigm represents a major innovation for scaling up cervical cancer screening in LMICs and has been adopted by many countries in subSaharan Africa and Asia.[26] Primary care practitioners should utilize every opportunity in routine health care interactions to screen women aged 30 years and above with VIA and investigate and treat screen positive women.
Despite all its limitations, implementing VIA screening in low-income countries provides a realistic approach to build up infrastructure and human resources that may facilitate the introduction of affordable HPV screening in future.
Visual inspection after application of Lugol’s iodine (VILI) is another simple inexpensive visual screening technique. The test relies on identification of Iodine negative areas on the cervix after application of Lugol’s Iodine [Figure 2]. VILI has not been widely evaluated and has high false positivity. The consumables are expensive and are not readily available. The test is not yet recommended by WHO for routine screening.
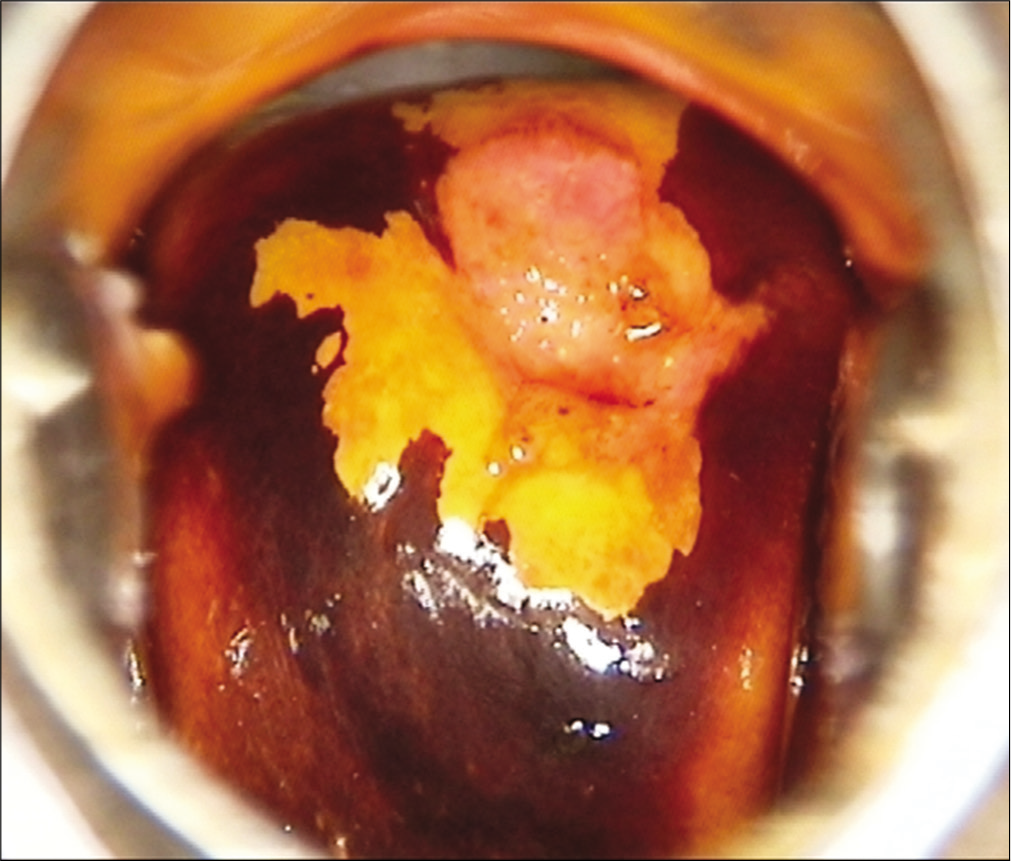
- Bright yellow Iodine negative area after application of Lugol’s Iodine.
Management of women with positive screening tests
The ultimate aim of cervical cancer screening is to reduce the disease burden by detecting the precancerous lesions early and managing them appropriately.
Management of women positive on HPV test
There is no single definitive strategy for management of HPV positive women. The management approaches generally depend on factors such as available resources, logistics, and program guidelines. As cervical cancer is a rare outcome of high risk-HPV infections, management strategies are based on assessment of risk thresholds that identifies the small percentage of women who are likely to develop cervical precancer or cancer. To allow risk stratification and subsequent identification of at-risk women, additional tests (Triage tests) are indicated depending on availability of the tests and feasibility of doing it. Triage tests like Cytology, HPV genotyping for HPV 16/18, test for E6/E7 mRNA, p16/Ki-67 dual staining and VIA may be used for risk-based management.
As majority of the HPV infections are transient, most of the HPV positive women will not have any clinically detectable disease on colposcopy. Referral of all HPV positive women for colposcopy will lead to over burdening of the system, particularly in context of LMICs with limited colposcopy facility. This approach is also likely to increase the cost of public health programs and should be considered for situations where performing triage tests are not feasible or ensuring follow-up of positive women is challenging.
Direct treatment of HPV positive women has been widely promoted, especially in LMICs, to ensure a linkage between screening and treatment and minimize loss to follow-up. In settings where colposcopy and histopathology services are not available, the WHO recommendation of “screen and treat” strategy has strongly encouraged treatment of HPV positive women by ablative treatment methods such as cryotherapy, provided the lesion suits the eligibility criteria for ablation.[4] Various management strategies for HPV positive women is summarized in [Figure 3].
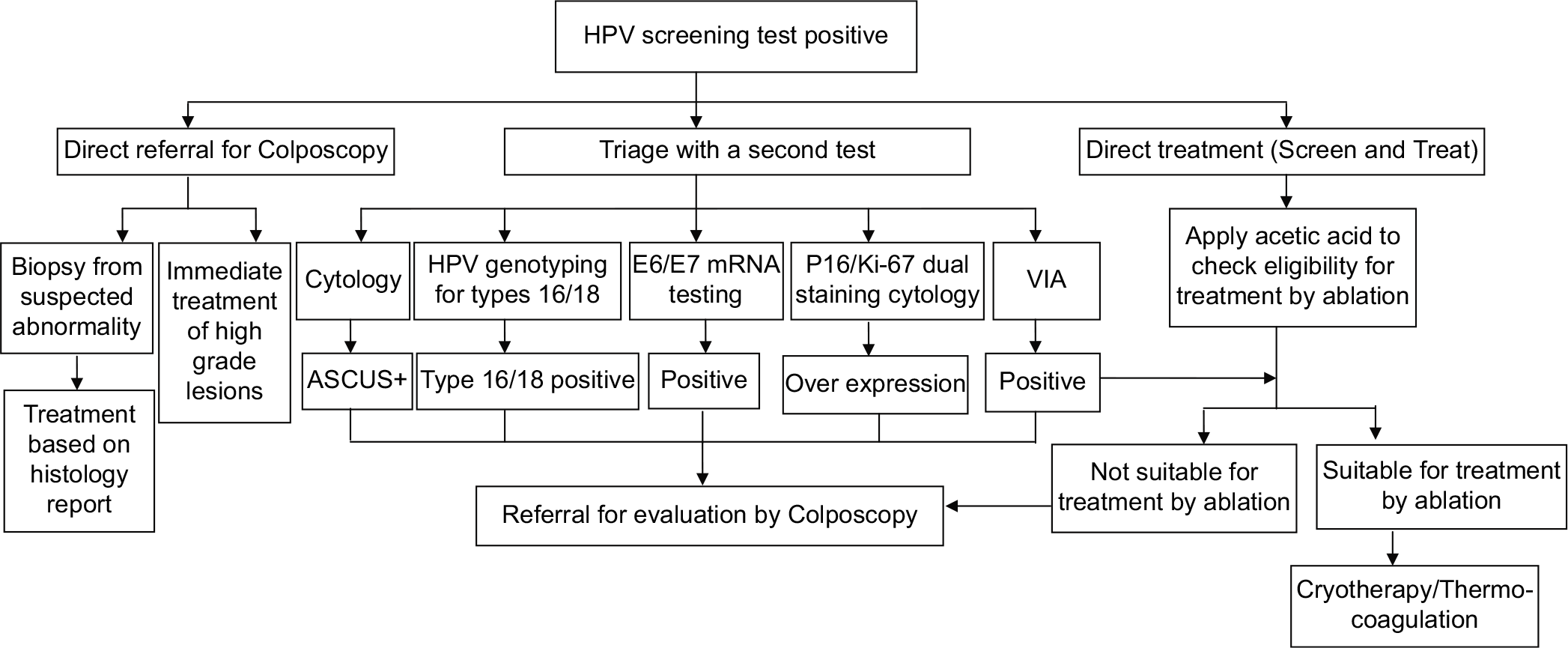
- Flowchart on management of women positive on HPV test.
Management of women positive on VIA test
VIA is a real time test that allows for taking treatment decisions in the same sitting as screening. In the context of LMICs where there is no or limited facility for colposcopy and histology, the WHO guidelines for “screen and treat” strategies recommend immediate treatment of VIA positive cases by ablative method if the lesion characteristic fulfills the criteria for ablative treatment.[4] In situations where Colposcopy and biopsy facilities are available, the VIA positive women can be referred for evaluation by Colposcopy. Treatment can be done either in the same sitting as Colposcopy (“See and treat”) or based on histopathology report. The management options for VIA positive women are given in [Figure 4].
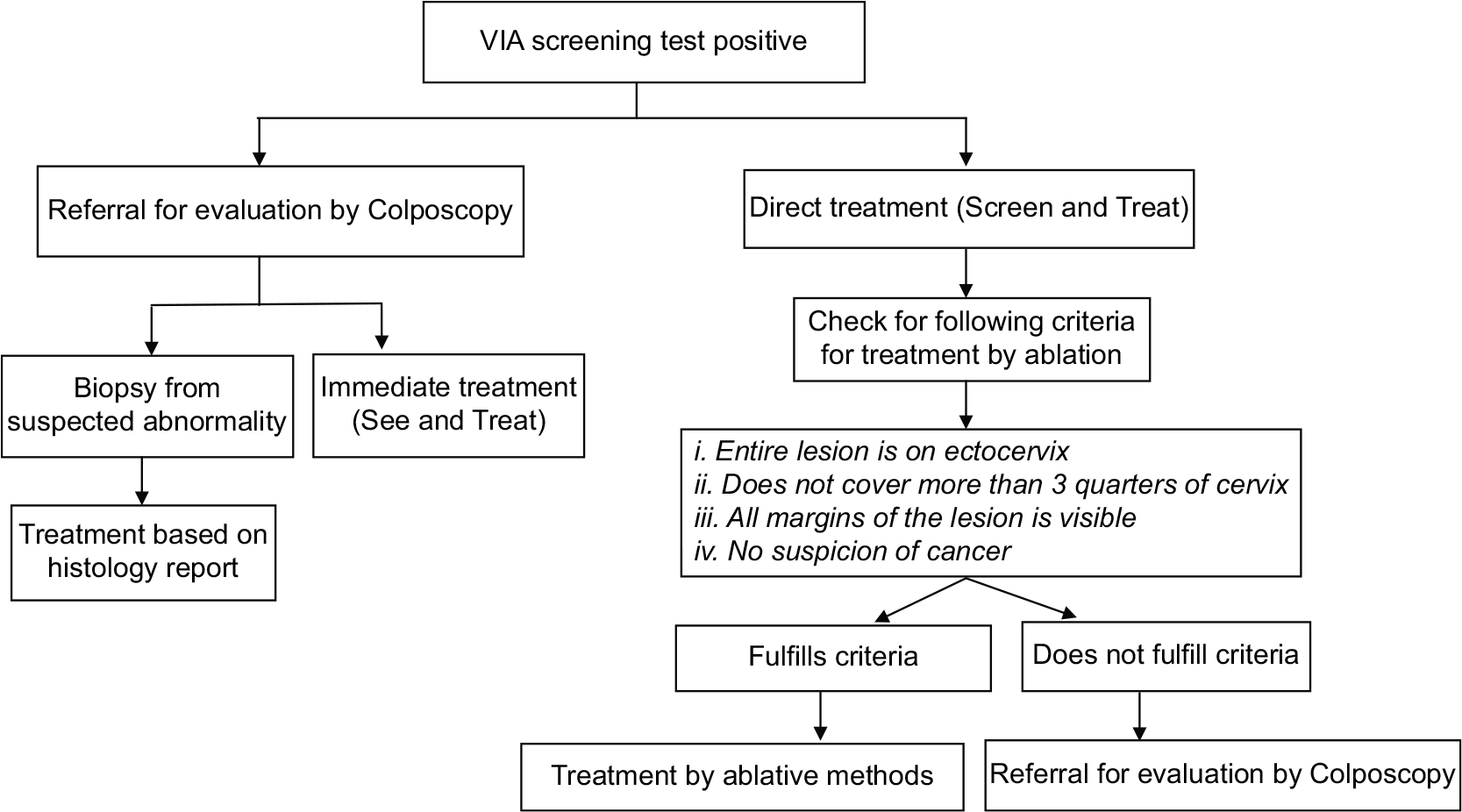
- Flowchart on management of women positive on VIA test.
Management of women with cytological abnormalities
The management options for women with cytological abnormalities are presented in [Figure 5] and discussed in details in the chapter “Role of colposcopy in management of women with abnormal cytology.”
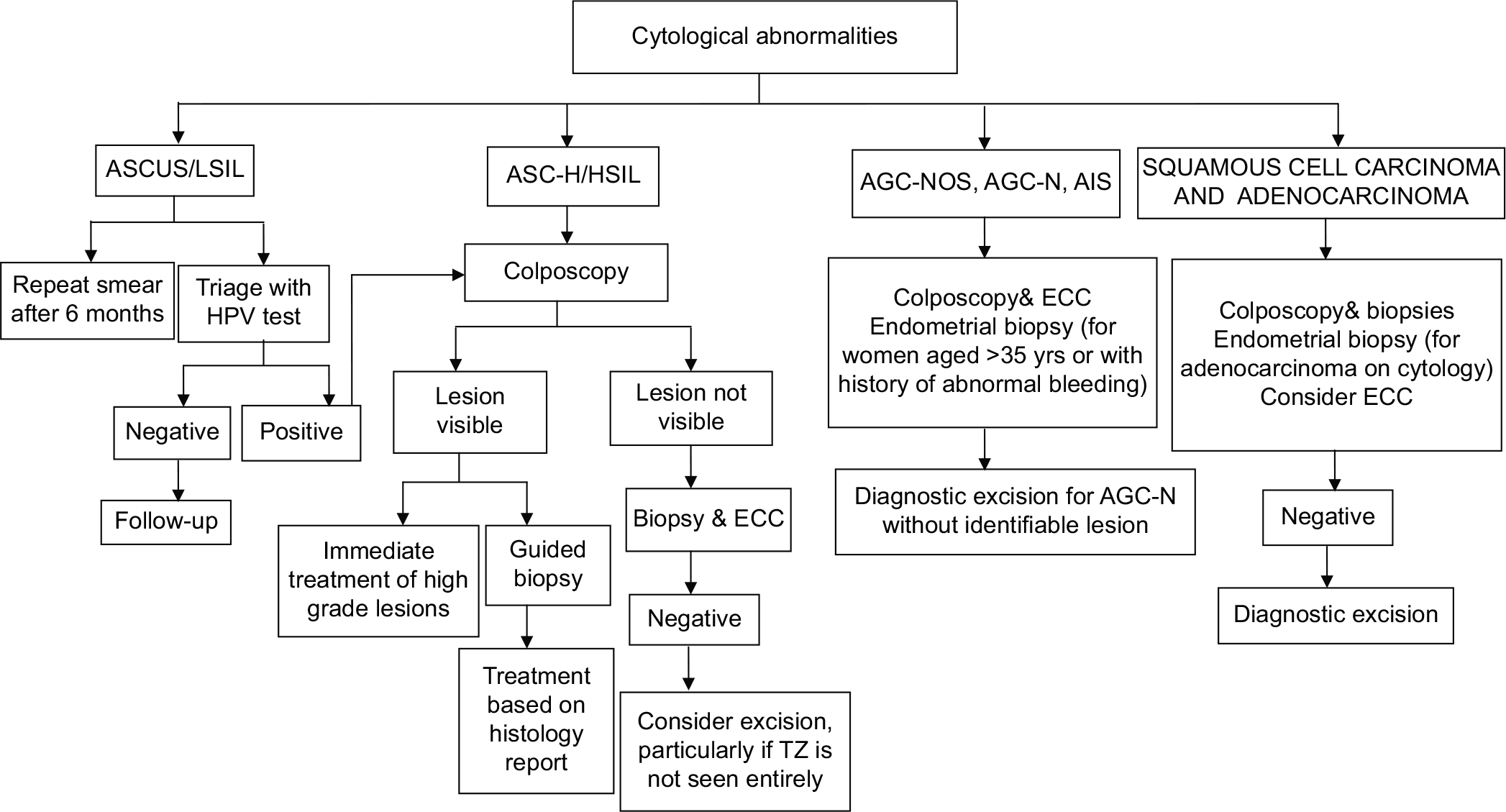
- Flowchart on management of women with cytological abnormalities.
CONCLUSION
Accumulated evidence on all cervical screening tools available shows that HPV testing is an acceptable, safe and highly efficacious procedure for detecting cervical cancer precursors. Sampling dependency and quality control is also favorable for HPV testing as compared to cytology. In middle income countries like India with lack of infrastructure and non availability of trained professionals, non cytological screening tests like VIA and HPV test are a viable alternative to cytologic screening. From the perspective of health policy, though at present HPV test as the primary screening test may have high upfront cost but because of its high sensitivity, specificity and negative predictive value, there is net cost saving in HPV detection based screening program due to high and prolonged protection that allows screening interval to be increased. HPV testing will be most suitable in the post vaccination era in view of low prevalence of HPV infection and neoplasia, when subjective tests like cytology and VIA will be more challenging with poor accuracy. Along with awareness and education change from a cellular to viral test will be more effective in controlling the huge burden of cervical cancer.
ACKNOWLEDGMENTS
None.
LIST OF ABBREVIATIONS (In alphabetic order)
ASC-US – Atypical squamous cells of undetermined significance
ASC-H – Atypical squamous cells-high grade
CIN – Cervical intraepithelial neoplasia
CKC – Cold knife conization
HSIL – High grade squamous intraepithelial lesion
HPV – Human papillomavirus
IARC – International agency for research on cancer
LLETZ – Large loop excision of transformation zone
LMIC – Low and middle income countries
LBC – Liquid based cytology
PAP smear – Papanicolaou smear
SCJ – Squamocolumnar junction
SVA – Single visit approach
TZ – Transformation zone
VIA – Visual inspectiononacetic acid
VILI – Visual inspection on lugol’s iodine
WHO – World health organization
LSIL – Low grade squamous intraepithelial lesion
AGC-NOS – Atypical glandular cells not otherwise specified
AGC – N – Atypical glandular cells favor neoplastic
AIS – Adenocarcinoma in situ
ECC – Endocervical curretage.
References
- WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. 2017. Available from: http://www.apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf [Last accessed on 2017 Aug 12]
- [Google Scholar]
- IARC Handbook of Cancer Prevention. 2017. 10 Available from: https://www.iarc.fr/en/publications/pdfs-online [Last accessed on 2017 Aug 12]
- [Google Scholar]
- Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: A systematic review. BJOG. 2014;121:929-42.
- [CrossRef] [PubMed] [Google Scholar]
- WHO Guidelines: WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention WHO. 2013.
- [Google Scholar]
- Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: A retrospective cohort study. Lancet Oncol. 2008;9:425-34.
- [CrossRef] [Google Scholar]
- IARC Handbooks of Cancer Prevention. Vol 10. Lyon, France: Cervix Cancer Screening IARC; 2005.
- [Google Scholar]
- Cervical cancer screening programs in Latin America and the Caribbean. Vaccine 26 2008(Suppl 11):L37-48.
- [CrossRef] [PubMed] [Google Scholar]
- A population-based evaluation of cervical screening in the United States: 2008-2011. Cancer Epidemiol Biomarkers Prev. 2014;23:765-73.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: A systematic review. Ann Intern Med. 2000;132:810-9.
- [CrossRef] [PubMed] [Google Scholar]
- Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: A systematic review for the U.S. preventive services task force. Ann Intern Med. 2011;155:687-97, W214-5
- [CrossRef] [PubMed] [Google Scholar]
- Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88-99.
- [CrossRef] [PubMed] [Google Scholar]
- A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20, 000 women in the Portland Kaiser cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:1398-409.
- [CrossRef] [PubMed] [Google Scholar]
- HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385-94.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet. 2014;383:524-32.
- [CrossRef] [Google Scholar]
- WHO Guidance Note: Comprehensive cervical Cancer Prevention and Control-a Healthier Future for Girls and Women. 2017. Geneva: World Health Organization; Available from: http://www.who.int/reproductivehealth/publications/cancers/9789241505147/en [Last accessed on 2017 Aug 15]
- [Google Scholar]
- Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai, Uganda. Sex Transm Dis. 2007;34:429-36.
- [CrossRef] [PubMed] [Google Scholar]
- A Practical Manual on Visual Screening for Cervical Neoplasia IARC Technical Publication No. 41 2003.
- [Google Scholar]
- Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet. 2011;113:14-24.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: A cluster-randomised trial. Lancet. 2007;370:398-406.
- [CrossRef] [Google Scholar]
- Screening of cervical neoplasia in HIV-infected women in India. AIDS. 2013;27:607-15.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of visual inspection with acetic acid and cervical cytology to detect high-grade cervical neoplasia among HIV-infected women in India. Int J Cancer. 2012;130:234-40.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness, safety and acceptability of 'see and treat' with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738-43.
- [CrossRef] [PubMed] [Google Scholar]
- Advancing cervical cancer prevention initiatives in resource-constrained settings: Insights from the cervical cancer prevention program in Zambia. PLoS Med. 2011;8:e1001032.
- [CrossRef] [PubMed] [Google Scholar]
- Population-level scale-up of cervical cancer prevention services in a low-resource setting: Development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS One. 2015;10:e0122169.
- [CrossRef] [PubMed] [Google Scholar]
- Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26:221-32.
- [CrossRef] [PubMed] [Google Scholar]








