Translate this page into:
Specimen-specific cell-blocking approaches

*Corresponding author: Vinod B. Shidham, Department of Pathology, Wayne State University School of Medicine, Karmanos Cancer Center, and Detroit Medical Center, Detroit, Michigan, United States. vshidham@med.wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Shidham VB. Specimen-specific cell-blocking approaches. CytoJournal 2020;17:28.
HTML of this article is available FREE at: https://dx.doi.org/10.25259/Cytojournal_75_2020
Abstract
The science of CellBlockistry highlights requirement for proper approach to process different types of cytopathology specimens with critical considerations during preparation of cell-blocks. Common cytopathology specimens which may be subjected for cell-blocking include FNA aspirates in addition to anterior fat pad aspirate, bone marrow aspirate, effusion fluids, and other fluids such as various washings and urine. In addition veterinary sciences and research fields including animal experiments and tissue/cell cultures may also be cell-blocked for improved diagnostic yield and research outcome.
Keywords
CellBlockistry
Cell-block
Cell-blocking
Cell-blocked
Cytopathology processing
Fat pad aspiration
Fine needle aspiration
FNA
CytoJournal monograph
Formalin-fixed paraffin-embedded
FFPE
Fixation

INTRODUCTION
The types of cytopathology specimens that may be cell-blocked include fine needle aspiration (FNA) aspirates in addition to anterior fat pad aspirate, bone marrow aspirate, effusion fluids, and other fluid such as various washings and urine. In addition, similar and comparable specimens from veterinary sciences and research fields including animal experiments and tissue/cell cultures can also be cell-blocked. Different types of cytology specimens may be processed for cell-blocking as mentioned in Table 1.
| 1. FNA aspirates a. Aspirates as suspension of needle rinses i. Aspirates without predominance of blood contamination[1-3] ii. Aspirates with predominance of blood contamination and BloodLyz[4] b.Dedicated aspirate with wider gauge needle (18 G) to be processed as natural clot in the syringe[5] 2. Serous fluids and other fluids Concentrate and process this sediment similar to needle rinses of FNA aspirates (with or without predominance of blood contamination) 3. Aspirates with fatty component, such as fat pad aspirate and bone marrow aspirate (not to centrifuge) a. Filtration of the aspirates[6] b. To process the aspirate as natural clot[5] 4. Veterinary sciences[8-14] 5. Research – Animal experiment and tissue/cell culture 6. Specimens with infectious potential[15] Bacterial and fungal infections Viruses Prions[16,17] |
FNA ASPIRATES
-
Aspirates as suspension of needle rinses
-
Aspirates without predominance of blood contamination
Concentrate the specimen by centrifuging at 2500 revolutions per minute (rpm) for 3 minutes (min) and discard the supernatant by inverting the tube or with the help of a pipette if the sediment is loose
Resuspend the sediments in the residual scant supernatant to be processed for making the cell-block
Select the best protocol that can achieve the best quantitative and qualitative outcome. For a variety of methodologies [Figures 1 and 2] with their limitations and benefits, please see Chapter 1
The most appropriate outcome with benefits of qualitative and quantitative excellence is achieved with Nano NextGen CelBloking™ kits with benefits of objective monitoring of the depth of cutting to select the initial sections with diagnostic cells [Figures 3-5].[1-3]
Aspirates with a predominance of blood contamination
Any specimen with a predominance of blood contamination would interfere with quantitative and qualitative outcomes.
Quantitatively, the diagnostic cells diluted in the blood-rich specimen will not be aligned along the bottoms of the wells (which correspond with the final cutting surface of the FFPE of the cell-block) in the Nano NextGen CelBloking™ kits after centrifugation but would be above the column of red blood cells like that of buffy coat.
Qualitatively, excess blood contamination will also interfere at the level of processing of such cell-block sections. For example, sections of blood-rich cell-blocks tend to float and fold during immunostaining with other interferences such as formalin pigment interference.
As discussed in detail in Chapter 1, blood interference should be removed for the best outcome [Figure 6]. The best approach that should not compromise the immunoprofile is similar to that practiced during flow cytometry with ammonium chloride-based lysis of red blood cells with a reagent such as BloodLyzTM [Figure 6].[4]
Concentrate the specimen by centrifuging and discarding the supernatant
Add 10 times the volume of working BloodLyzTM lysing reagent to the sediments
Mix the sediments with the lysing reagent and wait for 10 min at room temperature
Centrifuge at 2500 rpm for 3 min to get sediments rich in nucleated diagnostic cells without Red blood cells (RBCs)
Discard the supernatant with the lysed red blood cells
Process the sediment without blood contamination to make the cell-block with Nano NextGen CelBloking™ kits as described (Chapter 1).[2,3]
-
-
B. Dedicated aspirate with wider gauge needle (18G) to be processed as a natural clot in the syringe. See also the approach described as a second option (#b) for anterior fat aspirate [Figure 7].[5]
The FNA aspirate accumulated in the syringe is allowed to clot in the labeled syringe by waiting for 5–7 min (usual clotting time)
Aspirate 10% formalin in the syringe so that the clot separates from the wall of the syringe as a free-floating coagulum.[5]
Open the end of the syringe by removing the plunger and dislodge the clotted aspirate (blood with microtissue fragments) for transferring into the labeled 10% formalin container [Figure 7]
The intact transferred clot is usually firm after 15–20 min or longer in 10% formalin. If the clot is larger, it should be grossed like the surgical pathology biopsies by submitting 2–3 mm thick slices in labeled tissue cassette.
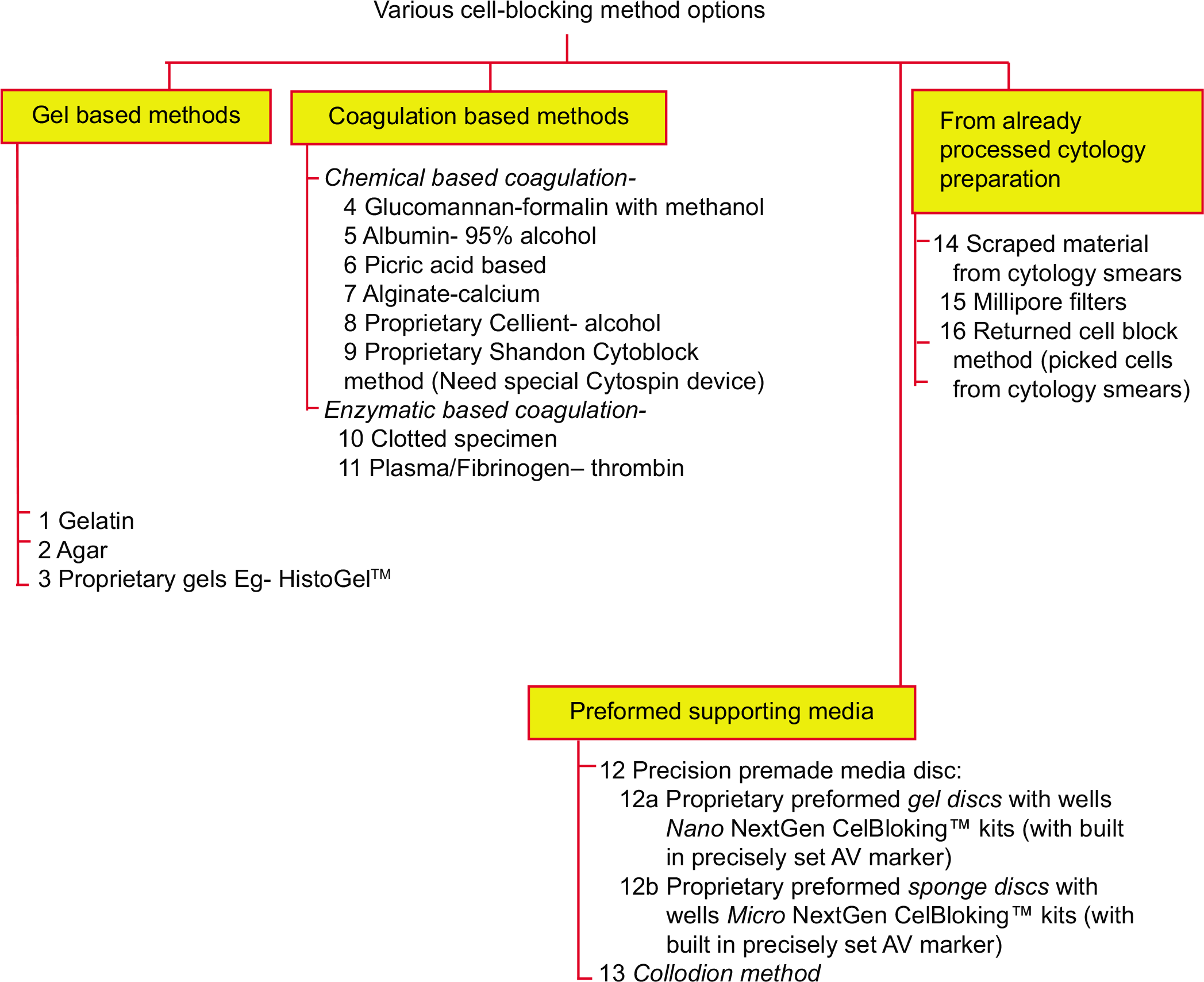
- Different types of approaches in cell-blocking (Reproduced from open access article Ref #11).
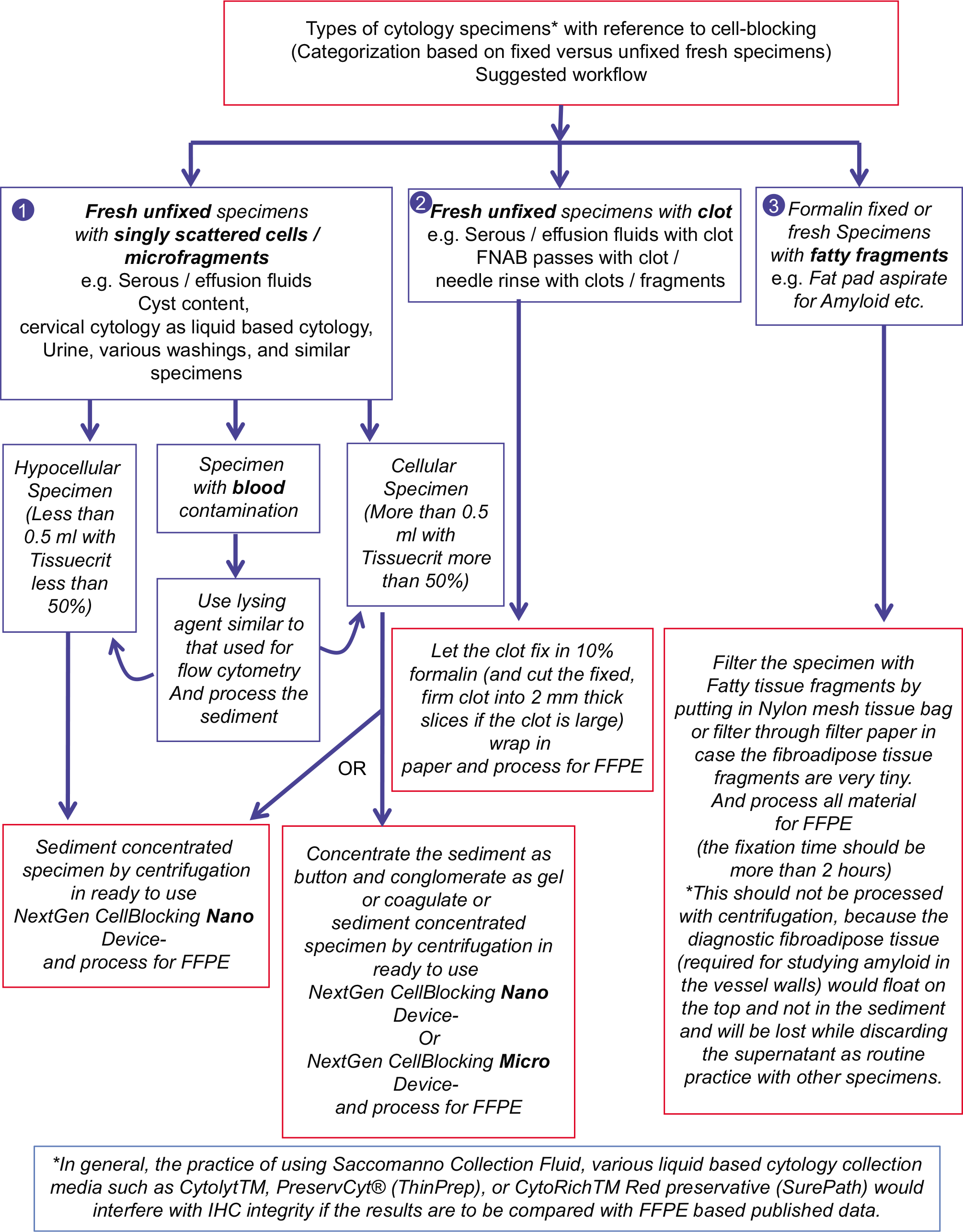
- The specimens may be divided into various categories (Reproduced from open access article Ref #11).
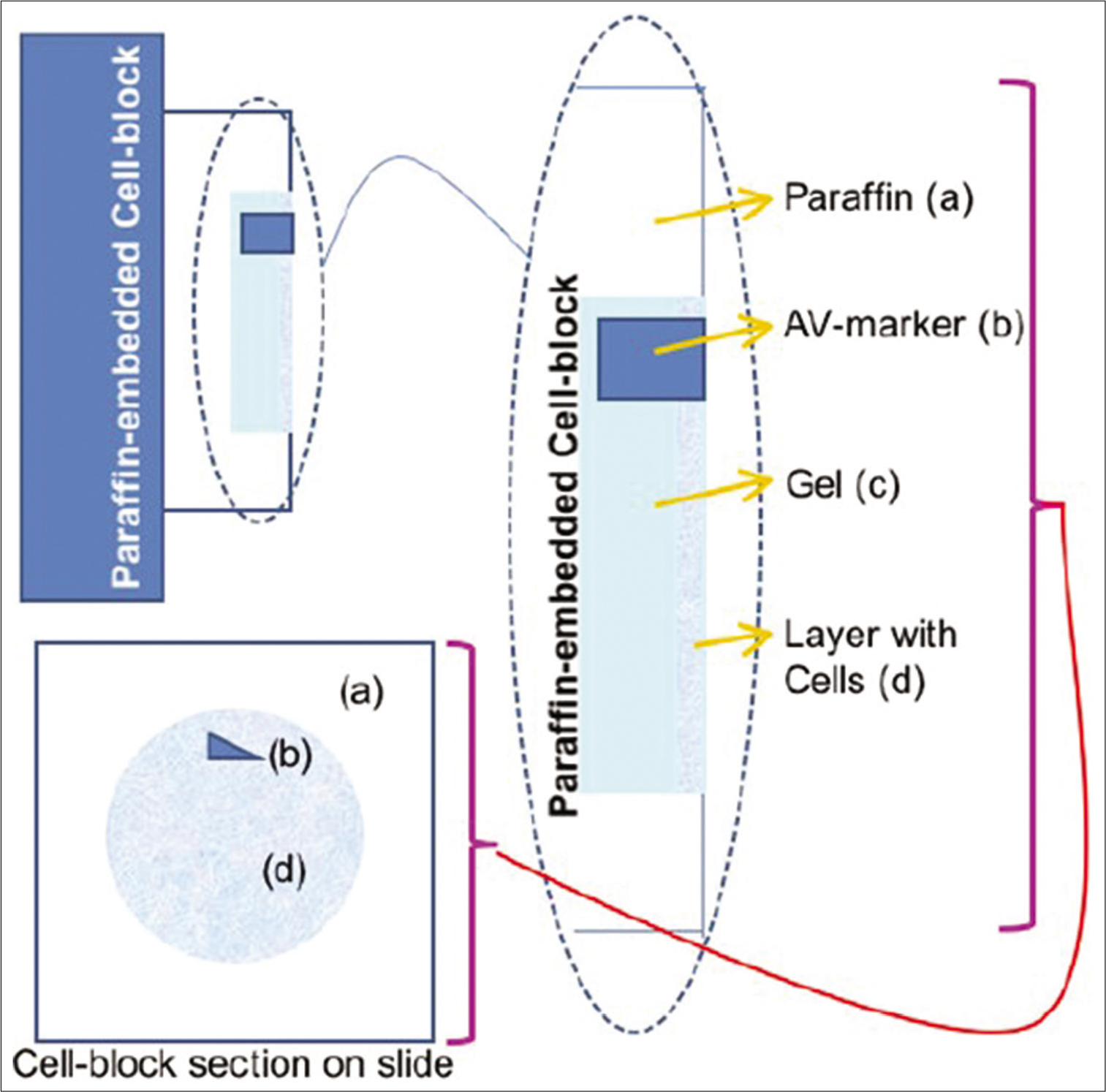
- Cell-blocking: AV Marker as a guide to monitor the depth of cutting. Reproduced from Open access publication: Varsegi and Shidham; Journal of Visualized Experiments; http://www.jove.com/index/Details.stp?ID=1316
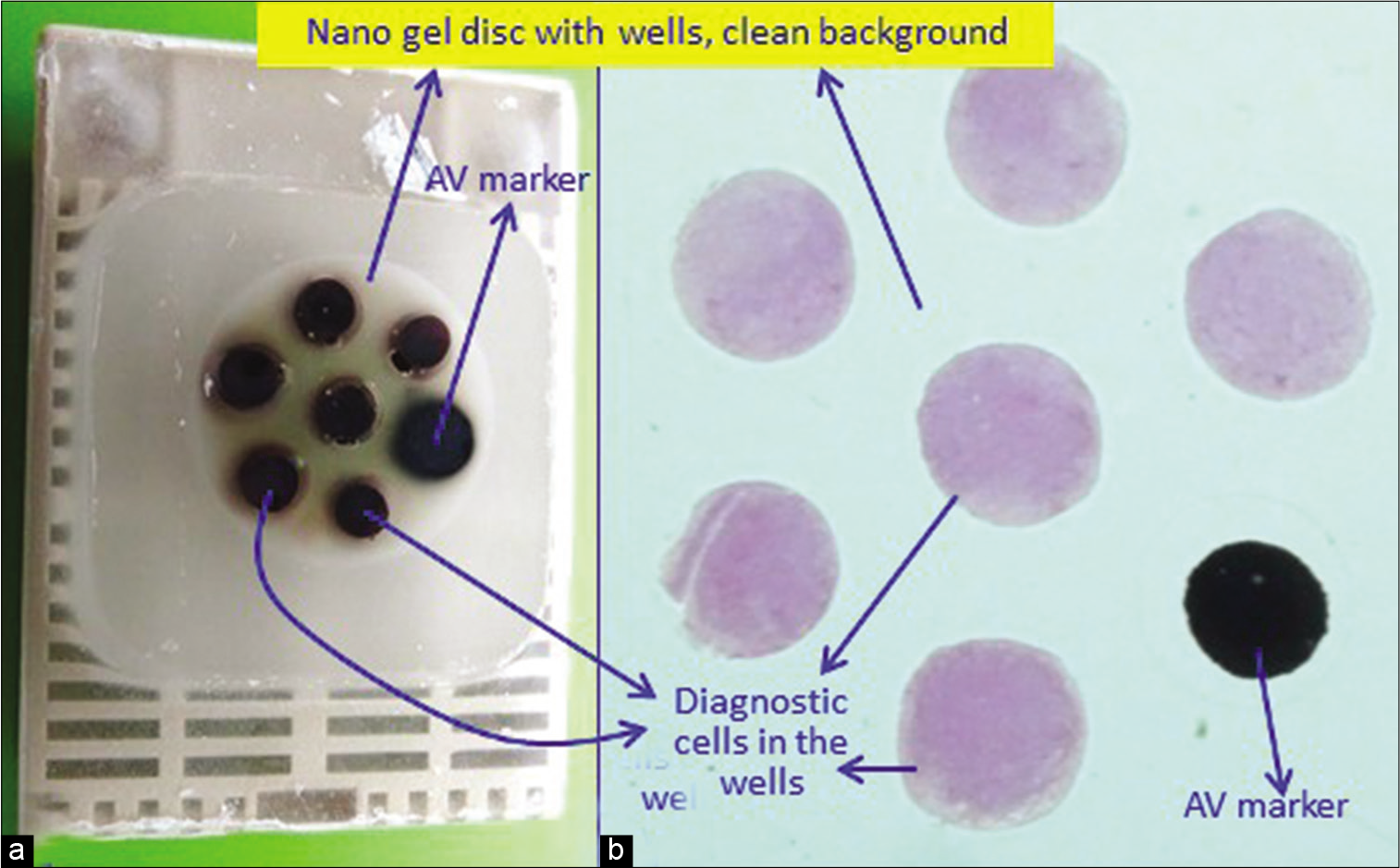
- (a) Final paraffin block; (b) scanning power view of HE stained section of cell-block prepared with Nano NextGen CelBloking™ kit. The preformed Nano gel disc is made of proprietary medium that allows the processing reagents to be exchanged freely but the diagnostic cells are retained and concentrated in the wells. The gel medium has (a) clean transparent property as a clean (b) background (pleural fluid). (Reproduced from open access article Ref #11).
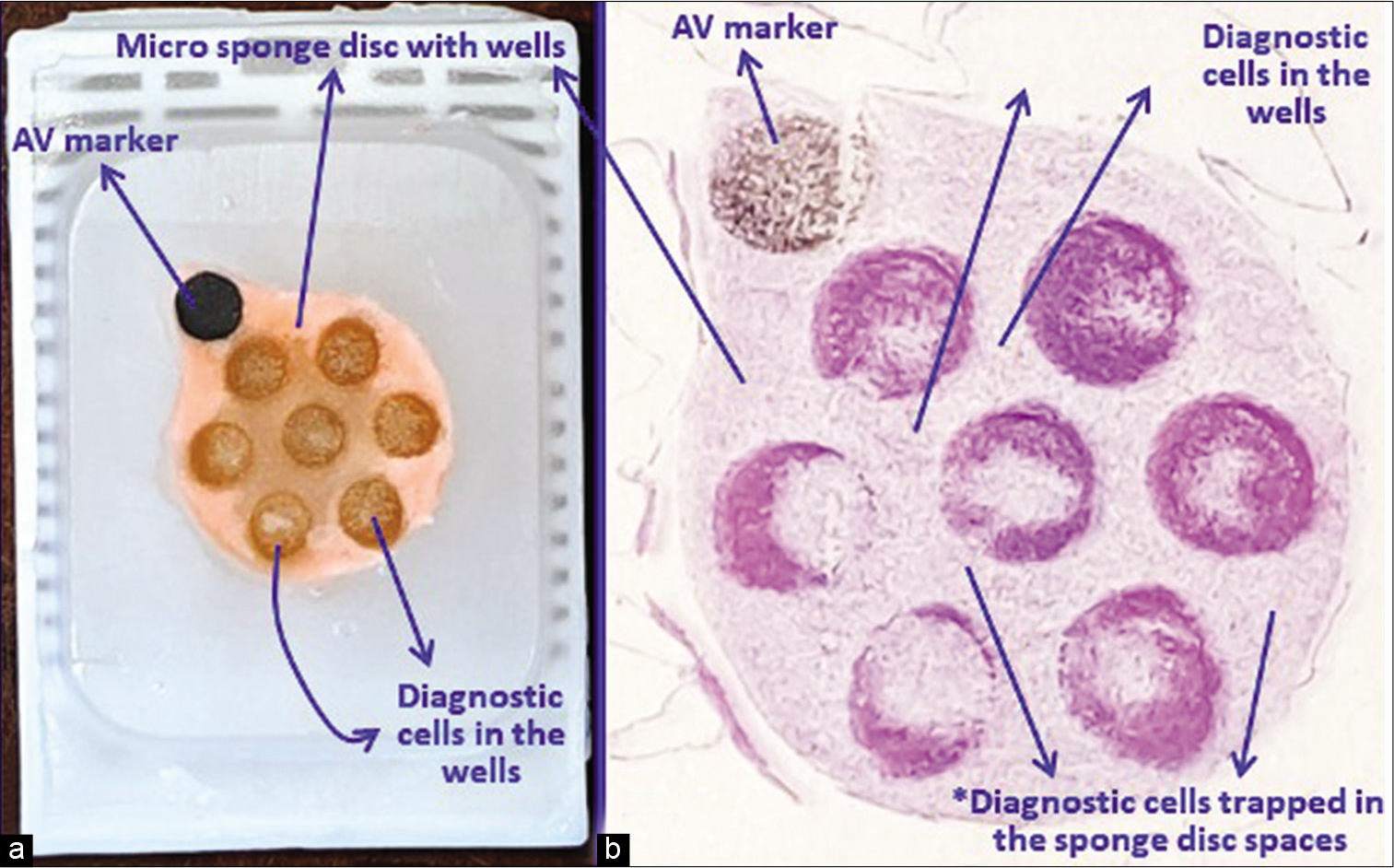
- (a) Final paraffin block; (b) scanning power view of HE stained section of cell-block prepared with Micro NextGen CelBloking™ kit. The preformed microsponge disc is made of proprietary porous medium that concentrates the diagnostic cells predominantly in the wells but the small groups of cells and singly scattered cells that wandered around during concentration process may also be seen in the sponge (b) spaces*. The sponge disc medium may stain faintly as background staining (pleural fluid). (Reproduced from open access article Ref #11).
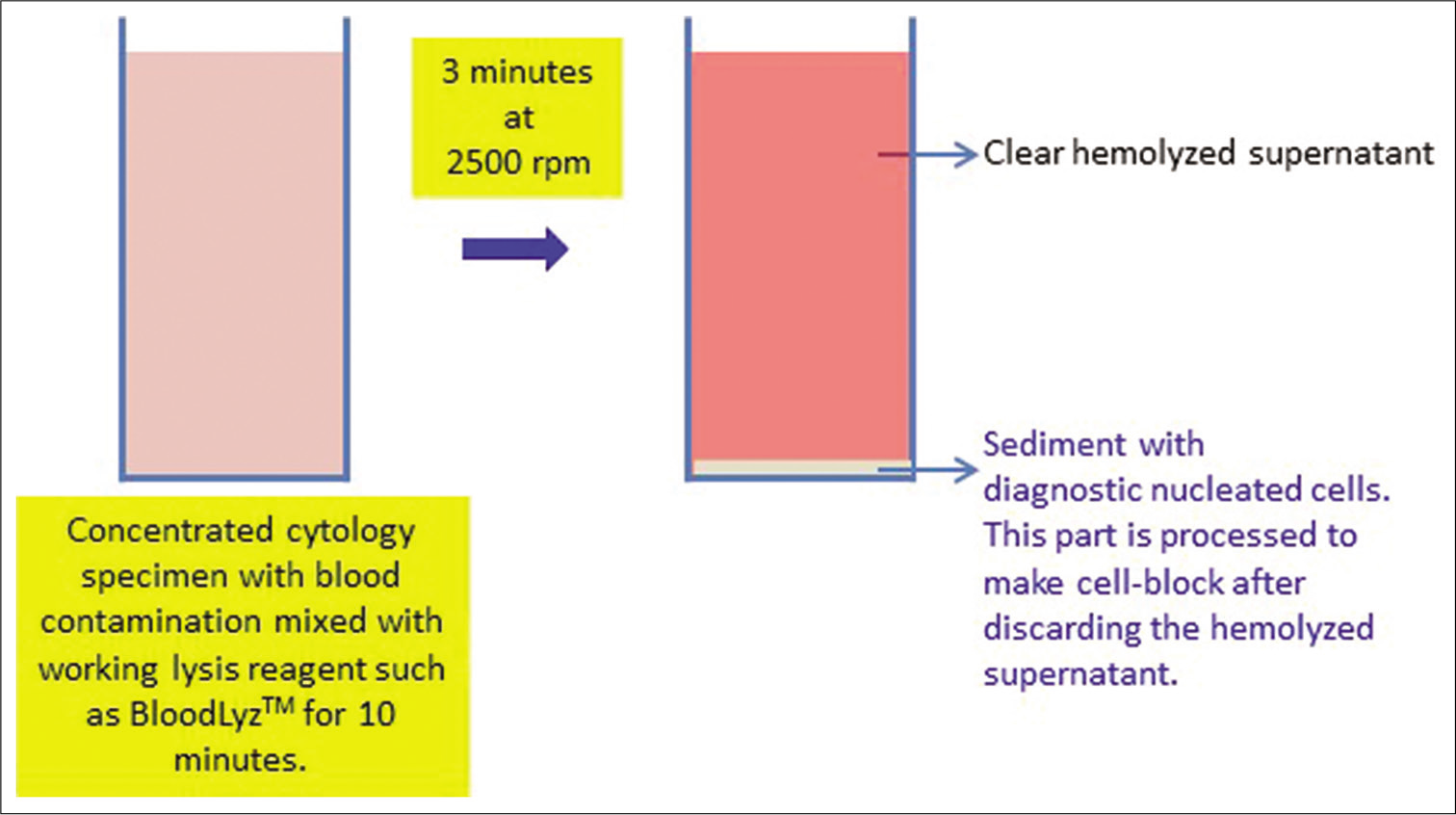
- Processing of blood contaminated cytology specimens with BloodLyz™ to nullify the problems related to red blood cell contamination. (Reproduced from open access article Ref #11).
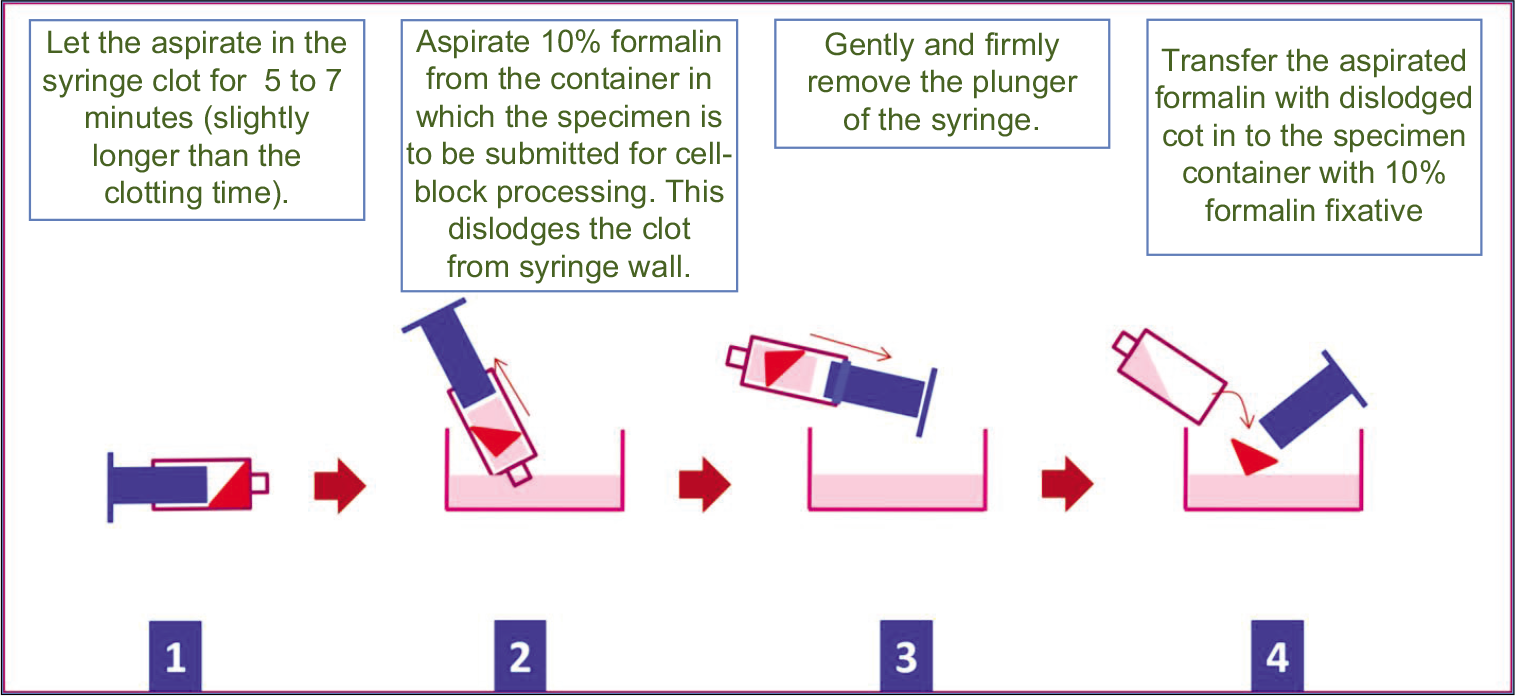
- Cell-blocking of clot in cytology specimen. Reproduced from open access publication: Shidham et al.; Journal of Visualized Experiments; http://www.jove.com/index/Details.stp?ID=1747
SEROUS FLUIDS AND OTHER FLUIDS
Concentrate such fluids by centrifuging as much specimen as possible to get the maximum amount of sediments.
Process this sediment similar to that of sediments from needle rinses of FNA aspirates (with or without predominance of blood contamination) described above.
FAT PAD ASPIRATE AND OTHER TISSUE WITH FAT SUCH AS BONE MARROW ASPIRATE (IMPORTANT CAVEAT: NOT TO CENTRIFUGE)
The most important precaution while processing fat pad aspirates (and specimens similar to this with fatty component) is that the tissue microfragments cannot be concentrated by centrifugation because the fatty tissue microfragments with lower specific gravity would float instead of settling down as sediments. Because of this, the tissue fragments would be discarded inadvertently with the supernatant and lost without including them in the cell-blocks made from such sediments.
This limitation may be resolved with any of the noncentrifugation approaches described below:
-
A simple approach is the filtration of fat pad aspirates for amyloid
The protocol standardized for fat pad aspirates for amyloid in our laboratory is mentioned below:[6]
The fat pad aspirate sampled for studying amyloid with a wider gauge (18 G) needle is transferred to the nylon tissue biopsy bag[7]
The excess blood and fatty component is squeezed out (amyloid is examined in the walls of small blood vessels/ capillaries in the fibrovascular component of fibroadipose tissue in the aspirate); specifically, by squeezing out the nylon tissue biopsy bag filled with fat fad aspirate on the firm, flat surface with filter paper underneath
The bag is then put in 10% formalin in a labeled container to be transferred to the cytology laboratory with other material with the proper requisition form
Transfer the nylon tissue biopsy bag with fat pad aspirate for relatively longer fixation (for at least 24 h) after pre-staining (with hematoxylin or eosin depending on individual laboratory protocol) to the labeled tissue cassette
Process the labeled tissue cassette with fat pad aspirate containing nylon tissue biopsy bag for tissue processing with protocol comparable to that used for fatty tissue such as breast biopsy
Rip open the nylon tissue biopsy bag and scrape off the paraffin-embedded fat pad aspirate to be embedded in paraffin block as usual.
Another previously described option is to process the aspirate as a natural clot [Figure 7][5] in the same way as mentioned for fine-needle aspiration with 18 G aspiration performed for cell-block (see #B above). This approach can be used for fat pad aspirates and bone marrow aspirates. The procedure details are mentioned below:
The aspirated contents in the syringe (sampled as aspiration procedure with a wider gauge: 18 G needle) are allowed to clot in the labeled syringe by waiting for 5–7 min (usual clotting time)
Aspirate 10% formalin in the syringe so that the clot separates from the wall of the syringe as a free-floating coagulum[5]
Open the back end of the syringe by removing the plunger and dislodge the clotted fibroadipose tissue to be transferred into the labeled 10% formalin container [Figure 7]
The intact transferred clot is usually firm after 15–20 min or longer in 10% formalin. If the clot is larger, it should be grossed like surgical pathology biopsies by submitting 2–3 mm thick slices in labeled tissue cassette
Process the labeled tissue cassette containing grossed clot of FNA aspirate for tissue processing with protocol comparable to that used for fatty tissue such as breast biopsy vi. Embed the processed specimen in the tissue cassette in paraffin as usual.
VETERINARY SCIENCES
The role of cell-blocks in Veterinary sciences is increasing in a fashion comparable to that applied by the medical sciences as mentioned above.[8-13]
RESEARCH – ANIMAL EXPERIMENT AND TISSUE/CELL CULTURE
Various specimens from different animal experiments can be processed for cell-blocking similar to Veterinary sciences specimens. A variety of testing could be performed on the cell-blocks by evaluating cells/tissue microfragments as cytopathological specimens with minimally invasive procedures as an ongoing follow-up in the same animal without sacrificing it.
Similarly, tissue cultures and cell cultures can also be cell-blocked for evaluation as formalin-fixed paraffin-embedded (FFPE) tissue. This is significant for a rapid transition to clinical application because the immunoprofile evaluation is predominantly performed as immunohistochemistry (IHC) on FFPE sections. Without this approach, many immunomarkers have been tested typically by immunofluorescence, the results of which may not be extrapolatable to IHC on FFPE. If the novel immunomarker is expected to be applied clinically, it would be ahead of time if that immunomarker can be evaluated at the research stage on FFPE sections. Besides, cell-blocks of the researched cell cultures as FFPE can be archived and evaluated at later stages for various purposes including simultaneous evaluation of various types of cell cultures.
Such cell-blocks may be used as a reliable source of positive controls during immunohistochemical evaluation in various settings including clinical testing. The simple approach is using the Nano NextGen CelBlokingTM kit with Gel-T as described below [Figure 8a and b]:
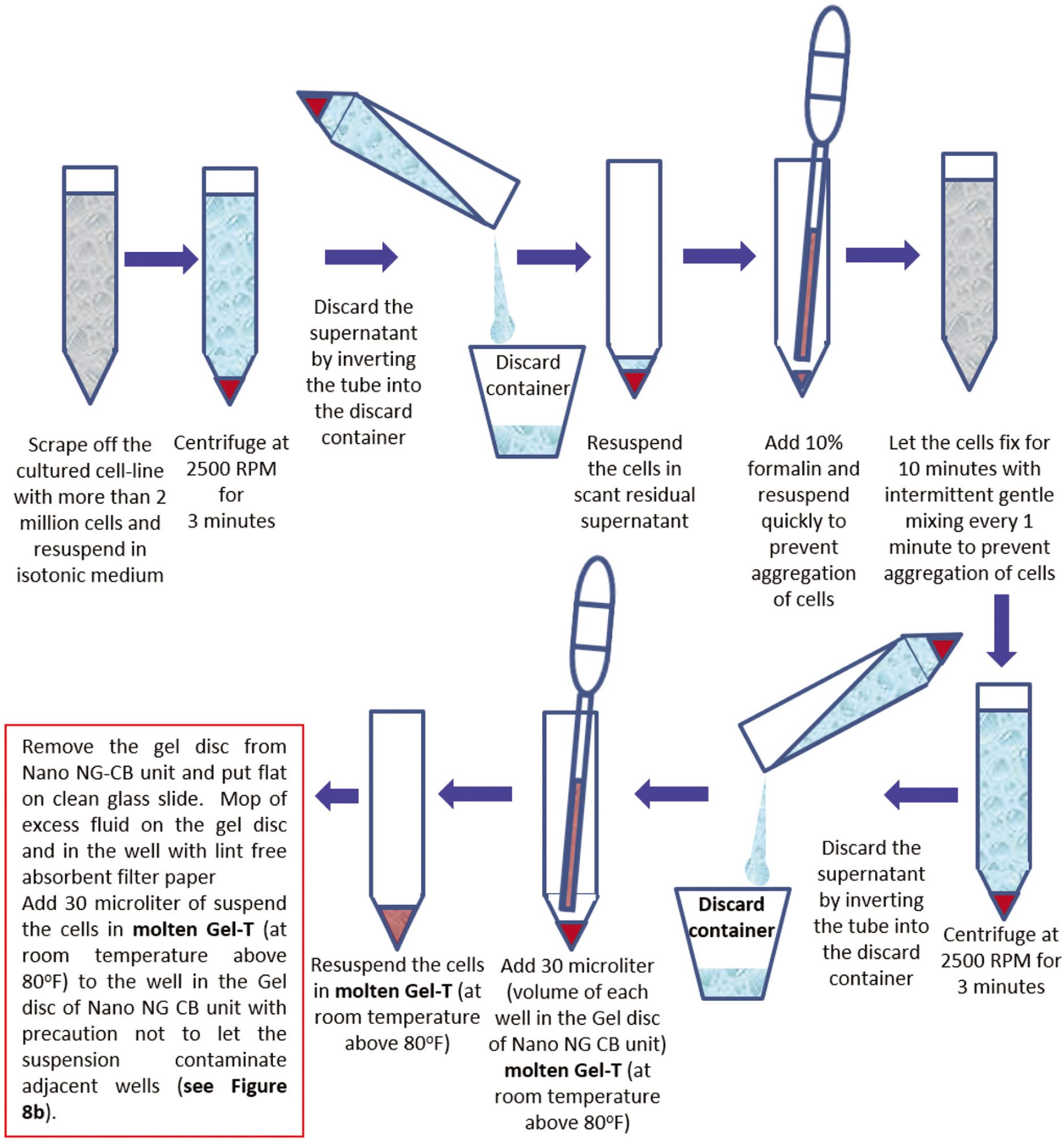
- Cell-blocking of Cell line Culture with Nano NG-CB kit and Gel-T.
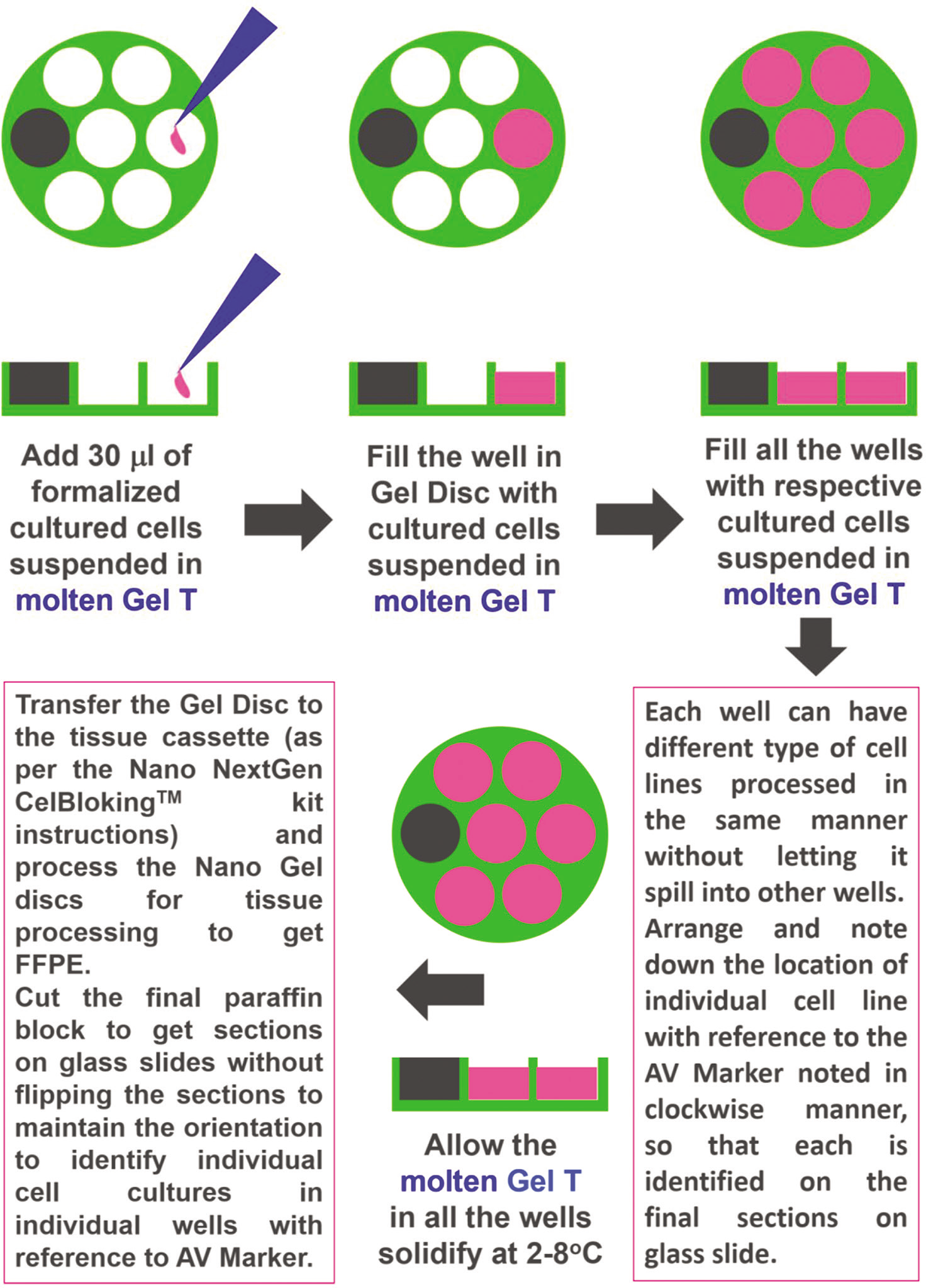
- Cell-blocking of Cell line Culture with Nano NG-CB kit and Gel-T.
Scrape off the culture lines from the culture plates
Count the number of cells in the suspension of the scrapped cell line
Centrifuge at 2500 rpm for 3 min
Discard the supernatant
Add 2 ml of 10% formalin to 2 million cells and resuspend the cells in 10% formalin
Let the cells fixed in 10% formalin for 10 min with periodic mixing every 1 min
Centrifuge and discard the supernatant
Add 30 microliter (μL) of molten Gel-T (kept warm at room temperature 80°F) to the sediment
Remove the individual gel disc from the Nano unit of Nano NG CB kit and remove excess transport fluid, if any. If the wells in the gel disc have any visible fluid, it should be mopped with the pointed tip of a narrow straw-like role of a filter paper sheet
Add 30 μL of molten Gel-T with 2 million cells in the well adjacent to AV marker of the gel disc of Nano NG CB kit
Like step #i through #x, add other cell lines to the remaining wells in the gel disc in an anticlockwise direction. Note the numbers and the position on a template with reference to the AV marker for the record
Instruct the histotechnologist to mount the section of the final FFPE of cell-block on a glass slide without flipping (the order of the wells with various cell lines added in an anti-clockwise fashion, corresponds with unflipped sections in a clockwise manner)
The number of wells in the section would be referred to in a clockwise manner to identify each cell line corresponding with each well in a clockwise manner [Figure 9].
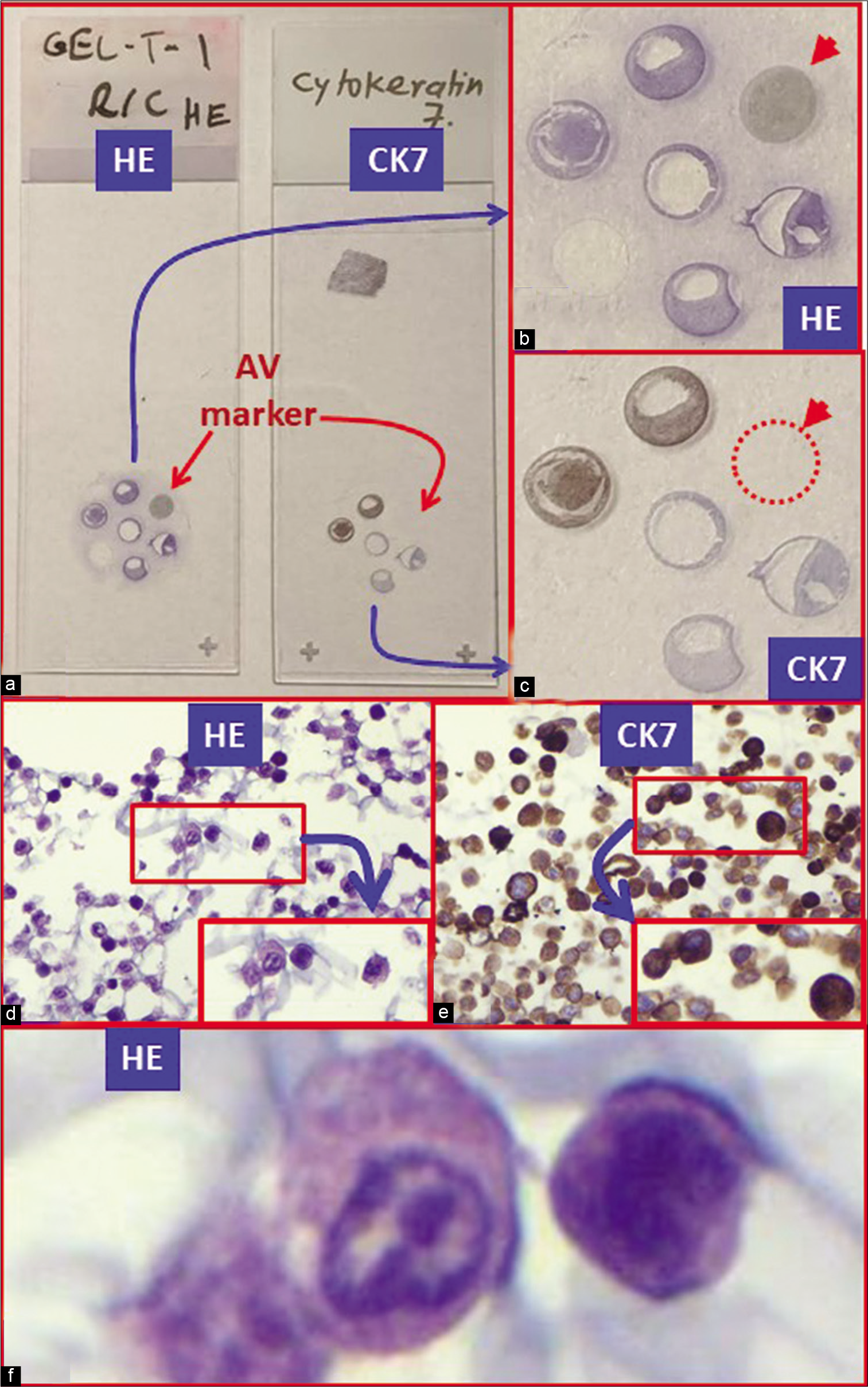
- Cell-block sections of cell lines: (a) Gross photo of glass slides with cell-block sections stained with hematoxylin and eosin (HE) on the left with IHC for cytokeratin (CK) 7 on the right. (b) Pancreatic adenocarcinoma cell line (HE stained). (c) Pancreatic adenocarcinoma cell line (immunostained for CK7). (d and f) Pancreatic adenocarcinoma cell line (HE stained) higher magnification (d). (e) Pancreatic adenocarcinoma cell line (immunostained for CK7) higher magnifications.
SPECIMENS WITH INFECTIOUS POTENTIAL
Bacterial and fungal infections
Viruses
Prions.
Unless a specific pathogen requires a special set of precautions, cell-blocking should be performed under usual universal and standard precautions.[14] However, depending on the known or suspected diagnosis for a particular pathogen, specific precautions should be followed.[14] Most of the pathogens, including viruses, are inactivated by routine formalin fixation used for routine FFPE cell-blocks.[14]
Special modification for specimens with prions or suspected to be positive for prions
Some unusual pathogens, such as prions, need special considerations because these structures do not lose their infectivity even after processing as formalin-fixed tissue. The cell-block has to be treated with formic acid. Depending on the type of processing, the specimens suspected to be positive for human prions should be processed under biosafety level (BSL) 2 or 3. In general, for experimental purposes, most human prions are processed under BSL-3 level. However, for cell-block making, the specimens with human prions may be manipulated at BSL-2.[15]
Prepare the cell-block and fix in 10% formalin for the duration as per the routine protocol
The tissue cassette with cell-block after formalin fixation is transferred to 50–100 ml of 95% formic acid for 1 hour[15,16]
Wash the tissue cassette with cell-block for 30 min in running tap water
Transfer the tissue cassette back to 10% formalin before proceeding for tissue processing.[15,16]
The cell-block sections of formic acid-treated FFPE would require pretreatment or a combination of pretreatments for IHC analysis for the best results.[17] Based on the study on IHC for the prion protein on the brain tissue of CreutzfeldtJakob disease (CJD) patient, hydrated steam autoclaving (HA) for 10 min at 121°C in 10 mM citric acid recovery buffer at pH 6 showed best results.[17] However, for other immunomarkers, it is recommended to standardize this antigen retrieval protocol with a positive control processed in the same manner as with formic acid-treated FFPE of cell-block.
ACKNOWLEDGEMENT
Author thanks Kathy Rost, Janavi Kolpekwar, and Dr. Moumita Saha Roy Chowdhury for the secretarial and copy-editing support.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The author’s (VS) spouse has stakes in AV BioInnovation LLC product(s) cited in this review.
LIST OF ABBREVIATIONS (IN ALPHABETIC ORDER)
BSL, Biosafety Level
CJD, Creutzfeldt-Jakob disease
FFPE, formalin-fixed paraffin-embedded
FNA, fine needle aspiration
G, gauge
IHC, immunohistochemistry
min, minutes
μL, microliter
NG CB kit, NextGen CelBloking kit
RBCs, Red blood cells
Rpm, revolutions per minute
References
- NextGen CelBlokingTM Kits, AV BioInnovation, USA. Available from: https://www.avbioinnovation.com/product/nano-nextgen-celbloking-kit [Last accessed on 2020 Dec 13]
- [Google Scholar]
- Processing of a Single Specimen of Any Cellularity to Make a Cell-block with Nano Unit. Available from: https://youtu.be/y29SS1NwO_8 [Last accessed on 2020 Dec 13]
- [Google Scholar]
- Simultaneous Processing of Multiple Specimens to Make Cell-blocks with Nano Units. Available from: https://youtu.be/ZPb0nq8MsLk [Last accessed on 2020 Dec 13]
- [Google Scholar]
- BloodLyz™. Available from: https://www.avbioinnovation.com/product/bloodlyz [Last accessed on 2020 Dec 13]
- [Google Scholar]
- Performing and processing FNA of anterior fat pad for amyloid. J Vis Exp. 2010;44:1747.
- [CrossRef] [PubMed] [Google Scholar]
- Updates in processing of anterior fat pad aspirate for amyloid (with video and sketches) Cytojournal. 2020;17:15.
- [CrossRef] [PubMed] [Google Scholar]
- Shandon™ Nylon Biopsy Bags. 2020. Available from: https://www.thermofisher.com/order/catalog/product/6774009#/6774009 https://www.archive.is/szap1 [Last accessed on 2020 Apr 22]
- [Google Scholar]
- Use of the agarose cell-block technique in veterinary diagnostic cytopathology: An old and forgotten method. Vet Clin Pathol. 2012;41:307-8.
- [CrossRef] [PubMed] [Google Scholar]
- Liquid-based cytology and cell-block immunocytochemistry in veterinary medicine: Comparison with standard cytology for the evaluation of canine lymphoid samples. Vet Comp Oncol. 2016;14(Suppl 1):107-16.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of carcinoma micrometastases in bone marrow of dogs and cats using conventional and cell-block cytology. Vet Clin Pathol. 2013;42:85-91.
- [CrossRef] [PubMed] [Google Scholar]
- CellBlockistry: Chemistry and art of cell-block making-a detailed review of various historical options with recent advances. Cytojournal. 2019;16:12.
- [CrossRef] [PubMed] [Google Scholar]
- CellBlockistry: Science of cell-block making as ancillary cytopathology component in the era of minimally invasive techniques with increasing role of molecular pathology (invited short communication) Clin Surg. 2019;4:2510. Available from: http://www.clinicsinsurgery.com/pdfs_folder/cis-v4-id2510.pd [Last accessed on 2020 Dec 13]
- [Google Scholar]
- Cell-block preparation methods and advanced techniques In: Önal B, ed. Techniques in Cytopathology-from Basic to Molecular. Ankara: Nobel Tıp Kitabevleri; 2019. p. :128-46.
- [Google Scholar]
- Severe acute respiratory syndrome Coronavirus 2 (the cause of COVID 19) in different types of clinical specimens and implications for cytopathology specimen: An editorial review with recommendations. Cytojournal. 2020;17:7.
- [CrossRef] [PubMed] [Google Scholar]
- APHA; Fixation, Tissue Processing, Histology and Immunohistochemistry Procedures for Diagnosis of Animal TSE (BSE, Scrapie, Atypical Scrapie, CWD) United Kingdom: Animal and Plant Health Agency; 2018. Available from: https://www.science.vla.gov.uk/tse-labnet/documents/histo%20&%20ihc%20protocols%20for%20tse%20diagnosis_rev_jan2019.pdf [Last accessed on 2020 Apr 16]
- [Google Scholar]
- WHO Infection Control Guidelines for Transmissible Spongiform Encephalopathies In: Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 2000. Available from: http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf [Last accessed on 2020 Apr 16]
- [Google Scholar]
- Antigen retrieval in prion protein immunohistochemistry. J Histochem Cytochem. 1999;47:1465-70.
- [CrossRef] [PubMed] [Google Scholar]








