Translate this page into:
Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman Disease): A case report and review of 49 cases with fine needle aspiration cytology
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Rosai–Dorfman disease (RDD), a rare, benign, self-limiting histiocytic proliferative disorder, can be encountered in both nodal and extranodal locations, and fine needle aspiration (FNA), a simple, accurate and economic tool, has been widely used for the diagnosis of superficial and deep-seated lesions. Familiarity with the cytomorphologic features of RDD is important as prognosis and treatment are quite different from other benign or malignant diseases for which it may clinically masquerade. Although large numbers of RDD cases have been reported, review of the literature has revealed 49 reported cases of RDD diagnosed by FNA. Here, we report a case of RDD with nasal and sinus involvement. The patient was seen at our institution, carrying a diagnosis of inflammatory pseudotumor rendered by an outside institution, based on material obtained by nasal and sinus surgical biopsies. Cervical lymph node FNA performed at our institution revealed typical features of RDD. The case, as well as a brief review of the literature and 49 RDD cases with FNA cytology, will be discussed.
Keywords
Fine needle aspiration
Rosai-Dorfman disease
Sinus histiocytosis with massive lymphadenopathy
CASE REPORT
The patient was a 79-year-old female who presented at an outside hospital with 2 years of nasal obstruction and epistaxis. She underwent surgery for removal of a right middle turbinate mass. Biopsies of a right septal lesion and the right ethmoid sinus were also performed at the same time. Pathologic findings noted were “spindle cell proliferation with abundant foamy macrophages and inflammatory cells including plasma cells and neutrophils.” Special stains for acid-fast bacilli, fungi and bacteria were all negative. An immunohistochemical stain for cytokeratin showed no immunoreactivity in the spindle cell component. Flow cytometry showed no evidence of B-cell or T-cell lymphoma. A diagnosis of “inflammatory pseudotumor (inflammatory myofibroblastic tumor)” was suggested by the outside institution. After surgery, the patient continued to experience nasal obstruction as well as alternating bilateral epistaxis and she eventually sought a second opinion at the Hospital of the University of Pennsylvania. Her physical examination was notable for bilaterally enlarged (3–5 cm), soft, smooth, mobile and non-tender submandibular lymph nodes. A fine needle aspiration (FNA) was performed on a left submandibular lymph node, and an onsite Diff Quik stained direct smear revealed numerous multinucleated giant cells with extensive emperipolesis in the background of mixed inflammatory cells [Figure 1]. An onsite, preliminary interpretation was reported as “submandibular lymph node aspiration with features suggestive of Rosai-Dorfman disease (RDD).” Subsequent specimen processing with Papanicolaou stained smears and a detailed examination of the Diff Quik smears revealed numerous multinucleated giant cells in a lymphohistiocytic background. Lymphocytes included larger and immature forms along with numerous neutrophils, a few plasma cells and rare eosinophils. The multinucleated giant cells showed anisocytosis, up to 80–100 μm in size, with vesicular, small monotonous nuclei. Emperipolesis of numerous neutrophils, variable numbers of lymphocytes and occasional plasma cells was noted. Ingested cells were well preserved; no mitosis or necrosis was observed. Immunocytochemical staining with appropriate controls was performed on cytospin preparations and revealed the multinucleated giant cells as positive for S100 and negative for CD1a. A diagnosis of sinus histiocytosis with massive lymphadenopathy (SHML; also known as RDD) was made. Review of the outside slides from the nasopharyngeal region revealed a mixed inflammatory infiltrate extensively involving the sinonasal mucosa and squamous lined mucosa with a moderate increase in fibrous tissue. Notably, scattered histiocytes with slightly atypical nuclei and ample foamy cytoplasm contain trafficking lymphocytes and neutrophils (emperipolesis), though not as prominently identified as in the lymph node FNA specimen. Additional immunohistochemical staining performed on the surgical biopsy material demonstrated that the histiocytes were positive for S100 and negative for CD1a, CD30 and ALK. A diagnosis of RDD involving the right middle turbinate, right nasal septum and right ethmoid sinus was subsequently rendered. Presently, the patient is being treated with prednisone. Additional sinus biopsies were performed at the Hospital of the University of Pennsylvania, which demonstrated the same morphologic and immunohistologic features as described above, lending further support for the diagnosis of extranodal RDD.
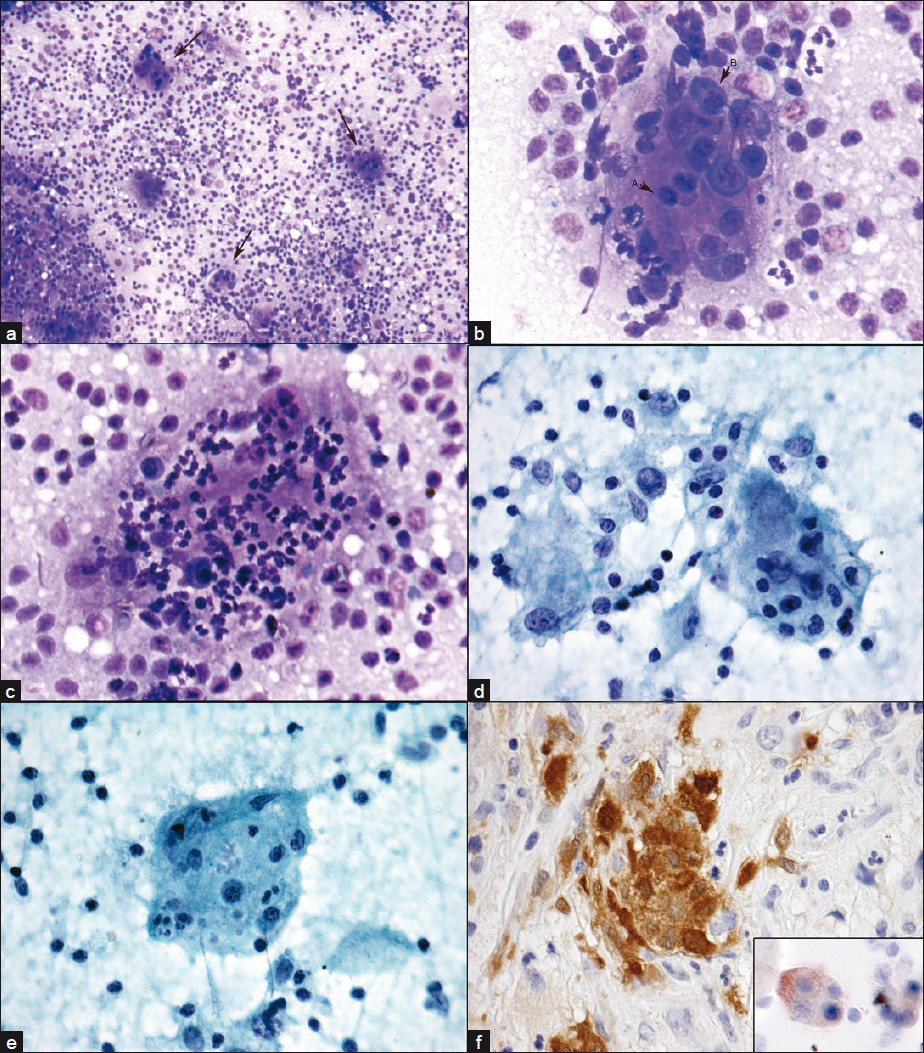
- Emperipolesis, RDD. FNA cervical lymph node. Emperipolesis in multinucleated giant cells (arrows) LP (a). Lymphocytes (arrow A) and plasma cells (arrow B) HP (b). Neutrophils and a few plasma cells (c) HP. Lymphocytes, neutophils, plasma cells and associated macrophages (d), HP. Lymphocytes and nuclear debris (e) high power. Nasal biopsy with S-100 positive cells, insert cytospin specimen with S-100 positive multinucleated giant cells. a–c) Diff Quik®; d-e) Papanicolaou stain; f) immunoperoxidase stain with DAB
DISCUSSION
Sinus histiocytosis with massive lymphadenopathy (SHML), also known as RDD, is a rare nonmalignant proliferative disorder first described by Rosai and Dorfman in 1969. Classically, it presents as prominent bilateral, massive, painless cervical lymphadenopathy; low-grade fever, weight loss, leukocytosis, elevated erythrocyte sedimentation rate and hypergammaglobulinemia are often found. Extranodal involvement of at least one site is identified in 43% of RDD cases and only 23% exclusively have extranodal disease.[1] Documented sites of extranodal involvement include skin, respiratory tract, bone, genitourinary system, oral cavity, central nervous system, eyes/orbit/ocular adnexa, salivary gland, tonsil, breast, soft tissue and heart. The bone marrow is rarely involved.[2] Most patients with RDD have a complete and spontaneous remission. Some may experience recurrent or persistent but stable lymphadenopathy. In rare cases, the disease follows an aggressive course and may be fatal[3] and involvement of kidney, lower respiratory tract, or liver has been found to be a poor prognostic sign.[1] Occasionally, RDD may be associated with autoimmune disorders and hematopoietic malignancies.[4–7] The etiology of RDD is unknown though it has been speculated that an occult chronic infection or an aberrant exaggerated immune response to an infectious agent or an antigen causes the initial histiocytic proliferation.[3]
RDD is notable for its varied clinical presentations which evoke a wide differential diagnosis. Although correlation of clinical presentation with radiologic and laboratory values is very helpful, the pathologic assessment is pivotal in making the diagnosis. The classic histology is characterized by effacement of nodal architecture and dilatation of lymph node sinuses by lymphocytes, plasma cells and numerous characteristic histiocytes with large vesicular nuclei and abundant clear cytoplasm. Many of these histiocytes, also known as RDD cells, contain intact lymphocytes, and sometimes plasma cells and red blood cells, within their cytoplasm. This process whereby cells enter and transit through a cell evading cellular degradation is known as emperipolesis and was first described by Humble et al.[8] When extranodal sites are involved, similar morphologic features to the nodal counterpart are seen although with more fibrosis, fewer typical RDD histiocytes, and less prominent emperipolesis.[3] Immunohistochemical stains are useful when diagnosing RDD as the RDD cells have been found to express pan-macrophage antigens (CD68, HAM56, CD14, etc.), antigens associated with phagocytosis (CD64, Fc receptor for immunoglobulin G), lysosomal activity (lysozyme alpha 1-antitrypsin, alpha1-antichymotrypsin), and immune activation and adhesion molecules (transferring receptor, interleukin 2 receptor).[3] The most consistent and reliable phenotype for RDD is S100(+), CD68(+) and CD1a(−).
RDD can be encountered in both nodal and extranodal locations, and FNA, a simple, accurate and economic tool, has been widely used for the diagnosis of superficial and deep-seated lesions. Familiarity with the cytomorphologic features of RDD is important as prognosis and treatment are quite different from other benign or malignant diseases for which it may clinically masquerade. Review of the literature has revealed 49 reported cases of RDD diagnosed by FNA [Table 1]. Typically, FNA specimens show non-cohesive, diffusely distributed, enlarged histiocytes. These cells have variable nuclei, abundant cyanophilic cytoplasm and demonstrate emperipolesis of lymphocytes, plasma cells and occasionally erythrocytes.[1933] Although the nuclear shapes vary from round to extremely bizarre configurations, the chromatin is fine and evenly distributed and the nucleoli are usually not prominent. Occasionally, atypical morphology may be seen and, when present, it can lead to a misdiagnosis of malignancy.[19] Lymphocytes, plasma cells, neutrophils and follicular center cells are often found in the background of the smear.[1933]
| Case | Author | Publication year | Sex (M/F) | Age (years) | FNA site | FNA diagnosis | Surgical diagnosis | Immunostains |
|---|---|---|---|---|---|---|---|---|
| 1 | Pettinato[9] | 1990 | (1/0) | 3 | LN | RDD | No | S100+, AIAT+, LYZ− (FNA) |
| 2 | Layfield[10] | 1990 | (1/0) | 5 | LN | Reactive lymphoid infiltrate with benign histiocytes | RDD | No |
| 3 | Trautman[11] | 1991 | (1/0) | 14 | LN | RDD | RDD (larynx) | No |
| 4 | Schmitt[12] | 1992 | (1/0) | 12 | LN | RDD | RDD | S100+, HAM56+, LYZ−, AlAT+ (biopsy) |
| 5 | Schmitt[12] | 1992 | (1/0) | 15 | LN | RDD | RDD | S100+, HAM56+, LYZ+, AlAT+ (biopsy) |
| 6 | Schmitt[12] | 1992 | (1/0) | 18 | LN | RDD | RDD | S100+, HAM56+, LYZ−, AlAT (biops |
| 7 | Chang[13] | 1993 | (1/0) | 30 | LN | RDD | RDD | No |
| 8 | Perez-Guillermo[14] | 1993 | (0/1) | 71 | Breast | RDD | RDD | No |
| 9 | Alvarez[15] | 1995 | (0/1) | 62 | LN | RDD | RDD | S100+, LYZ+ (biopsy) |
| 10 | Gupta[16] | 1996 | (1/0) | 12 | LN | RDD | RDD | No |
| 11 | Patel[17] | 1996 | (1/0) | 12 | LN | RDD | RDD | No |
| 12 | Norman[18] | 1997 | (1/0) | 71 | Parotid | Suggestive of malignancy | RDD | No |
| 13 | Deshpanande[19] | 1998 | (1/0) | 40 | LN | RDD | No | No |
| 14-19 | Deshpanande[19] | 1998 | (4/2) | 1.5–40 | LN | RDD | RDD | No |
| 20 | Deshpanande[19] | 1998 | (1/0) | 21 | LN | Suggestive of Hodgkin’s lymphoma | RDD | No |
| 21 | Hummel[20] | 1999 | (0/1) | 52 | Breast | Atypical lymphohistiocytic proliferation | RDD | No |
| 22 | Soares[21] | 1999 | (0/1) | 65 | Breast | Centroblastic non-Hodgkin’s malignant lymphoma | RDD | No |
| 23-26 | Deshpande[22] | 2000 | (3/1) | 12–17 | LN | RDD | RDD | No |
| 27 | Juskevicius[23] | 2001 | (0/1) | 48 | Parotid | RDD | RDD | S100+, HAM56+, CDla- (biopsy) |
| 28 | Das[24] | 2001 | (0/1) | 14 | LN | RDD | No | No |
| 29 | Das[24] | 2001 | (0/1) | 35 | LN | RDD | No | S100+ (FNA) |
| 30 | Goel[25] | 2003 | (0/1) | 7 | Bone | RDD | No | No |
| 31 | Cocker[26] | 2003 | (0/1) | 38 | Thyroid | Lymph node | RDD | S100+ (biopsy) |
| 32 | Singh[27] | 2004 | (0/1) | 35 | Skin | RDD | RDD | S100+, CD68+, CDIIc+, CDIa- (FNA) |
| 33 | Panikar[28] | 2005 | (0/1) | 45 | LN | RDD | No | No |
| 34 | Ruggiero[29] | 2006 | (0/1) | 12 | LN | RDD | No | S100+, CD68+ |
| 35 | Ruggiero[29] | 2006 | (1/0) | 9 | LN | RDD | No | S100+, CD68+, CDIa- (FNA) |
| 36 | Maiur[30] | 2007 | (0/1) | 50 | Orbit | RDD | RDD | No |
| 37 | Sachdev[31] | 2007 | (0/1) | 47 | LN | RDD | No | S100+, CD68+, CDIa- (FNA) |
| 38 | Bist[32] | 2007 | (1/0) | 20 | LN | RDD | RDD (nasal mass) | No |
| 39, 40 | Kumar[33] | 2008 | (0/2) | 35–50 | LN | RDD | RDD | No |
| 41, 42 | Kumar[33] | 2008 | (2 0) | 6–75 | LN | RDD | No | No |
| 43 | Pinto[34] | 2008 | (1/0) | 43 | LN | Non-conciusive | RDD | No |
| 44 | Jing[35] | 2009 | (0/1) | 51 | Bone | Acute and chronic inflammation | RDD | S100+, CDIa-(biopsy) |
| 45 | Farkash[36] | 2009 | (0/1) | 52 | LN | RDD | RDD | No |
| 46 | Tseng[37] | 2009 | (1/0) | 55 | Subglottis | RDD | RDD | No |
| 47 | Morkowski[38] | 2010 | (0/1) | 53 | Breast | Reactive lymphoid hyperplasia | RDD | S100+, CD68+, CDIa-(excision) |
| 48 | Bansai[39] | 2010 | (1/0) | 35 | Breast | RDD | No | No |
| 49 | Li[40] | 2010 | (0/1) | 28 | Bone | RDD | No | S100+, CDIa- (FNA) |
LN, Lymph node; FNA, Fine needle aspiration; RDD, Rosai-Dorfman Disease
Misdiagnosis of RDD can be rendered in both FNA and surgical biopsy specimens. When compared to surgical core or excisional biopsy, FNA can at times be misinterpreted due to limited or non-representative sampling and, as FNA does not permit examination of the tissue architecture, diagnosis can be further confounded. Despite these potential limitations, FNA is still a very useful tool for the diagnosis of RDD. In fact, emperipolesis, the hallmark for RDD, tends to be more prominent in FNA material than on tissue sections. This is believed to be due, in part, to the fact that in FNA smears, an entire cell may be observed by focusing through the different planes of the slide, whereas in surgical pathology sections, generally a single plane of tissue is available for review. Furthermore, the physical action of spreading the sample into a smear of single cells renders the cellular borders of histiocytes more clearly defined and, thereby, emperipolesis may be more readily and definitively identified. It is important to note, however, that the occurrence of emperipolesis per se should not be considered diagnostic of RDD. Instead, diagnosis requires correlation of the appropriate clinical presentation with an adequately preserved and properly prepared FNA sample of consistent cytomorphologic features. In addition, numerous other disease states either demonstrate emperipolesis or represent cytomorphologic mimics, including lymphoma, malignancy, hemophagocytic syndrome, infection, Langerhans histiocytosis and various other reactive processes [Figure 2].
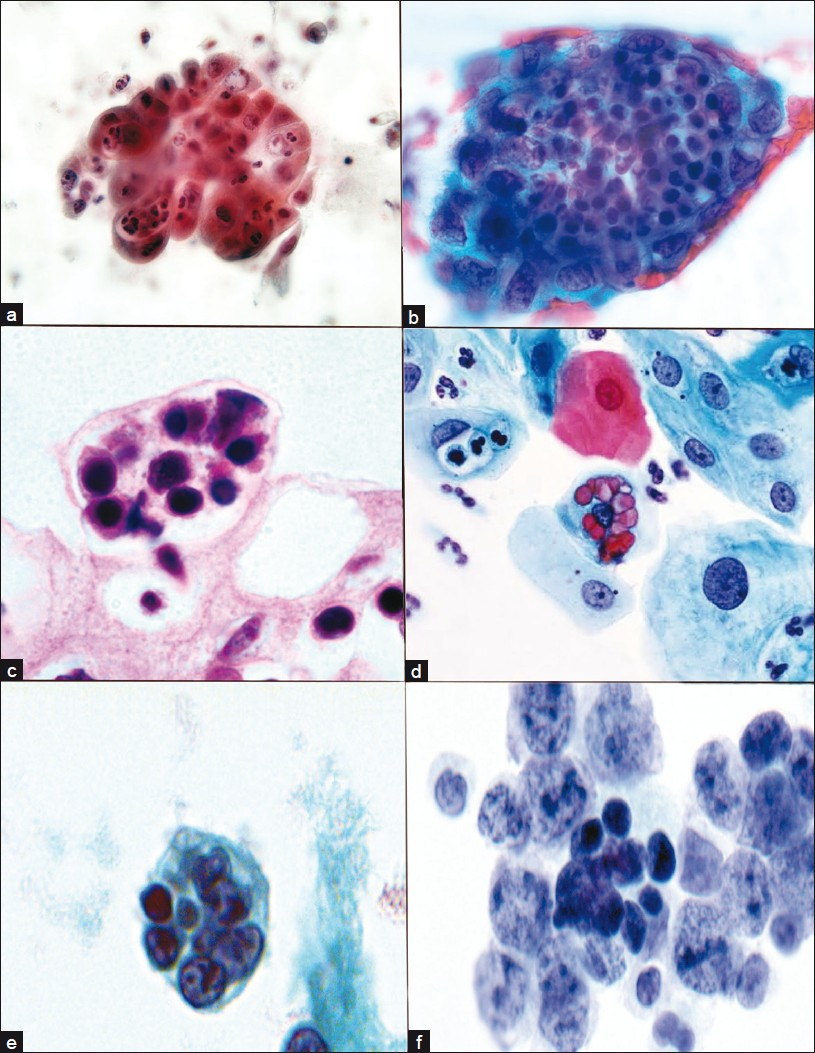
- Emperipolesis in other disorders. Acute and chronic cervicitis. Endocervical cells with indigested neutrophils, cervical smear (a). Endometrial adenocarcinoma tissue fragment with intracytoplasmic lymphocytes and neutophils, cervicovaginal smear (b). Intracytoplasmic lymphocytes, cytomegalovirus (CMV) infection, cervical biopsy (c). Ingested erythrocytes metaplastic endocervical cells, HSV, cervical smear (d). Ingested lymphocytes and debris, lymphoma cerebrospinal fluid (e). Ingested lymphocytes, B cell lymphoma, cervical lymph node FNA (f). (a, b, d, e, f) Papanicolaou; (c) H/E stain
Significantly, misdiagnosis of RDD by FNA more often occurs in extranodal rather than in nodal disease. In the cases with biopsy confirmation [Table 1], 3 out of 25 (12%) lymph node aspirations were misdiagnosed or inconclusive and 6 out of 12 (50%) extranodal aspirations were misdiagnosed. Therefore, in the case where extranodal RDD is suspected, careful clinical lymph node examination should be undertaken and FNA performed in order to maximize diagnostic accuracy. Finally, as RDD is infrequently suspected clinically and is a rare disease process, an awareness of the entity along with its clinical profile is essential in proper specimen evaluation, interpretation and diagnosis.
CONCLUSION AND SUMMARY
In summary, RDD is a rare disease of unknown etiology, typically presenting in young adulthood and following a relatively benign clinical course. As a diagnostic entity it is significant in that it has the readily recognizable, although not specific, cytomorphologic feature of emperipolesis on FNA. This finding should also bring to mind a wide differential diagnosis including lymphoma, malignancy, hemophagocytic syndrome, infection, Langerhans histiocytosis and various other reactive processes. Lymph node specimens are most helpful diagnostically and should be sampled, in addition to extranodal sites, when possible. FNA represents an efficient, minimally invasive, cost-effective and reliable, though not infallible, technique for the diagnosis of RDD. However, awareness of the entity and consideration of it as a diagnosis in the review and histiocytic and lymphocytic pathologies is of utmost importance if one is to be successful in the diagnosis of RDD by FNA.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
No competing interest to declare by any of the authors.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version was read and approved. All authors qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from institutional Review Board (IRB) (or its equivalent) of all the institutions associated with this study.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model(authors are blinded for reviewers and reviewers are blinded for authors)through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2011/8/1/3/
REFERENCES
- Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73.
- [Google Scholar]
- Extranodal Rosai-Dorfman disease involving the bone marrow: a case report. Am J Surg Pathol. 2006;30:1189-92.
- [Google Scholar]
- Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy. Arch Pathol Lab Med. 2007;131:1117-21.
- [Google Scholar]
- Focal changes of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) associated with nodular lymphocyte predominant Hodgkin’s disease. Hum Pathol. 1995;26:1378-82.
- [Google Scholar]
- Clinical quiz.Large anaplastic lymphoma coexisting with Rosai-Dorfman disease. Pediatr Radiol. 2004;34:509-10.
- [Google Scholar]
- Sinus histiocytosis with massive lymphadenopathy and malignant lymphoma involving the same lymph node: a report of four cases and review of the literature. Mod Pathol. 2000;13:414-9.
- [Google Scholar]
- Focal changes of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) associated with nodular lymphocyte predominant Hodgkin’s disease. Hum Pathol. 1995;26:1378-82.
- [Google Scholar]
- Biological interaction between lymphocytes and other cells. Br J Haematol. 1956;2:283-94.
- [Google Scholar]
- Fine needle aspiration cytology and immunocytochemical characterization of the histiocytes in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman syndrome) Acta Cytol. 1990;34:771-7.
- [Google Scholar]
- Fine needle aspiration cytologic findings in a case of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman syndrome) Acta Cytol. 1990;34:767-70.
- [Google Scholar]
- Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): diagnosis by fine-needle aspiration. Diag Cytopathol. 1991;7:513-6.
- [Google Scholar]
- Sinus Histiocytosis with massive lymphadenopathy (Rosai-Dorfman Disease): Cytomorphologic analysis on fine needle aspirates. Diag Cytopathol. 1992;8:596-9.
- [Google Scholar]
- Sinus histiocytosis with massive lymphadenopathy. Report of a case with fine needle aspiration cytology. Acta Cytol. 1993;37:186-90.
- [Google Scholar]
- Malacoplakia and Rosai-Dorfman disease: two entities of histiocytic origin infrequently localized in the female breast-the cytologic aspect in aspirates obtained via fine-needle aspiration cytology. Diag Cytopathol. 1993;9:698-704.
- [Google Scholar]
- Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): diagnosis with fine-needle aspiration in a case with nodal and nasal involvement. Diagn Cytopathol. 1995;13:333-5.
- [Google Scholar]
- Cytologic appearance of sinus histiocytosis with massive lymphadenopathy: a case report. Acta Cytol. 1996;40:595-8.
- [Google Scholar]
- Fine-needle aspiration cytology of sinus histiocytosis with massive lymphadenopathy: a case report. Diagn Cytopathol. 1996;15:221-3.
- [Google Scholar]
- Rosai-Dorfman disease presenting as a parotid mass. J Laryngol Otol. 1997;111:1091-8.
- [Google Scholar]
- Fine needle aspiration (FNA) cytology of Rosai Dorfman disease. Cytopathol. 1998;9:329-35.
- [Google Scholar]
- Fine-needle aspiration cytology of Rosai-Dorfman disease of the breast: A case report. Diagn Cytopathol. 1999;21:287-91.
- [Google Scholar]
- Cytology of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) Diagn Cytopathol. 2000;22:181-5.
- [Google Scholar]
- Rosai-Dorfman disease of the parotid gland: cytologic and histopathologic findings with immunohistochemical correlation. Arch Pathol Lab Med. 2001;125:1348-50.
- [Google Scholar]
- Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of two cases with fine-needle aspiration cytology. Diag Cytopathol. 2001;24:42-5.
- [Google Scholar]
- Primary Rosai-Dorfman disease of bone without lymphadenopathy diagnosed by fine needle aspiration cytology. A case report. Acta Cytol. 2003;47:1119-22.
- [Google Scholar]
- Rosai-Dorfman disease. Report of a case presenting as a midline thyroid mass. Arch Pathol Lab Med. 2003;127(4):e197-200.
- [Google Scholar]
- Rosai-Dorfman disease manifesting as multiple subcutaneous nodules. Report of a case with diagnosis on a fine needle aspiration. Acta Cytol. 2004;48:215-8.
- [Google Scholar]
- Salivary gland manifestations of sinus histiocytosis with massive lymphadenopathy: fine needle aspiration cytology findings. A case report. Diag Cytopathol. 2005;33:187-90.
- [Google Scholar]
- Rosai-Dorfman disease. Two case reports and diagnostic role of fine-needle aspiration cytology. J Pediatr Hematol Oncol. 2006;28:103-6.
- [Google Scholar]
- Orbital Rosai-Dorfman disease: report of a case with fine needle aspiration cytology and histopathology. Acta Cytol. 2007;51:581-2.
- [Google Scholar]
- Sinus histiocytosis with massive lymphadenopathy. Is the lymph node enlargement always massive? Med Oral Pathol Oral Cir Bucal. 2007;12:198-200.
- [Google Scholar]
- Rosai Dorfman syndrome with extranodal manifestation. J Assoc Physicians India. 2007;55:445-7.
- [Google Scholar]
- Diagnosis of sinus histocytosis with massive lymphadenopathy (Rosai-Dorfman Diseases) by fine needle aspiration cytology. Diag Cytopathol. 2008;36:691-5.
- [Google Scholar]
- Rosai-Dorfman disease in the differential diagnosis of cervical lymphadenopathy. Braz J Otorhinolaryngol. 2008;74:632-5.
- [Google Scholar]
- Fine-needle aspiration cytology of Rosai-Dorfman disease of bone. Diag Cytopathol. 2008;36:516-8.
- [Google Scholar]
- Emperipolesis in Rosai-Dorfman disease presenting as a peritonsillar mass. Diag Cytopathol. 2009;38:349-50.
- [Google Scholar]
- Rosai-Dorfman disease of the subglottis diagnosed by ultrasound-guided fine-needle aspiration biopsy: a case report. J Clin Ultrasound. 2010;38:103-6.
- [Google Scholar]
- Rosai-Dorfman disease of the breast in a male: a case report. Acta Cytol. 2010;54:349-52.
- [Google Scholar]
- Fine needle aspiration diagnosis of Rosai-Dorfman disease in an osteolytic lesion of bone. Cyto journal. 2010;7:12.
- [Google Scholar]








