Translate this page into:
Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System

*Corresponding author: Ahmed Alrajjal, MD, Department of Pathology, Wayne State University School of Medicine, Karmanos Cancer Center, Detroit Medical Center, Detroit, Michigan, United States. dr.airajjal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Alrajjal A, Pansare V, Choudhury MS, Khan MY, Shidham VB. Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System. CytoJournal 2021;18:16.
HTML of this article is available FREE at: https://dx.doi.org/10.25259/Cytojournal_24_2021
Abstract
For every 100,000 women in the United States, eight new cervical cancer cases and two deaths are reported as per the most recent (2017) Center of Disease Control and Prevention statistics. Of all the gynecologic cancers (ovary, uterus, cervix, vagina, and vulva), only cervical cancer has a screening test. Cervical Pap test (or Pap smear) is the best screening method for cervical precancerous lesions and is best reported using a unified and a well-established reporting system like The Bethesda System. In this system, “Epithelial cell abnormality: Squamous” includes squamous intraepithelial lesion (SIL) category which encompasses a spectrum of squamous cell lesions starting from the precancerous lesions of low-grade SIL (LSIL) to high-grade SIL (HSIL), and ultimately invasive squamous cell carcinoma. However, depending on the qualitative and quantitative limitations with the specimen, some equivocal morphological features suggestive of squamous cell abnormality may fall under equivocal category: “Atypical Squamous Cells” (ASCs), which are subdivided into two categories; “Atypical Squamous Cells of Undetermined Significance” (ASC-US) or “Atypical Squamous Cells, HSIL cannot be excluded” (ASC-H), based on the suspected underlying lesion LSIL versus HSIL, respectively. This review provides the key cytologic features that distinguish Bethesda squamous categories from other important entities, using algorithmic approach and illustrations of common cytomorphologic patterns for clear identification of those entities in practice. The important mimickers which may be considered during the differential interpretation of SIL are discussed and presented here in a brief cytomorphologic review.
Keywords
Cervical cancer
Pap test
Cervical cytology
Cervical intraepithelial neoplasia
Human papilloma virus
HPV
This article may be reproduced under ‘open access charter’ and included in the multi-chapter, multiauthor CytoJournal’s CMAS (CytoJournal Monograph and Atlas Series) book series or part of other book as one of the chapters after minor modifications as required.
If included in CMAS, the monograph will be available as e-book version on CytoJournal website (www.CytoJournal.com) and also as in print version.
INTRODUCTION
According to the most recent (2014-2018) Center of Disease Control and Prevention statistics, eight new cervical cancer cases and two deaths are reported per 100,000 women in the United States.[1] Pap test (or cervical Pap smear) is the time tested screening method for cervical precancerous. The Bethesda “Epithelial cell abnormality: Squamous” category encompasses a spectrum of squamous cell lesions starting from the precancerous lesions of low-grade dysplasia associated with transient human papilloma virus (HPV) infection to higher grade lesions, including cervical intraepithelial neoplasia 2 and 3 (CIN 2 and 3) and ultimately invasive squamous cell carcinomas. Based on our current understanding of carcinogenic potential of HPV, a dichotomous diagnostic terminology for squamous lesions of the lower anogenital tract has been recommended for both mucosal and cutaneous lesions, regardless of the specimen type (histologic and cytologic specimens).[2]
The two-tiered system of low-grade SIL (LSIL) and high-grade SIL (HSIL) matches the HPV carcinogenic potential and allows for better communication between pathologists and other patient care providers. [3] The Bethesda System (TBS) for reporting cervical cytology follows this approach (updated in 2015) [Figure 1]. This system is also applicable with minor modifications for vaginal cytology[4] and anal Pap.[5]

- The cytology reporting of cervicovaginal specimens has changed over the past 6 decades. The previous systems and the current WHO are linear with only definitive diagnostic categories. While The Bethesda System is non-linear and introduced atypical squamous cells, which can include changes from benign to the neoplastic spectrum.
One of the most important diagnostic features used during the interpretation of epithelial cell abnormality is based on nuclear enlargement compared with the size of intermediate cell nuclei (ICN) as internal ruler [Figure 2].

- (a) ThinPrep Pap showing binucleation with nuclear features, including enlargement, hyperchromasia, and contour irregularities. (b) ThinPrep Pap with thick clumps of koilocytes of varying sizes and clear halos and binucleation. The sketch on right compares nuclear area (size) of LSIL nucleus against the area of intermediate cell nucleus “internal ruler.”
In general, hyperchromatic nuclei with coarse chromatin in cells of SIL lack nucleoli. However, this simple and important morphological clue may be difficult to be evaluated due to subtle overlap of nucleolar morphology with prominent chromocenters in some nuclei [Figure 3]. Chromocenters are heterochromatic, punctate, condensed blocks of chromatin. These should not be confused with nucleoli, which are usually sharply delineated eosinophilic round structures in contrast to relatively smaller and ill-defined chromocenters with staining pattern similar to the adjacent chromatin [Figure 3].

- Morphological features of nucleolus versus chromocenter in Pap-stained preparation.
LSIL is now recommended to be used as a diagnostic category to describe HPV transient infection-related changes, while HSIL is used to categorize true precancerous lesion.[2] However, depending on qualitative and quantitative factors, some equivocal morphological features may fall under the category “Atypical Squamous Cells” (ASCs), which are subdivided into two categories; “Atypical Squamous Cells of Undetermined Significance” (ASC-US) or “Atypical Squamous Cells-HSIL cannot be excluded” (ASC-H), based on the suspected underlying lesion LSIL versus HSIL, respectively.
In addition, although not considered by TBS for reporting cervical cytology updated in 2015, some cases may show unequivocal features of LSIL with definitive interpretation, in addition to the equivocal squamous cell abnormality suspicious for ASC-H (LSIL-H). The management of this category would be comparable to HSIL and ASC-H, but for statistical purposes, this would be a definitive interpretation in the LSIL category. This is a brief cytomorphologic review of these SILs, including LSIL-H.[6,7]
HPV and SIL
The relative risk for developing cervical cancer with high-risk HPV (HR-HPV) is greater than 70 as compared to less than 5 with other risk factors such as smoking, high parity, prolonged use of oral contraceptives, and other sexually transmitted infections. Hence, there is a wide acceptance of cervical cancer as a sexually transmitted disease that should be diagnosed at an early stage through screening methods utilizing a combination of Pap test and HR-HPV DNA testing (contesting). The initial cytological findings suggestive of gray-zone interpretations such as ASC-US and ASC-H may be categorized into one of the definitive interpretations by downgrading or upgrading the initial impression depending on HR-HPV status along with other clinical details as ancillary support. This is now possible in cases where HPV test results are available as part of cotesting. However, it is important not to allow negative or positive bias to compromise the final cytomorphological interpretation in concert with this ancillary support.
Another important point to be considered while practicing cotesting is that negative HR-HPV test does not rule out low-risk HPV. Due to this, it is not rare to detect LSIL in HPV negative cases due to low-risk HPV. In addition, a rare possibility of false-negative HR-HPV in higher grade lesions should be kept in mind, especially in some late cases of invasive carcinoma.[8-10]
LSIL
LSIL refers to morphologic changes along the lower end of the spectrum of SIL. About 1.7% of all PAPs are interpreted as LSIL, the majority of which (>80%) are positive for HR-HPV [Table 1]. One of the major cytomorphologic and an easily identifiable feature of LSIL is koilocytosis. Koilocytes show raisinoid nuclei with sharply delineated perinuclear cytoplasmic clearing with irregular outline including focal angulations [Figure 4]. The nuclei show nuclear enlargement, hyperchromasia, and nuclear membrane irregularities. Binucleation and multinucleation are frequent [Figure 5].
| Cytology | Frequency (%) | Frequency of HR-HPV+ |
|---|---|---|
| NILM | ≅ 94 | 4 |
| ASCUS | 3.6 | 54 |
| LSIL | 1.7 | 87 |
| ASC-H | 0.3 | 82 |
| HSIL | 0.3 | 95 |
Adopted from the (2019) ASCCP management consensus guidelines. Egemen D et al. “Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines.” Journal of lower genital tract disease 24.2 (2020): 132

- (a) Perinuclear cytoplasmic clearing (koilocytosis) (b and c) sharply demarcated irregular outline with focal angulation is characteristic of HPV cytopathic effect. (Halo is not required for LSIL. Nuclear features are sufficient. Compare with benign intermediate cells)

- (a) Halo is not required for LSIL if dysplastic nuclear features are present. (b) Binucleation and (c) hyperchromasia are common features of LSIL.
The cytomorphological distinction between HPV-related changes and mild dysplasia (CIN1) is poorly reproducible without useful practical application in management algorithm.[11] On an average, LSIL lesions are found in women 10 years younger than those with invasive cancer.[11] Moreover, LSIL cases are caused by a transient self-limiting HPV infection that usually lasts less than 2 years. On the other hand, persistent infection carries higher risk of progression to neoplastic transformation.[12]
HPV status plays a major role in predicting the immediate risk of HSIL. HPV-positive LSIL cases are associated with a higher immediate risk of CIN3 as compared to HPV-negative ones. As a result, based on HPV status, the immediate risk of CIN3 on a PAP test interpreted as LSIL ranges from 1 to 4.3% [Table 2]. Immediate CIN3 risk is defined as the probability of having clinically detectable CIN 3 if referred to colposcopy at the time of PAP test.[12-14] The immediate risk of CIN3 is regarded as the most relevant determinant for indicated clinical intervention.
| Cytologic interpretation | Immediate risk of CIN3+ | |
|---|---|---|
| HR-HPV (–) | HR-HPV (+) | |
| NILM | 0.00 | 2.1 |
| ASC-US | 0.04 | 4.4 |
| LSIL | 1.1 | 4.3 |
| ASC-H | 3.4 | 25 |
| HSIL | 25 | 48 |
The patient management for abnormal PAP relies on the immediate risk of CIN3+. Adopted from the (2019) ASCCP management consensus guidelines. Egemen D et al. “Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines.” Journal of lower genital tract disease 24.2 (2020): 132
APPROACH FOR CYTOMORPHOLOGICAL INTERPRETATION OF LSIL
On low-power magnification, most LSIL cells with significant proportion of cytoplasm similar to superficial and intermediate squamous cells should stand out with larger and darker nuclei either as groups or as single cells. In addition to other features, enlargement of the nuclei, at least 3 times the size of ICN, is important feature of LSIL cells. Multinucleation with 2–3 nuclei may be seen. The chromatin is usually coarse but might be hazy and very dark with raisinoid appearance (especially related to HPV-related lesion). The dysplastic cells may also show features of koilocytosis with a large, clear, perinuclear intracytoplasmic space, or pocket with irregular accentuated outline with focal angulation [Figure 4].
Koilocytic change is very helpful for the diagnosis of LSIL. However, it is not required for diagnosis nor is it diagnostic without other nuclear features. Similarly, the cytomorphological features of LSIL may not always be definitive, with some gray-zone findings that are suspicious but not definitive for LSI and fall under ASC-US category. LSIL with immature metaplastic cytoplasm may be difficult to distinguish from ASC-H or HSIL. This may be associated with eosinophilic dysplasia[15] or from acanthotic component of condylomatous lesion.[16]
SUMMARY OF CYTOMORPHOLOGICAL FEATURES OF LSIL CELLS
Nuclear enlargement (nuclear size more than 3 times ICN) with intermediate/superficial cell-like cytoplasm with relatively low N/C ratio, as compared to intermediate cells [Figure 2]
Nuclear hyperchromasia with occasional binucleation [Figures 4 and 5]
Variably irregular nuclear contours with sharp angulations and indentations
Coarse chromatin
May show increased keratinization seen as dense orangeophilia (atypical parakeratosis)[11]
Sharply demarcated perinuclear cytoplasmic clearing (koilocytosis) with irregular outline with focal angulation is characteristic of HPV cytopathic effect [Figure 4]
LSIL cells with immature metaplastic cytoplasm (small atypical parakeratotic [SAPK] cells) may be difficult to distinguish from ASC-H or HSIL. This may be associated with eosinophilic dysplasia[15] or from acanthotic component of condylomatous lesion.[16]
DIFFERENTIAL DIAGNOSIS
A few important entities which may be considered during the differential interpretation of LSIL in cytology are listed below:
Parakeratosis/pseudoparakeratosis/atypical parakeratosis [Figure 6]
Parakeratosis is seen as clusters of cells with dense orangeophilic cytoplasm and small pyknotic nuclei with low N/C ratio. These may be misinterpreted as LSIL (or ASC) if the nuclear features are equivocal
Parakeratosis is not pre-neoplastic by itself and should not be included in LSIL/ASC-US categories. However, parakeratosis can be seen in the background of infections including an HPV infection, and hence, careful search for LSIL should be done when one encounters parakeratosis
Pseudoparakeratotic cells are small squamous cells with intense orange cytoplasm and pyknotic nuclei [Figure 6][15]
If nuclear atypia is present in otherwise keratinized clusters, the case should be assigned ASC, LSIL, or HSIL based on the degree of nuclear abnormalities of dysplasia.

- (a and b) Pseudoparakeratosis with dense orangeophilic cytoplasm with small pyknotic nuclei and low N/C ratio. (c and d) Pseudoparakeratosis might be misinterpreted atypical if with relatively larger and darker nuclei.
Pseudokoilocytosis [Figure 7]
Koilocytic change or perinuclear cytoplasmic clearing can be seen without dysplasia. By itself, koilocytic change should not be interpreted as LSIL. Characteristic nuclear abnormalities, as described above, are required for diagnosis of LSIL
Glycogenated cells may be associated with pregnancy and atrophy with so-called “Navicular cells” [Figure 7]
Navicular cells and cells with tight halos are distinguished from koilocytes by lacking a sharp “cookie-cutter” edge and lacking associated dysplastic nuclear features
Similarly, perinuclear halo noted in cases with infections and inflammation such as Candida and Trichomonas should not be confused with koilocytic space (especially when this space is relatively larger in some cells than the subtle faint perinuclear area). It is a cytomorphologic feature observed as a space around the nucleus secondary to nuclear shrinking related to processing for staining.

- (a) Pseudokoilocytes “tight halos” (long arrow) are common findings in atrophic specimens and with infections (e.g., Candida). (b) Glycogenated “navicular” cells (short arrow) also show perinuclear space occupied by yellow tinged “glycogen,” however, nuclei are negative for dysplastic features.
Herpes simplex virus (HSV) cytopathic effects [Figure 8]
Classic 3 M’s: Multinucleation, Margination (of chromatin), and Molding (of nuclei)
Multinucleation with multiple nuclei should not be mistaken for multinucleation seen in LSIL
Chromatin margination to the nuclear membrane periphery giving rise to central ground glass appearance is a characteristic feature of HSV effect
Early HSV infection may not show all three classic features and may manifest only as nuclear hyperchromasia mimicking with some nuclear features of ASC, LSIL, or HSIL.

- (a and b) Herpes simplex viral cytopathic effects. Chromatin margination to the nuclear membrane periphery with ground-glass appearance and inclusions in some cases. Nuclei mold into each other. All three classic nuclear features “3 Ms” multinucleation, molding, margination may not be seen in early herpes infection.
Radiation changes
Clusters and sheets of cells or isolated cells
Cytomegaly with enlargement of both nucleus and cytoplasm without significant change in N/C ratio in contrast to SIL
Characteristic two-toned, vacuolated cytoplasm
Multinucleation
Hyperchromatic smudged chromatin
Some cases post-radiation therapy may show both tumor cells and reactive cells with radiation effects. Careful search for distinction should be made.
HSIL
HSIL refers to morphologic changes associated with higher end of the SIL spectrum and includes both CIN 2 and CIN 3 (with CIS). About 0.3% of all PAPs are interpreted as HSIL, almost all (95%) of which are HR-HPV positive [Table 1]. HSIL has a higher rate of progression to cancer and a lower rate of regression. Long-term progression to invasive cancer is estimated at 30% for 30 years.[14]
One may encounter HSIL with any other squamous or glandular abnormalities. The goal is to communicate the potential higher risk in these cases. Cases where HSIL cells are present with predominance of LSIL in the background should be interpreted as “HSIL.” On the other hand, if only a few questionable HSIL cells are present in a background of LSIL, then such cases may be categorized by some as ASC-H or alternatively LSIL, cannot rule out high-grade dysplasia (LSIL-H).[6,17,18]
Approach for cytomorphologic Interpretation of HSIL
Under low-power view, two-dimensional sheets of cells or multiple three-dimensional hyperchromatic crowded groups (HCGs) seen as a syncytium can be identified. In general, CIN 2 cells may be seen as checkerboard pattern [Figure 9] and CIN 3 cells as the syncytial aggregates [Figure 10]. In liquid-based cytology (LBC) preparations, HSIL cells tend to present as scattered single dysplastic cells, which if scant in number, may be easily missed [Figure 11].

-
Checkerboard pattern of HSIL, showing dark nuclei, smudgy chromatin, nucleoli, randomly distributed apoptotic bodies (arrow) (in contrast to MGH with apoptosis in the area corresponding with nucleus). This pattern correlates with CIN2 on biopsy. Adopted from CytoJournal open access. Chivukula M, Shidham VB. Cytojournal 2006;3:14.

-
Syncytial pattern of HSIL showing hyperchromatic crowded groups of cells without distinct cell borders. The hyperchromatic nuclei vary in size and show coarsely granular chromatin. Adopted from CytoJournal open access. Chivukula M, Shidham VB. Cytojournal 2006;3:14.

-
Singly scattered/isolated cell pattern (so called “litigation”) HSIL cells featuring scattered, isolated, atypical cells show high N/C ratio. The nuclei have coarse chromatin without nucleoli. Adopted from CytoJournal open access. Chivukula M, Shidham VB. Cytojournal 2006;3:14.
Under high-power view, one can easily identify significant nuclear atypia characteristic of HSIL. Careful examination of nuclear membrane and chromatin pattern would differentiate dysplastic cells from reactive cells, especially the smaller sized cells such as basal and parabasal cells seen in atrophic smears [Figures 6 and 12]. In general, HSIL cells are smaller than LSIL, both in terms of cytoplasmic volume (maturity) and the actual nuclear size (but still 3 times ICN) with high N/C ratio. However, the nuclei display significant dysplastic features such as hyperchromasia, coarse but delicate chromatin, and nuclear membrane irregularities without nucleolar prominence. Longitudinal nuclear grooves (Tulip flower-like) and indentations are seen typically. These key nuclear features may not be necessarily displayed all together in the same HSIL case, rather, one or more of the characteristic features tend to predominate.

- Various normal cellular components seen on Pap test compared to HSIL. The cell sizes and how they relate to each other are shown in the drawings. Actual pictures of superficial, intermediate, parabasal, metaplastic, and HSIL are shown below the drawings.
Apoptotic bodies with mitotic figures can be seen in some HSIL groups. Features of squamous cell carcinoma such as necrotic debris, dysplastic tadpole-shaped cells, or fiber cells when seen, should be classified as squamous cell carcinoma or HSIL with features suspicious for invasive squamous cell carcinoma, based on the degree of severity. Dysplastic nucleoli in early invasion may show nucleoli. However, HSIL involving cervical glands may show groups of atypical HCG with nucleoli in the cells along the periphery of HSIL cells without nucleoli [Figure 13].

- HSIL cells with endocervical gland involvement seen as (a) hyperchromatic crowded groups (HCG) of cells with epigenetic features showing (b) high N: C ratio, hyperchromatic nuclei evenly distributed coarse chromatin and prominent nucleoli at the periphery of the group (arrow in c). The dysplastic cells in the center (arrowhead in c) show features of HSIL without nucleoli.
Other common problematic patterns of HSIL include cases where HSIL is arising in a background of atrophy leading to equivocal interpretation of ASC-H [Figures 14 and 15] and keratosis, referred to as “Keratinizing high-grade lesions” [Figure 16]. Scant, single scattered HSIL cells can be challenging to detect, especially in LBP, as mentioned above. Such singly scattered rare cells with unequivocal features of HSIL may be a critical liability in Pap test screening, hence the designation of these cells as “litigation cells” [Figure 11].[19] Specimens with LSIL, ASCUS, and ASCH may be associated with HSIL cells. Because of this, such Pap smears should be scrutinized and screened with extra precautions. Some cases may show only bare dysplastic nuclei with HSIL-like features. Such cases, there may be a few intact HSIL cells for definitive interpretation. Otherwise, such cytology preparations only with bare nuclei should be categorized as ASC-H.

- ASC-H. Atrophy-like pattern showing cohesive hyperchromatic crowded groups of small parabasal cells with slightly enlarged darker nuclei, with nucleoli (although relatively small). N/C ratio may appear higher due to smaller size of cells. Adopted from CytoJournal open access. Chivukula M, Shidham VB. Cytojournal 2006;3:14.

- (a and b) ASC-H: Atrophy can present as sheets of atrophic squamous cells raising the differential of HSIL “syncytial pattern.”

- Keratinizing HSIL cells showing pyknotic and hyperchromatic nuclei with high N: C ratio with coarse and irregularly distributed chromatin with attenuated cytoplasm. The numerous bizarre keratinizing (orange) cells and the granular background are highly suggestive of invasion. Image from The Bethesda System for reporting cervical cytology.
Summary of cytomorphological features of HSIL cells
Singly scattered HSIL cells [Figure 11], as sheets with checkerboard pattern [Figure 9] or in syncytial aggregates of HCG [Figure 10]
HSIL cells are smaller and show less cytoplasm than cells of LSIL [Figure 17]
High N/C ratio compared to LSIL
Irregular nuclear contours with frequent indentations and longitudinal nuclear grooves [Figure 18]
Usually hyperchromatic nuclei, but occasionally normochromic or rarely even hypochromatic
Typically, evenly distributed coarse chromatin; but sometimes can be fine [Figure 19]
Nucleoli are generally absent but may be seen along the periphery of HSIL cell groups in cases with endocervical glandular extension
Cytoplasm is variable from “immature” dense “metaplastic” with focal vacuolation to occasionally densely keratinized cytoplasm (in contrast to adenocarcinoma in situ [AIS] with lacy and delicate cytoplasm) [Figure 19].

- Various normal cellular components seen on Pap test against squamous intraepithelial lesions (SILs). The cell sizes and how they relate to each other are shown in the drawings. Microscopic images (all at the same magnification) of superficial, intermediate, parabasal, LSIL, and HSIL are shown below the drawings.

- HSIL showing irregular nuclear contours with subtle indentations (some nuclei may show longitudinal Tulip flower bud-like folds).

- Cytoplasm of HSIL versus AIS cells: (a and b) Groups of HSIL cells. The metaplastic cytoplasm of cells as seen in the cells at the periphery (arrow) is dense and homogeneous. (c and d) Groups of AIS cells. The cytoplasm shows lacy appearance (arrow).
DIFFERENTIAL DIAGNOSIS
A few important entities which may be considered during the differential interpretation of HSIL in cytology are listed below:
Atrophy [Figure 15; also Figures 14 and 20]
HCG of parabasal cells associated with atrophic cellular changes may mimic HSIL on low power, but they lack the characteristic nuclear dysplastic features of HSIL on high power
Nuclei of parabasal cells are small with smooth nuclear contours and fine chromatin pattern with nucleoli although indistinct
Postmenopausal women can have sheets of parabasal cells mimicking syncytial variant of HSIL.

- Squamous metaplastic cells show mild nuclear enlargement, with mild nuclear contour irregularity and a distinctive cytoplasmic turquoise color with occasional cytoplasmic processes (arrow) “spider cells.”
Squamous metaplasia [Figures 20 and 21]
Mild nuclear enlargement, not more than 50% N/C ratio
Mild nuclear contour irregularity
Cytoplasm: Distinct turquoise color, with extensions “cytoplasmic processes” “Spider cells”
Lack of marked nuclear atypia, as seen in HSIL.

- ASC-H. Atrophy-like pattern showing isolated hyperchromatic metaplastic cells (may resemble parabasal cells) with atypical nuclei and smudgy chromatin. Adopted from CytoJournal open access. Chivukula M, Shidham VB. Cytojournal 2006;3:14.
AIS [Figure 19]
AIS is relatively rare as compared to HSIL. AIS cells exhibit nuclear atypia characterized by hyperchromasia without nucleoli similar to that seen in HSIL, but with cigar-shaped elongated nuclei with lacy cytoplasm (as compared to dense, metaplastic cytoplasm with focal vacuolation in HSIL cells) [Figure 19]
Most distinguishing feature of AIS cells is palisading with nuclear feathering (better seen in Pap-stained direct smears). Without these features, the hyperchromatic cluster under examination may resemble HSIL, especially in LBC preparations
However, some cases may have AIS and HSIL synchronously [Figure 22].

- Synchronous HSIL with AIS.
Exfoliated endometrial cells [Figure 23]
Exfoliated endometrial cells appear as dark, tight balls of small cells (exodus)
Cells are fairly small in size, compared to HSIL, even the small cell variant
Nuclear hyperchromasia may be seen, but they lack all the other dysplastic features of HSIL
The nuclei are irregular but fairly uniform with less dense cytoplasm with focal vacuolation. HSIL cells tend to show more nuclear variation and a denser cytoplasm.

- HSIL. Specimen with CIN2 and CIN3 cells. The CIN3 cells may resemble HCG of endometrial cells with high N: C ratio, tight ball of small cells, hyperchromatic, and dysplastic nuclei (with lack of nucleoli).
Endocervical polyp
Disorganized clusters often with intermixed neutrophils and florid reactive/reparative changes and bland nuclei
Prominent nucleoli may be seen
Nuclei lack dysplastic features of HSIL such as coarse chromatin and significant nuclear contour irregularities.
Microglandular hyperplasia (MGH)
The cytomorphological features of squamous metaplastic cells in MGH may overlap with HSIL, especially when the nuclei are slightly larger and with degenerative hyperchromasia
The presence of centrally located apoptosis in area corresponding with nucleus in occasional metaplastic cells is usually seen in MGH as compared to randomly scattered apoptotic bodies in HSIL [Figures 24 and 25].[20]

- Apoptosis pattern in HSIL (CIN2) versus microglandular hyperplasia (MGH). Single-cell apoptosis in HSIL is not uncommon and typically shows dispersed nuclear fragments in the cytoplasm. In contrast, MGH cells which can also show single-cell apoptosis, show the apoptotic bodies restricted in the area of nucleus similar that observed in the normoblasts.
![Apoptosis in microglandular hyperplasia (MGH) (vs. apoptosis in HSIL): mercury droplet-like apoptotic bodies are [Figure 24] restricted to the area of nucleus (normoblast-like pattern) in MGH, in contrast to the randomly scattered apoptotic bodies in HSIL cells.](/content/105/2021/18/1/img/Cytojournal-18-16-g025.png)
- Apoptosis in microglandular hyperplasia (MGH) (vs. apoptosis in HSIL): mercury droplet-like apoptotic bodies are [Figure 24] restricted to the area of nucleus (normoblast-like pattern) in MGH, in contrast to the randomly scattered apoptotic bodies in HSIL cells.
Chronic follicular cervicitis (CFC) [Figure 26]
Mixture of small and large (polymorphic) lymphocytes admixed with epithelial cells
They display high N/C ratio with coarse chromatin pattern
When the reactive lymphocytes are the dominant cell type, some may mimic singly scattered HSIL cells
One must look for other squamous cells which may or may not be dysplastic
Tingible body macrophages, when seen, are assurance in favor of CFC.

- (a) Follicular cervicitis showing singly scattered reactive lymphocytes (red arrow) with round nuclei and thin rim of fluffy cytoplasm may resemble single-cell pattern of HSIL. (b) Reactive endocervical cells (black arrow) adjacent to reactive lymphocytes, mimicking HCG pattern of HSIL. (c) Reactive lymphocytes in follicular cervicitis can be confused with ASC-H or HSIL. (d) HSIL single-cell pattern (thick arrows) for comparison (same magnification).
Macrophages
Floridly reactive macrophages may be misinterpreted as singly scattered HSIL cells, especially in LBP
Eccentric oval/round or reniform nuclei with foamy cytoplasm.
ATYPICAL SQUAMOUS CELLS (ASC): INCLUDES ASC-US AND ASC-H
ASC interpretation should be used to designate ASCs that fall short of LSIL and HSIL features due to quantitative or qualitative limitations.
The current terminology divides ASC into two distinct subcategories:
ASC-US (ASCs of undetermined significance)
ASC-H (ASCs, cannot be excluded HSIL).
Although this category is created, it is expected to be used carefully and not as non-specific dustbin interpretation. This category should be reported with appropriate management recommendation under the comment section as indicated. The frequency of ASC interpretation among laboratories differs based on the patient population and the interpretation skill level of the cytopathology laboratory.
In general, the ASC category is the most frequent interpretation in abnormal category (around 4%) [Table 1]. ASC category should not exceed 5% based on expert consensus. However, for laboratories with higher risk population, ASC/SIL ratio of 3:1 can be used as a QA measure to monitor over and under interpretation. The currently available parameter related to ASC incidence in relation to HPV positivity is additional QA measure.[21]
ASC-US
ASC-US is relatively more prevalent with cytomorphological features revealing higher nuclear atypia than reactive changes. The atypical features are equivocal for definitive dysplasia due to qualitative reasons with or without quantitative limitations such as atypical changes only in scant cells.
However, well-defined criteria for ASC-US are not established. The features may range from marginal increase in size of the nucleus to other features such as nuclear hyperchromasia and irregular nuclear membranes. There may be an overlap of some cytomorphologic features from non-dysplastic etiologies such as Vitamin B12/folate deficiency associated megaloblastic nucleomegaly and age-related increase in nuclear size.[11] Atypical parakeratosis and atypical repair are included under ASC-US category.
Difficulty may arise especially in specimens with atrophic cellular pattern and infection-related reactive changes such as squamous metaplasia and parakeratosis. In such clinical scenarios, including a comment explaining potential differential interpretation with recommendation to improve further management may be useful. For example, cases of ASC-US with atrophic cellular pattern/atrophic vaginitis may benefit from repeating the Pap smear after estrogen therapy (systemic or local). Similarly, ASC-US in association with infection may benefit from repeating the Pap smear after treating the infection.
Cytomorphological features of ASC-US
Enlarged nuclear size 2.5–3 times ICN
Mild increase in N/C ratio
Mild hyperchromasia and nuclear irregularity
Atypical parakeratosis with orangeophilic cytoplasm and equivocally atypical nuclei
Atypical repair with “School of Fish” streaming pattern with prominent nucleoli.
Differential diagnosis of ASC-US
-
Infections such as Candida, Trichomonas vaginalis, and HSV [Figure 8].
Squamous cells show tight perinuclear halos
Hyperkeratosis
Moth-eaten cytoplasm.
-
ii. Reactive inflammatory changes
Nuclear size 1.5–2.5 times ICN with anisonucleosis
Hypochromatic nuclei with clearing
Multinucleation
Nuclear degeneration (pyknosis)
May mimic ASC-US or ASC-H.
-
iii. Repair [Figure 27]
Features of reactive cells with inflammation
Enlarged cells (nuclei smaller than 2 times ICN)
Nucleolar prominence
“School of fish” appearance with spindle-shaped epithelial cells streaming in one direction.
-
iv. Parakeratosis
Small “miniature” cells in clusters with densely orangeophilic cytoplasm
Can be seen in whorls of keratin (squamous pearls)
Nuclear atypia not seen.

- “Repair-like” pattern (ASC-H, favor repair) featuring cohesive groups of cells with ill-defined school of fish pattern with relatively polarized cells with pointed ends show relatively low N/C ratio. Adopted from CytoJournal open access. Chivukula M, Shidham VB. Cytojournal 2006;3:14.
ASC-H
ASC-H is designated for cases with cellular changes that are equivocal for high-grade dysplasia because of either qualitative or quantitative limitations. An ASC-H is a significant interpretation. Proper designation of ASC-H cases is necessary as it might be downstream to a completely different route of management.
In contrast to ASC-US, a study categorizing cytomorphological spectrum of ASC-H based on correlation with biopsy results is reported.[6] The described features are summarized in Table 3. The spectrum may allow proper management by adding comment in the cytopathology report.[6,18] The cytomorphological features range from reactive to LSIL to HSIL potential [Table 3, Figures 9, 10, 11, 21, 27-29].
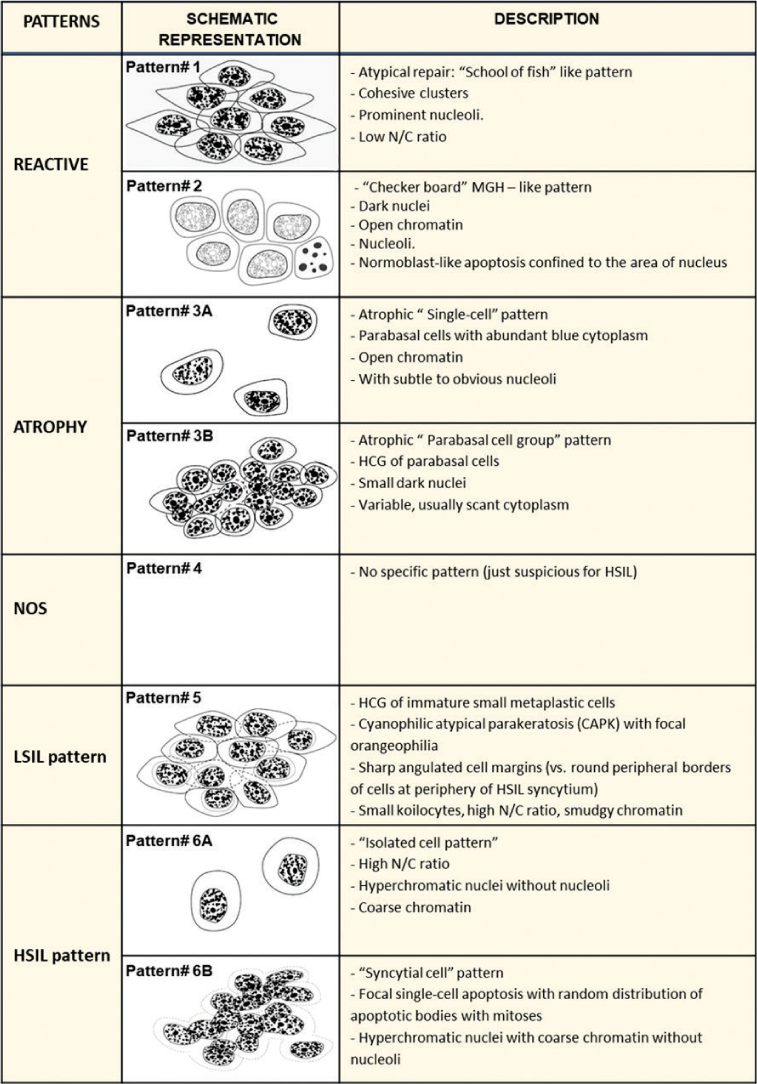 |
Modified from open access article CytoJournal 2006, 3:14[7]

- ASC-H Small atypical parakeratotic (SAPK) cells. HCG of small parakeratotic cells showing cohesive groups of hyperchromatic small cyanophilic atypical parakeratotic (CAPK) cells with ill-defined to well-defined cell borders, which are straight with angulations better seen at periphery. N/C ratio is higher. Chromatin is smudgy. Some cells may show koilocytic space around nuclei. Adopted from CytoJournal open access. Chivukula M, Shidham VB. Cytojournal 2006;3:14.

- ASC-H. Atrophic smear with single atypical squamous metaplastic cells (ASMs): Parabasal cells can also mimic single-cell pattern “litigation cells” of HSIL. Atrophic cells lack nuclear dysplastic features. Compared to HSIl cells with hyperchromatic nuclei with irregular nuclear membrane and higher N/C ratio.
Similar to other squamous categories, HPV status plays a major role in determining the immediate risk of HSIL in cases with ASC-H interpretation. HPV positivity in ASC-H conveys a higher risk of precancerous lesions, that is, HSIL compared to HPV-positive ASC-US and LSIL interpretation, but still carries lower risk than HSIL. In fact, the immediate risk of HSIL in HR-HPV-positive ASC-H cases is closer to HSIL as compared to other SIL interpretations [Table 2].
American Society for Colposcopy and Cervical Pathology (ASCCP) 2019 shifted the management style with “equal management for equal risks” principal utilized comprehensively as per the recent most guidelines. Specifically, management options are based on a patient’s risk of high-grade dysplasia (HSIL/CIN-III), regardless of the method used. For example, clinicians could start treatment directly for patients with PAP results of HR-HPV-positive ASC-H, while HR-HPV-negative LSIL patients have to be followed by colposcopy/biopsy before initiating treatment plans.[13]
Cytomorphological features of ASC-H: [Table 3]
Nuclear size 2.5–3 times ICN
Mild increase in N/C ratio
Mild hyperchromasia and nuclear irregularity
Other features overlapping with HSIL but not unequivocal
Differential Diagnosis of ASC-H
Please see differential diagnosis of HSIL discussed above and various cytomorphological patterns reported with reference to different biopsy outcomes [Table 3].
LSIL-H (LSIL, CANNOT RULE OUT HSIL)
ASC-H is not a definitive interpretation but is related to an increased risk of higher grade lesions on biopsy.[22] On the contrary, LSIL is a definitive interpretation with relatively lower prevalence of high-grade lesion on subsequent biopsy. At present, the reporting pattern to communicate this concurrence varies among different cytopathology laboratories.
Although not covered under The Bethesda System, LSIL-H is an interpretation used for cervical cytology exhibiting unequivocal features of LSIL, but is also admixed with some cells suspicious for, but not sufficient to be interpreted as HSIL. A few studies have shown that LSIL-H cases overlapped with LSIL and ASC-H.[6,18] A management algorithm comparable to ASC-H and HSIL appears to be appropriate in LSIL-H cases. Histopathologic correlation studies have shown that LSIL-H category lies between LSIL and ASC-H in relation to HSIL on histology and in terms of HR HPV positivity rate.[18]
This concurrence has been identified recently by many laboratories, but it’s reporting is not addressed in the 2001 Bethesda System terminology (Bethesda 2001).[22-24] Lack of standardized method of reporting, however, may affect proper application of ASCCP guidelines based on Bethesda 2001.[25,26] A few studies explain the cytomorphology of this category with clinical significance and suggest management algorithm which is closer to ASC-H (but with definitive statistical inclusion in LSIL category).[5,18] The study also recommends the management algorithm with reference to ASCCP guidelines [Figures 30 and 31].[18,27]

- Based on the recent ASCCP management guideline principle of ‘equal risk = equal management’, if the chance of HSIL on biopsy for LSIL-H and ASC-H are same as HSIL, the management algorithm of LSIL-H should be comparable to the management of ASC-H.

- The ASCCP 2006 management guidelines for ASC-H have not been changed in the most recent guidelines (2019). Following ASC-H interpretation, two negative specimens are needed to return to routine screening, immediate biopsy, and a negative cytology of negative HPV testing within a year. Adopted from J Low Genit Tract Dis. 2007 Oct;11(4):201-22 (28).
In some institutions, for statistical and quality assurance reasons, we report this concurrence under definitive interpretation as LSIL-H with comment that LSIL cells are present with ASC-H cells. Some interpreters may choose to combine the associated ASC-H component with LSIL and report the combination as HSIL, which may lead to a potentially high false positivity rate. Others essentially follow a reverse approach and report it as ASC-H with the comment that LSIL cells are also present.
Rarely, ASC-H component may be downgraded to ASCUS with the final interpretation as LSIL, with potentially false-negative results for high-grade lesions. LSIL-H interpretation should be reported with citation of appropriate reference which provides management algorithm [Figures 30 and 31].[17,27]
Acknowledgments
Authors thank Kathy Rost for the secretarial support during this project and Janavi Kolpekwar for copy editing expertise.
LIST OF ABBREVIATIONS (In alphabetic order)
ASC – Atypical squamous cell
ASM – Atypical squamous metaplastic cell
ASCCP – American Society for Colposcopy and Cervical Pathology
ASC-H – Atypical squamous cells, HSIL cannot be excluded
ASC-US – Atypical squamous cells of undetermined significance
CFC – Chronic follicular cervicitis
CIN – Cervical intraepithelial neoplasia
HCG - hyperchromatic crowdwd groups
HPV – Human papilloma virus
HR-HPV – High-risk HPV
HSIL – High-grade squamous intraepithelial lesion
ICN – Intermediate cell nuclei
LBC – Liquid-based cytology
LSIL – Low-grade squamous intraepithelial lesion
LSIL-H – LSIL, cannot rule out HSIL
N/C ratio – Nuclear/cytoplasmic ratio
SAPK – Small atypical parakeratotic.
References
- U. S. Cancer Statistics Data Visualizations Tool, Based on 2019 Submission Data (1999-2017): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Available from: https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/ [Last accessed on 2020 Jun 27]
- [Google Scholar]
- The lower anogenital squamous terminology standardization project for HPV-associated lesions. Int J Gynecol Pathol. 2013;32:76-115.
- [CrossRef] [PubMed] [Google Scholar]
- The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-9.
- [CrossRef] [PubMed] [Google Scholar]
- A common clinical dilemma: Management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-70.
- [CrossRef] [PubMed] [Google Scholar]
- The anal pap smear: Cytomorphology of squamous intraepithelial lesions. Cytojournal. 2005;2:4.
- [CrossRef] [PubMed] [Google Scholar]
- Low-grade squamous intraepithelial lesion, cannot exclude high-grade: TBS says Don't Use It! Should I really stop it? Cytojournal. 2017;14:13.
- [CrossRef] [Google Scholar]
- ASC-H in pap test-definitive categorization of cytomorphological spectrum. Cytojournal. 2006;3:14.
- [CrossRef] [PubMed] [Google Scholar]
- HPV-ISH-negative invasive cervical squamous cell carcinoma: Histologic and Pap test results. Acta Cytol. 2019;63:417-23.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 2017;4:85-9.
- [CrossRef] [PubMed] [Google Scholar]
- HPV-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG. 2015;122:119-27.
- [CrossRef] [PubMed] [Google Scholar]
- The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes. 2015 Softcover ISBN 978-3319-11073-8
- [CrossRef] [Google Scholar]
- Impact of the national breast and cervical cancer early detection program on cervical cancer mortality among uninsured low-income women in the U.S. 1991-2007. Am J Prev Med. 2014;47:300-8.
- [CrossRef] [PubMed] [Google Scholar]
- 2019 ASCCP risk-based management consensus guidelines: Methods for risk estimation, recommended management, and validation. J Low Genit Tract Dis. 2020;24:90-101.
- [CrossRef] [PubMed] [Google Scholar]
- Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24:132-43.
- [CrossRef] [PubMed] [Google Scholar]
- Eosinophilic dysplasia of the cervix: A newly recognized variant of cervical squamous intraepithelial neoplasia. Am J Surg Pathol. 2004;28:1474-84.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical condylomatous atypia and its relationship to cervical neoplasia. Am J Clin Pathol. 1981;76:255-62.
- [CrossRef] [PubMed] [Google Scholar]
- Cyanophilic Small Atypical Parakeratotic Cells. 2021. Available from: https://www.scholar.google.com/scholar?hl=enandas_sdt=0%2c23andq=cyanophilic+small+atypical+parakeratotic+cells+in+thinprep+cervical+cytology%3a+a+pitfall+leading+to+hsil+or+asc-h+misinterpretation and btng. [Last accessed on 2021 May 05]
- [Google Scholar]
- Should LSIL with ASC-H (LSIL-H) in cervical smears be an independent category? A study on SurePath specimens with review of literature. Cytojournal. 2007;4:7.
- [CrossRef] [PubMed] [Google Scholar]
- Litigation cells: Their incidence and classification in gynecologic smears. Diagn Cytopathol. 2002;26:345-8.
- [CrossRef] [PubMed] [Google Scholar]
- Microglandular hyperplasia has a cytomorphological spectrum overlapping with atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H) Diagn Cytopathol. 2004;30:57-61.
- [CrossRef] [PubMed] [Google Scholar]
- Human papillomavirus testing and reporting rates: Practices of participants in the college of American pathologists interlaboratory comparison program in gynecologic cytology in 2006. Arch Pathol Lab Med. 2008;132:1290-4.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol. 2000;157:1055-62.
- [CrossRef] [Google Scholar]
- Types of human papillomavirus revealed in cervical adenocarcinomas after DNA sequencing. Oncol Rep. 2003;10:175-9.
- [CrossRef] [PubMed] [Google Scholar]
- Papillomavirus life cycle organization and biomarker selection. Dis Markers. 2007;23:297-313.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-53.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer statistics for the year 2020: An overview. Int J Cancer 2021
- [CrossRef] [PubMed] [Google Scholar]
- 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201-22.
- [CrossRef] [PubMed] [Google Scholar]
- 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201-22.
- [CrossRef] [PubMed] [Google Scholar]








