Translate this page into:
The endometrial cancer detection using non-invasive hypermethylation of CDO1 and CELF4 genes in women with postmenopausal bleeding in Northwest China

*Corresponding authors: Zhaoyan Meng, Department of Obstetrics and Gynecology, Gansu Provincial Maternity and Child-care Hospital, Lanzhou, China. 540486046@qq.com
Qing Liu, Department of Obstetrics and Gynecology, Gansu Provincial Maternity and Child-care Hospital, Lanzhou, China. 230547180536@qq.com
-
Received: ,
Accepted: ,
How to cite this article: Bingxin C, Jun D, Yishan W, Zhijie L, Yan W, Liuyu L, et al. The endometrial cancer detection using noninvasive hypermethylation of CDO1 and CELF4 genes in women with postmenopausal bleeding in Northwest China. CytoJournal. 2024;21:15. doi: 10.25259/Cytojournal_78_2023
Abstract
Objective:
The objective of this study was to verify the clinical predictive performance of methylated cysteine dioxygenase type 1 (CDO1m) and CUGBP Elav-like family member 4 (CELF4m) in endometrial cancer (EC) women with postmenopausal bleeding (PMB).
Material and Methods:
A single-center, prospective, and case–control study was conducted in the Gansu Provincial Maternity and Child-care Hospital with 138 female postmenopausal patients enrolled in 2022. All patients underwent body mass index (BMI) detection, transvaginal ultrasonography (TVUS) detection, carbohydrate antigen 125 detection, and the cervical exfoliated cell CDO1/CELF4 gene methylation detection to analyze the sensitivity, specificity, and accuracy of different screening tests statistically with the biopsy and/or dilation and curettage (D&C) pathological diagnosis under hysteroscopy as the gold standard.
Results:
There was no significant difference in age between the EC group and the non-EC group, P = 0.492. Using quantitative polymerase chain reaction (qPCR) technology, we validated the CDO1 and CELF4 methylation detection with 87.5% sensitivity and 95.9% specificity as a useful strategy for the triage of women with PMB for the detection of EC. In addition, 100% of type II EC (n = 6) were positively detected by the CDO1 or CELF4 methylation test.
Conclusion:
The CDO1 and CELF4 methylation test with high specificity as an auxiliary diagnostic tool or alternative method provides physicians with a reference to distinguish between benign and malignant tumors in women with postmenopausal bleeding, to justify the necessity of using invasive methods to confirm diagnosis.
Keywords
Endometrial cancer
Methylation
CDO1
CELF4
Postmenopausal bleeding
INTRODUCTION
Endometrial cancer (EC) stands as the most prevalent gynecologic malignancy of the female genital tract in developed countries. It ranks as the sixth most common cancer in women, with 417,000 new diagnoses globally in 2020, and is anticipated to rise over the next 10 years.[1,2] The increased incidence of EC is primarily attributed to the rising prevalence of obesity, aging, early menarche, late menopause, diabetes, metabolic syndrome, nulliparity, and other associated risk factors.[3] The survival rate of EC patients is significantly contingent on the stage at diagnosis, and any delay in diagnosis can exert substantial adverse effects on survival.[4] As per the International Federation of Gynecology and Obstetrics (FIGO) diagnostic staging, the 5-year survival rate for EC patients decreases from 85% in stage I to 25% in stage IV.[5] Consequently, lower mortality and extended disease-free survival appear to correlate with early diagnosis, underscoring the pivotal role of high-precision detection and screening programs.[6]
Postmenopausal bleeding (PMB) affects up to 10% of women, constituting about two-thirds of gynecological visits for postmenopausal women and serving as a common clinical symptom of EC.[7] Given the prevalence of PMB and its association with both EC and benign diseases, it is crucial to accurately quantify the cancer risk in women experiencing PMB.[8] The PMB symptom is highly sensitive, even in the early stages of EC, and the American College of Obstetricians and Gynecologists recommends that women with PMB should be promptly evaluated.[9] Women with PMB should undergo standardized clinical testing procedures, including the use of transvaginal ultrasonography (TVUS) to assess endometrial thickness (ET), which is the most frequently used initial investigation. This should be followed by invasive hysteroscopy, endometrial biopsy, and/or dilation and curettage (D&C). It is important to note that the inspection and results may vary widely among different settings.[9,10]
For the anatomical continuity between the uterine cavity and the cervix, brushing the exfoliated cells of the cervix can also collect abnormal exfoliated endometrial cells to assess signs of endometrial disease. Routine cervical cytology can detect EC in nearly half of the patients, particularly among experienced cytopathologists.[11] Recent literature indicates that specific deoxyribonucleic acid (DNA) methylation markers in cervical specimens surpass cytological examination in detecting EC.[12] Two hypermethylated candidate genes, namely, cysteine dioxygenase type 1 (CDO1) and CUGBP Elav-like family member 4 (CELF4), were identified in patients with EC based on existing literature and data from the cancer genome atlas database.[13]
The inverse relationship between the incidence of EC, its mortality rate, and the socioeconomic index is particularly concerning. Women in low-income and middle-income countries (LMICs) are significantly more prone to succumb to EC than their counterparts in high-income countries, indicative of inadequate access to timely evidence-based medical care and high-quality early diagnosis of EC.[14] The gross domestic product of Gansu Province in China is ranked 27th out of the 31 provinces in the country. Due to the geographical location in the northwest and the distinctive characteristics of people’s diet and lifestyle, EC is escalating rapidly, especially among postmenopausal women. At present, there exists an urgent need to identify a noninvasive, accurate, and cost-effective EC screening method for outpatients with PMB and validate its clinical feasibility.
This study aims to validate the clinical predictive efficacy of methylated CDO1 (CDO1m) and methylated CELF4 (CELF4m) in women with EC presenting with PMB syndromes in a single hospital in Gansu, China. We assessed the discriminatory potential of CDO1m/CELF4m in distinguishing between benign and malignant tumors in cases of EC and non-EC. Finally, we compared their methylation performance with the non-invasive capabilities of TVUS, body mass index (BMI), carbohydrate antigen 125 (CA125), and other relevant factors.
MATERIAL AND METHODS
Study design and sample collection
This prospective cross-sectional study employs a noninvasive clinical method to validate the clinical efficacy of detecting CDO1m and CELF4m in a single hospital. The study received approval from the Institutional Review Board (IRB) of the Ethics Committee at Gansu Provincial Maternity and Child-care Hospital, Gansu, China (IRB No.: 2022-GSFY-49). PMB patients were enrolled and provided informed consent according to the Standards for Reporting of Diagnostic Accuracy in clinics.[15] The inclusion criteria were as follows: Postmenopausal women experiencing symptoms of vaginal bleeding. Exclusion criteria encompassed women with a prior diagnosis of cancer in any organ, those who had undergone hysterectomy, individuals who did not complete all the required examinations, those receiving hormonal therapy for menopausal symptoms within the past year, and those undergoing immunosuppressive therapy within the year preceding enrollment.
The cervical brush was inserted into the cervix and swirled clockwise 5–6 times to collect exfoliated cells, which would be preserved in the PreservCyt solution (Hologic, Bedford, MA, USA) in the gynecology clinic. Patients’ information, including TVUS, hysteroscopy, endometrial biopsy, and/or D&C, was collected. The results were confirmed through biopsy or surgical histopathology.
CDO1 and CELF4 hyper-methylation detection
Methylation detection was conducted in a certified DNA laboratory, with operators and staff members blinded to the clinical information. CDO1 and CELF4 methylation detection (CISENDO® test) utilized the same specimens collected in the PreservCyt solution.
Genomic DNA (gDNA) was extracted from the exfoliated cervical sample using the JH-DNA Isolation and Purifying kit (OriginPoly Bio-Tec Co., Ltd., Beijing, China) per the manufacturer’s instructions. The DNA concentration was quantified using the NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, DE, USA). Briefly, 500 ng of gDNA were subjected to bisulfite conversion using JHDNA Methylation-Lightning MagPrep (OriginPoly Bio- Tec Co., Ltd., Beijing, China). Subsequently, the levels of CDO1m and CELF4m were determined using the CISENDO® DNA Methylation Detection Kit for EC (Real-time polymerase chain reaction) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control (OriginPoly Bio-Tec Co., Ltd., Beijing, China) by the ABI 7500 real-time PCR System platform (Life Technology, Foster City, CA, USA). The hypermethylation level of the CDO1/CELF4 gene was determined by the difference between the two ΔCp values (ΔCpC1 = Cp CDO1-Cp GAPDH and ΔCpC4= Cp CELF4-Cp GAPDH). A positive result of the CISENDO® methylation test is defined as either ΔCpC1 ≤8.4 or ΔCpC4 ≤8.8.
Demographic characteristics and clinical assessment
Demographic characteristics and clinical evaluation data on age, race, weight, height, waist circumference, heart rate, blood pressure, current or past occupation, drug treatment, abnormal uterine bleeding, and history of gynecological diseases were collected for each participant woman. For EC patients, the EC tissue type, FIGO grade and stage, and tumor history were also recorded. BMI was calculated as weight in kilograms divided by height in meters squared. The level of CA125 in serum was detected using the CA125 ELISA kit (Coibo Bio Co. Shanghai, China) according to the manufacturer’s instructions. ET was measured by TVUS.
Statistical analysis
The participants were characterized using descriptive statistics. The ΔCp values of CDO1m and CELF4m were compared using the Mann–Whitney U-test. Differences in proportions between independent groups were compared using the Wald (continuity corrected) test. Receiver operating characteristic (ROC) curves were used to evaluate the areas under the curve (AUCs) of BMI, ET, CA125, CDO1m, and CELF4m. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for detecting EC and their confidence intervals were calculated. The Statistical Package for the Social Sciences 27.0 (IBM Corp., Armonk, NY, USA) and R (version 4.1.2, Vienna, Austria) were used to do the statistics. The methylation cutoff value for the final clinical statistical analysis was based on the CISENDO® test defined as either ΔCpC1 ≤8.4 or ΔCpC4 ≤8.8. All differences were considered two-sided and statistically significant at P < 0.05.
RESULTS
Participant characteristics
The enrollment flow charts and participant characteristics are reported in Figure 1 and Table 1, respectively. A total of 138 women (median [range] 58.0 [54.0–66.0] years) were enrolled and analyzed in the study: 40 (29.0%) with EC (58.0 [55.9– 66.4] years) and 98 (71.0%) with no EC (58.0 [53.0–66.0] years). No significant age difference was observed between the EC and non-EC groups (P = 0.492) in Table 2. Among the 40 women with EC, 32 (80%) had stage I disease, 1 (2.5%) had stage II, 6 (15%) had stage III disease, and 1 (2.5%) had stage IV, according to the FIGO classification. Regarding tumor grading, 18 (45.0%) were G1, 12 (30.0%) were G2, and 10 (25.0%) were G3. There were 34 (85.0%) type I and 6 (15.0%) type II EC. Of the six patients with type II EC, two had clear cell carcinoma, two had serous carcinoma, and two had mixed carcinoma (endometrioid carcinoma and sarcoma, and serous tumor and sarcoma).
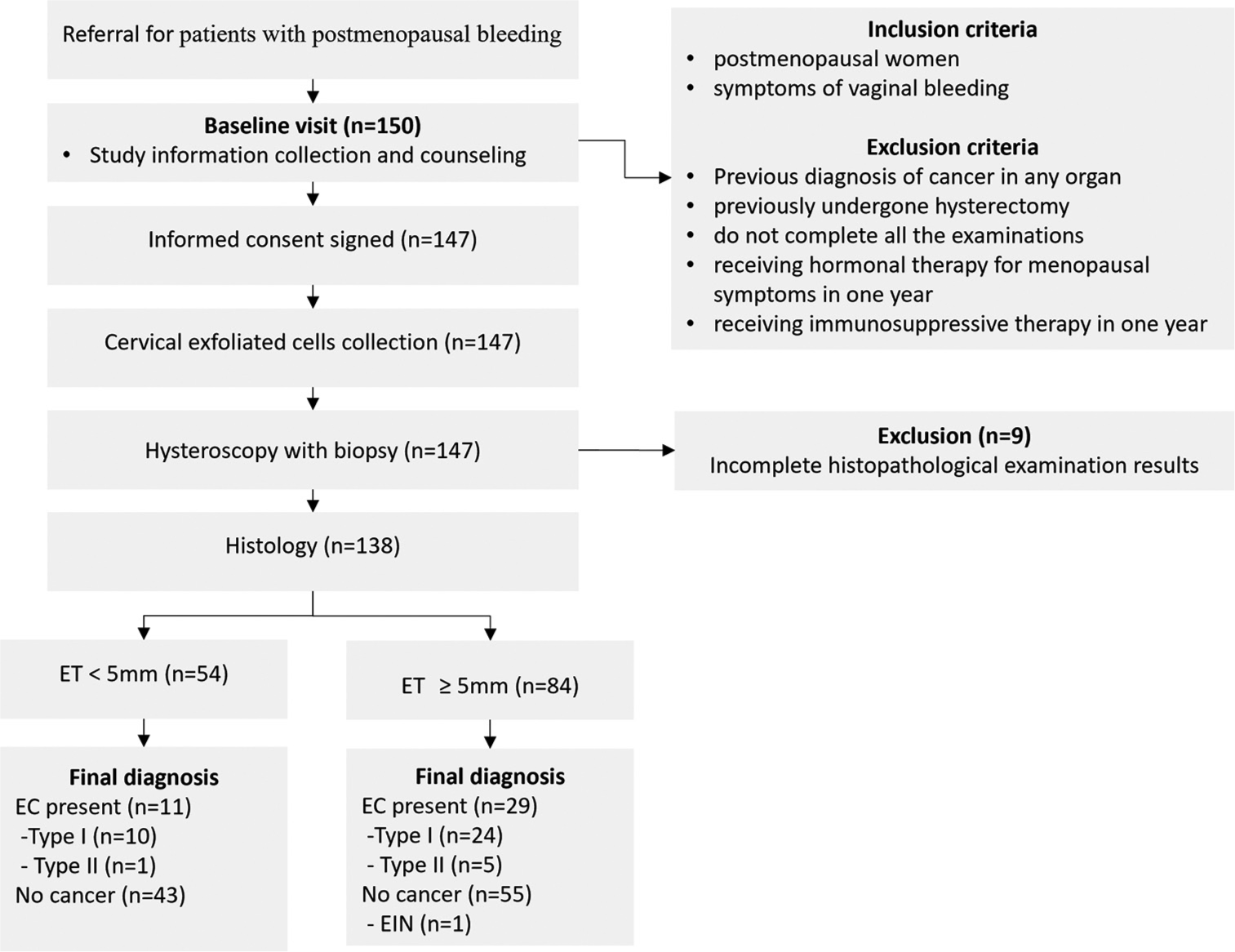
- Diagram showing the flow of participants according to Standards for Reporting Diagnostic accuracy studies (STARD). (ET: Endometrial thickness, EC: Endometrial cancer, n: number, EIN: Endometrial intraepithelial neoplasia).
| Characteristic | Frequency | Percent |
|---|---|---|
| Age, median (IQR) | 58.0 (54.0–66.0) | |
| BMI (kg/m2), median (IQR) | 24.5 (22.7–26.8) | |
| Endometrial thickness (mm), median (IQR) | 6.0 (3.0–10.0) | |
| CA125 (u/mL), median (IQR) | 14.14 (9.00–23.50) | |
| CDO1m, median (IQR) | 15.94 (9.03–17.92) | |
| CELF4m, median (IQR) | 13.50 (9.61–17.27) | |
| Pathology | ||
| Non-endometrial cancer | 98 | 71.0 |
| Endometrial cancer | 40 | 29.0 |
| IA | 24 | 60.0 |
| IB | 8 | 20.0 |
| II | 1 | 2.5 |
| III | 6 | 15.0 |
| IV | 1 | 2.5 |
| Tumor grading | ||
| G1 | 18 | 45.0 |
| G2 | 12 | 30.0 |
| G3 | 10 | 25.0 |
| Subtype | ||
| Type I | 34 | 85.0 |
| Type II | 6 | 15.0 |
IQR: Interquartile range, G: Tumor grading, BMI: Body mass index, CA125: Carbohydrate antigen 125, CDO1m: Methylated cysteine dioxygenase type 1, CELF4m: Methylated CUGBP Elav-like family member 4, G1: Grade 1, G2: Grade 2, G3: Grade 3.
| Characteristic | Non-EC (n=98) | EC (n=40) | P |
|---|---|---|---|
| Age, median (IQR) | 58.0 (53.0–66.0) | 58.0 (55.9–66.4) | 0.492 |
| BMI (kg/m2), median (IQR) | 24.1 (22.0–26.4) | 25.8 (23.2–28.0) | 0.004 |
| ET (mm), median (IQR) | 5.0 (3.0–9.0) | 10.0 (3.5–19.0) | 0.001 |
| CA125 (u/mL), median (IQR) | 12.42 (8.30–19.80) | 18.90 (13.25–36.35) | <0.001 |
| CDO1m, median (IQR) | 17.15 (14.00–18.41) | 5.55 (2.65–8.27) | <0.001 |
| CELF4m, median (IQR) | 16.22 (11.58–17.66) | 6.03 (3.39–11.78) | <0.001 |
Mann-Whitney U-test, IQR: Interquartile range, P:P value, BMI: Body mass index, ET: Endometrial thickness, CA125: Carbohydrate antigen 125, CDO1m:Methylated cysteine dioxygenase type 1, CELF4m: Methylated CUGBP Elav-like family member 4, EC: Endometrial cancer
Figure 2 showed that the AUCs from high to low were 0.932 (0.878–0.985) for CDO1m, 0.863 (0.789–0.938) for CELF4m, 0.704 (0.610–0.797) for CA125, 0.672 (0.553–0.792) for ET, and 0.656 (0.559–0.752) for BMI, respectively.
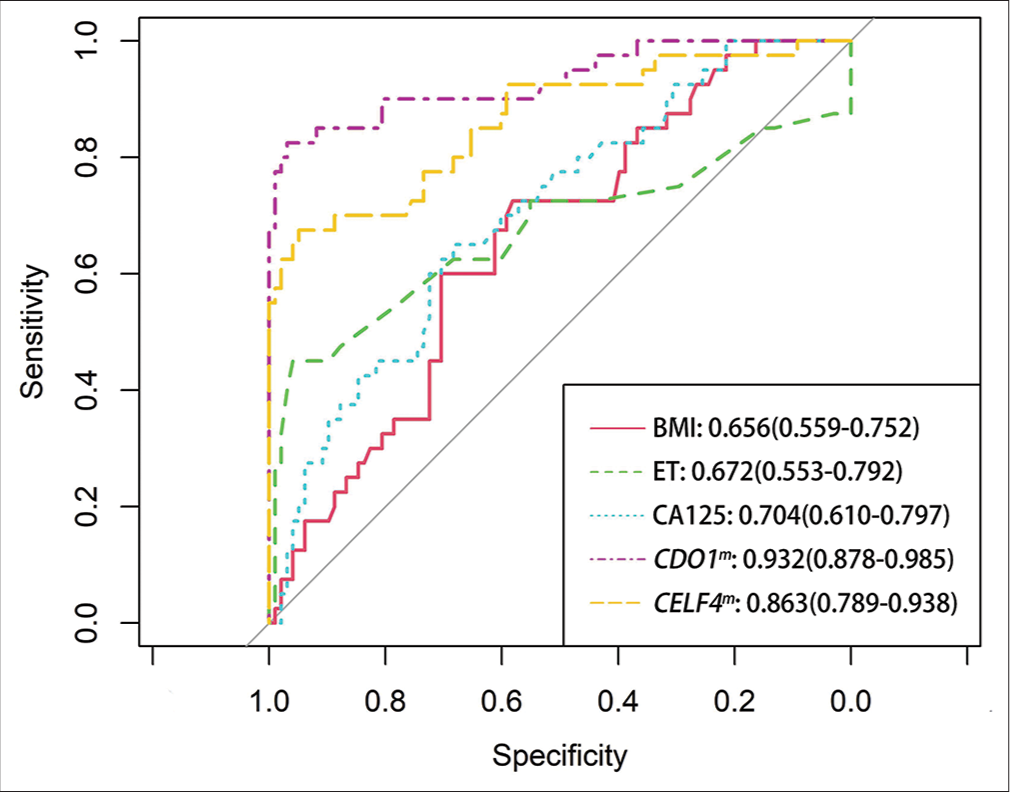
- Receiver operating characteristic (ROC) curve analysis of different tests for EC. (EC: Endometrial cancer, BMI: Body mass index, ET: Endometrial thickness, CA125: Carbohydrate antigen 125, CDO1m: Methylated cysteine dioxygenase type 1, and CELF4m: Methylated CUGBP Elav-like family member 4. The area under the ROC area under the curve of each test was calculated for the diagnosis of EC).
Characteristics of endometrial carcinoma and nonendometrial carcinoma
Compared with the non-EC group, women with EC had higher detection values in BMI, ET, and CA125, but had lower values in CDO1m and CELF4m [Table 2 and Figure 3]. The BMI values in non-EC and EC were 24.1 (22.0–26.4) and 25.8 (23.2–28.0), respectively. The ET values in non-EC and EC were 5.0 (3.0–9.0) and 10.0 (3.5–19.0), respectively. The CA125 values in non-EC and EC were 12.42 (8.30–19.80) and 18.90 (13.25–36.35), respectively.
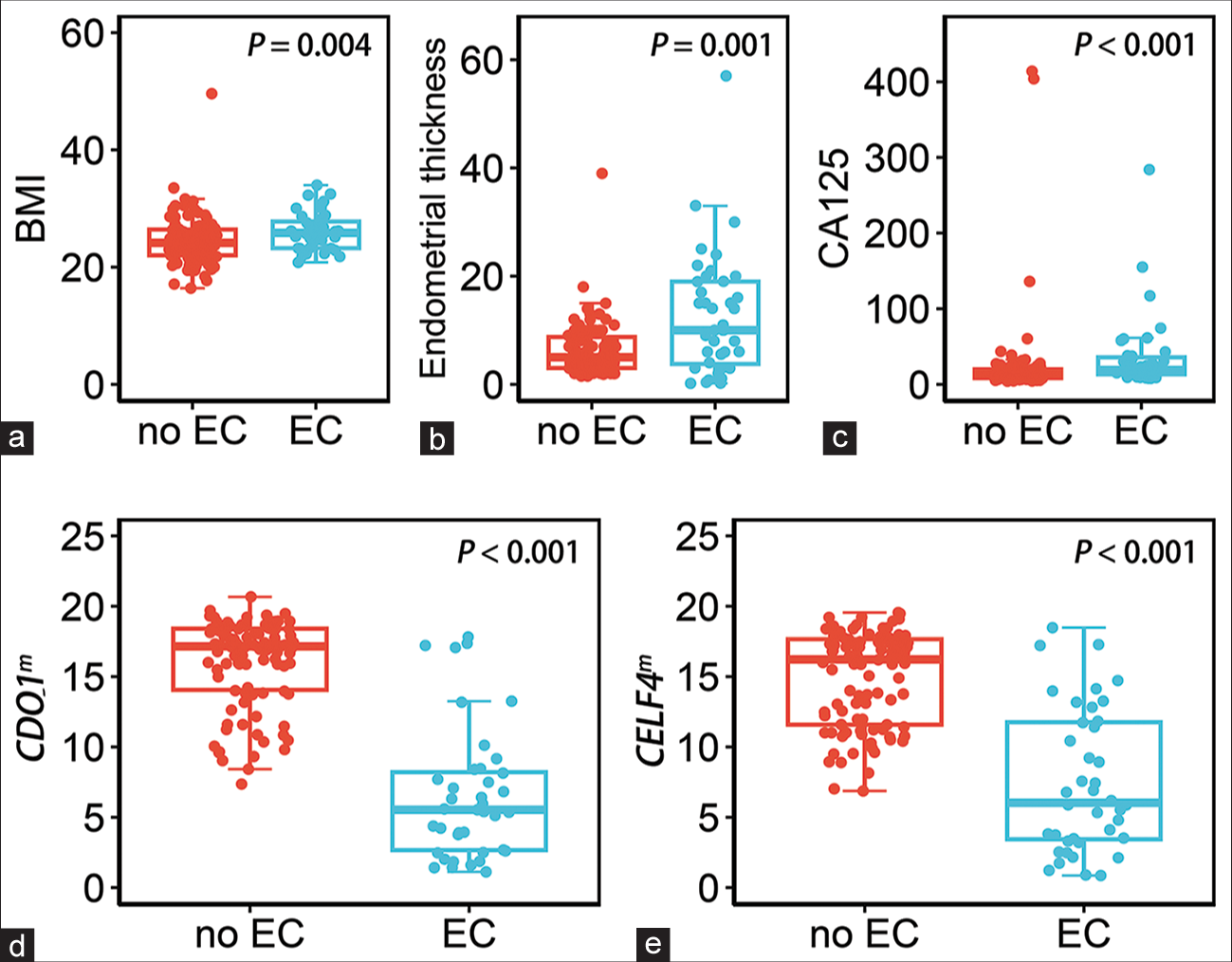
- The dot plots show the distribution of the five testing in non-endometrial cancer (EC) and endometrial cancer (EC) groups, the median and interquartile ranges are depicted by boxes. (a) Distribution of the value of BMI, ≥25 kg/m2. (b) Distribution of the value of endometrial thickness, ≥5 mm. (c) Distribution of the value of CA125, >35 u/mL. (d) Distribution of the value of methylated CDO1, ΔCp ≤ 8.4. (e) Distribution of the value of methylated CELF4, ΔCp ≤ 8.8. (EC: Endometrial cancer, BMI: Body mass index, CA125: Carbohydrate antigen 125, CDO1m: Methylated cysteine dioxygenase type 1, and CELF4m: Methylated CUGBP Elav-like family member 4).
In contrast, only the positive numbers of ET in EC patients and non-EC patients were not significantly different, based on the definition of clinical routine use in Table 3. The positive frequency of CDO1m and CELF4m was 77.5% (63.9–89.7) and 62.5% (46.9–77.3) in the EC group; 1.0% (0.0–3.3) and 3.1% (0.0–6.8) in the non-EC group, P < 0.001, respectively. Among these tests, the top three largest differences between the EC group and non-EC group were 76.5 (61.6–91.3) for CDO1m, 59.4 (42.3–76.6) for CELF4m, and 26.2 (6.8–45.6) for BMI, which are higher than ET (recommended in the guideline).
| Characteristic | Non-EC (N=98) n, %±SE |
EC (N=40) n, %±SE |
Difference (95% CI) | P-value |
|---|---|---|---|---|
| Definition of clinical routine use1 | ||||
| BMI (≥25 kg/m2) | 38, 38.8±4.9 | 26, 65.0±7.5 | 26.2 (6.8–45.6) | 0.007 |
| ET (≥5 mm) | 55, 56.1±5.0 | 29, 72.5±7.1 | 16.4 (−2.4–35.1) | 0.091 |
| CA125 (>35 u/mL) | 6, 6.1±2.4 | 11, 27.5±7.1 | 21.4 (5.0–37.8) | 0.009 |
| CDO1m (ΔCp C1≤8.4) | 1, 1.0±1.0 | 31, 77.5±6.6 | 76.5 (61.6–91.3) | <0.001 |
| CELF4m (ΔCp C4≤8.8) | 3, 3.1±1.7 | 25, 62.5±7.7 | 59.4 (42.3–76.6) | <0.001 |
| Definition of this study cohort2 | ||||
| BMI (kg/m2) ≥25.52 kg/m2 | 29, 29.6±4.6 | 24, 60.0±7.7 | 30.4 (11.0–49.8) | 0.001 |
| ET (≥8 mm) | 31, 31.6±4.7 | 25, 62.5±7.7 | 30.9 (11.5–50.2) | 0.001 |
| CA125 (>16 u/mL) | 31, 31.6±4.7 | 26, 65.0±7.5 | 33.4 (14.2–52.5) | <0.001 |
| CDO1m (ΔCp≤10.2) | 8, 8.2±2.8 | 34, 85.0±5.6 | 76.8 (62.8–90.9) | <0.001 |
| CELF4m (ΔCp≤10.47) | 11, 11.2±3.2 | 28, 70.0±7.2 | 58.8 (41.5–76.1) | <0.001 |
1“Definition of clinical routine use” is based on the guidelines, clinical use behavior, and the cutoff value recommended by the kit. The positive result was defined: BMI≥25 kg/m2, ET≥5 mm, CA125>35 u/mL, CDO1m, ΔCp C1≤8.4, and CELF4m, ΔCp C4m≤8.8. 2“Definition of this study cohort” is the best cutoff value determined by the AUC calculation of this clinical study. The positive result was defined: BMI≥25.52 kg/m2, ET≥8 mm, CA125>16 u/mL, CDO1m, ΔCp C1≤10.2, and CELF4m, ΔCp C4m≤10.47. N: Total numbers of the group, n: number, %: Percentage, SE: Standard error, CI: Confidence intervals, EC: Endometrial cancer, BMI: Body mass index, ET: Endometrial thickness, CA125: Carbohydrate antigen 125,CDO1m: Methylated cysteine dioxygenase type 1, CELF4m: Methylated CUGBP Elav-like family member 4,
The positive number and percentage of BMI and ET of this study cohort decreased compared to this study cohort, while CA125, CDO1m, and CELF4 m in this study increased [Table 3]. These results indicate that the positive cutoff values in the cohort for BMI and ET are higher than those recommended in the guidelines. The positive cutoff values of CA125, CDO1m, and CELF4 m are lower than the recommended values in the CISENDO® DNA Methylation Detection Kit [Table 3].
Clinical performance of different testing for EC detection
Table 4 reports the diagnostic performance for each test when applied to the study. In the single test, CDO1m had the best sensitivity (77.5 [61.5–89.2]) and the best specificity of (99.0 [94.4–100.0]). In the combined testing, CDO1m or ET had the highest sensitivity of 95.0 (83.1–99.4), but the specificity is only 43.9 (33.9–54.2). The CDO1m or CELF4m combined test, without a missed diagnosis of type II EC, has the highest specificity of 95.9 (89.9–98.9) and a reasonable sensitivity of 87.5 (73.2–95.8), with10% sensitivity higher than CDO1m single testing. The highest PPV of positive CDO1m was 96.9 (81.4–99.5), while the best NPV of CDO1m/CELF4m/ET was 97.6 (85.4–99.7), respectively.
| Test | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|
| BMI (kg/m2) ≥ 25 kg/m2 | 26/40, 65.0 (48.3-79.4) |
60/98, 61.2 (50.8-70.9) |
26/64, 40.6 (32.8-48.9) |
60/74, 81.1 (73.2-87.1) |
0.631 (0.529-0.733) |
| ET ≥ 5 mm | 29/40, 72.5 (56.1-85.4) |
43/98, 43.9 (33.9-54.3) |
29/84, 34.5 (28.9-40.6) |
43/54, 79.6 (69.3-87.1) |
0.582 (0.479-0.685) |
| CA125 > 35 u/ml | 11/40, 27.5 (14.6-43.9) |
92/98, 93.9 (87.1-97.7) |
11/17, 64.7 (42.1-82.2) |
92/121, 76.0 (72.2-79.4) |
0.607 (0.497-0.717) |
| CDO1m ΔCp≤8.4 | 31/40, 77.5 (61.5-89.2) |
97/98, 99.0 (94.4-100.0) |
31/32, 96.9 (81.4-99.5) |
97/106, 91.5 (85.8-95.0) |
0.882 (0.803-0.962) |
| CELF4m ΔCp≤8.8 | 25/40, 62.5 (45.8-77.3) |
95/98, 96.9 (91.3-99.4) |
25/28, 89.3 (72.7-96.3) |
95/110, 86.4 (80.9-90.4) |
0.797 (0.701-0.894) |
| CDO1m or CELF4m | 35/40, 87.5 (73.2-95.8) |
94/98, 95.9 (89.9-98.9) |
35/39, 89.7 (76.9-95.8) |
94/99, 94.9 (89.2-97.7) |
0.917 (0.853-0.91) |
| CDO1m or Endometrial Thickness | 38/40, 95.0 (83.1-99.4) |
43/98, 43.9 (33.9-54.2) |
38/93, 40.9 (36.4-45.5) |
43/45, 95.6 (84.5-98.8) |
0.694 (0.606-0.782) |
| CELF4m or Endometrial Thickness | 36/40, 90.0 (76.3-97.2) |
41/98, 41.8 (31.9-52.2) |
36/93, 38.7 (34.1-43.5) |
41/45, 91.1 (79.7-96.4) |
0.659 (0.566-0.753) |
| CDO1m or CELF4m or Endometrial Thickness | 39/40, 97.5 (86.8-99.9) |
41/98, 41.8 (31.9-52.2) |
39/96, 40.6 (36.5-44.9) |
41/42, 97.6 (85.4-99.7) |
0.697 (0.610-0.783) |
PPV, positive predictive value; NPV, negative predictive value; AUC, the area under the Receiver operating characteristic (ROC) curve; BMI, body mass index; ET, endometrial thickness; CA125, carbohydrate antigen 125;CDO1m, methylated cysteine dioxygenase type 1; CELF4m, methylated CUGBP Elav-like family member 4;m, methylated.
DISCUSSION
The histological examination of endometrial specimens by invasive hysteroscopy or D&C remains the gold standard in the diagnosis of EC. Such a diagnosis is limited by sampling invasiveness, sampling error, repeated sampling, the experience of pathologists, and poor reproducibility of the histological examination. 20–36% of the negative results in D&C were due to sampling failure, especially in the corners of the endometrial cavity.[16] Thus, many patients undergo repeated invasive evaluation through endometrial sampling or D&C with an accuracy of about 60%, causing inconvenience, pain, stress, injury, and high cost. This makes it more challenging to implement EC diagnosis in economically underdeveloped areas.[17] Although there have been great efforts in non-invasive EC screening research, no reasonable methods or biomarkers have been validated to date, let alone in economically underdeveloped areas. Furthermore, some women consider this invasive diagnosis procedure embarrassing, which is another factor contributing to a late-stage diagnosis of EC.[18] The high survival rate of EC in the early stage highlights the importance of developing non-invasive detection and even self-sampling in the future.[19] It is crucial to develop and validate the non-invasive EC diagnosis with high-precision testing in outpatient or health centers in developing regions.
This study demonstrates that the CDO1 or CELF4 methylation test (CISENDO® test), which detects the DNA methylation status from cervical scraping cells, can be useful biomarkers for the triage of women with PMB. It quantitatively detects the hypermethylation levels of CDO1 or CELF4 genes in the cervical scraping samples of women with PMB syndrome. Using quantitative polymerase chain reaction technology, the CDO1 or CELF4 combination test can reach 87.5% sensitivity and 95.9% specificity as triage biomarkers to predict EC for women with PMB in hospital outpatient settings. In addition, 100% of type II EC cases (n = 6) were positively detected by the CDO1 or CELF4 methylation test.
According to a large-scale systematic review of 44 studies (including 1341 cases and 15998 controls), the cutoff value of 5 mm ET evaluated by TVUS for triage of suspected EC has 96.2% sensitivity, but the specificity is low (about 51.5%).[20,21] Thus, the high sensitivity would ensure that most cases of EC are captured, but the low specificity of TVUS may lead to many unnecessary invasive hysteroscopy follow-up procedures. In this study, the sensitivity of ET was 72.5%, which is 23.7% lower than that reported in the systematic review. We also found that 27.5% (n = 11/40) of EC patients had ET <5 mm, and 39.9% (n = 55/138) of non-EC patients had ET >5 mm, resulting in many false positives and false negatives, respectively. Therefore, TVUS may lead to too many invasive biopsy referrals and missed diagnoses. In conclusion, the CDO1 and CELF4 methylation tests outperformed TVUS in all clinical performance aspects for detecting EC [Table 4]. These results indicate that the testing of ET may still be limited by the experience of medical staff and instruments in the LMICs.
In addition to ET, many studies reported on the use of serum CA125 and cervical exfoliated cytology for non-invasive detection of EC. In this study, the lower sensitivity (27.5%) of CA125 in detecting EC is similar to that reported in previous literature, because CA125 is easily confused with benign diseases (such as adenomyosis, hysteromyoma, and endometriosis).[22] Therefore, guidelines do not recommend CA-125 for cancer screening. The previous proof-of-concept studies of noninvasive testing showed that EC can be distinguished from benign diseases by brushing exfoliated cervical cells, tampons, or vaginal self-samples for cell morphology, and genomic and epigenomic testing.[23,24] A meta-analysis of 45 studies (with a total of 6599 EC patients) showed that 45% (95% confidence interval [CI], 40–50%) of participants observed abnormal Pap test results before diagnosis or surgery of EC. This rate was significantly higher than the results for endometrioid subtypes, with 77% (66–87%) versus 44% (34–53%), P < 0.001. In conclusion, routine cervical cytology can detect EC in almost half of the patients, but it is more sensitive in patients with nonendometrioid histology or advanced cancer.[11]
Detection of EC methylation biomarkers using minimally invasive or non-invasive sampling devices such as Tao-brush, cervical brush, vaginal tampon, and self-sampling devices has been reported.[25,26] Liew et al. reported that hypermethylation BHLHE22/CDO1/HAND2 (87.0% sensitivity and 86.0% specificity) and BHLHE22/CDO1/TBX5 (89.1% sensitivity and 80.0% specificity) can differentiate benign and malignant endometrial lesions.[26] A validation study based on multi-hospitals in Asia showed that the sensitivity and specificity of methylated BHLHE22 and CDO1 in the detection of Pap samples from women with abnormal uterine bleeding were 92.5~92.9% (0.610-0.783) PPV, positive predictive value; NPV, negative predictive value; AUC, the area under the Receiver operating characteristic (ROC) curve; BMI, body mass index; ET, endometrial thickness; CA125, carbohydrate antigen 125; CDO1m, methylated cysteine dioxygenase type 1; CELF4m, methylated CUGBP Elav-like family member 4;m, methylated. and 71.5~73.8%, as a useful strategy for the triage of women with AUB for the detection of EC, respectively.[27] In this study, we validated the CDO1m and CELF4m test with 87.5% sensitivity and 95.9% specificity as a useful strategy for the triage of women with PMB for the detection of EC. Compared with other research results, the high specificity of CDO1m and CELF4m test is a reasonable method for diagnosis of EC in PMB patients.
This is the first study to validate the effectiveness of methylation test in routine clinical practice of PMB women in northwest China. The CDO1m and CELF4m combination test with high specificity as an auxiliary diagnostic tool or alternative method provides physicians with a reference to distinguish between benign and malignant tumors in women with PMB, to justify the necessity of using invasive methods to confirm diagnosis. Furthermore, the methylation test is a pre-invasive diagnostic assist or an alternative to reduce women’s fear or embarrassment and mitigate the possible risks of EC. The test results should be used in combination with the doctor’s assessment and the patient’s risk factors to guide the patient’s individualized management. If confirmed in subsequent studies embedded in PMB clinics, this novel methylation test for EC could transform clinical practice by accurately selecting women with malignant pathology for urgent diagnostic workups while safely reassuring. These results need to be reproduced in the future in large practical diagnostic studies of multiple clinics, which are designed to rapidly track symptomatic women. Therefore, based on the real-time PCR system widely used during the epidemic of COVID-19 in China, the test can provide real-time results in primary or secondary care in the coming future. Further, research should also evaluate whether methylation tests can distinguish different molecular profiles of EC in the real-world setting.
This study has limitations. First, the study does not indicate the potential confounding effect of other diseases such as diabetes and hypertension. Second, this study does not discuss the potential relationship between CDO1m and CELF4m results and different molecular profiles of EC. Finally, only a few selected patients in one hospital may not be representative of women in northwest China. In the future, multi-center research will be carried out, delving into the CDO1m and CELF4m methods from various perspectives, such as larger sample size, different populations, and diverse medical environments. This comprehensive exploration aims to eliminate biases inherent in single-center data, ensuring a more reliable description and application of the new diagnostic and therapeutic methods.
SUMMARY
This pioneering research has made a potential breakthrough in the early diagnosis of EC through the non-invasive detection of methylation of cervical scraping cells in Gansu, China. The CDO1 and CELF4 methylation test with high diagnostic accuracy, objectivity, and repeatability may become an effective triage test for women with PMB, or a screening test for asymptomatic women at risk of EC in the future.
AVAILABILITY OF DATA AND MATERIALS
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
AUC - Areas under the curve
BMI - Body mass index
CA125 - carbohydrate antigen 125
CDO1 - Cysteine dioxygenase type 1
CDO1m - CDO1 methylation detection
CELF4 - CUGBP Elav-like family member 4
CELF4m - CELF4 methylation detection
D&C - Dilation and curettage
EC - Endometrial cancer
ET - Endometrial thickness
FIGO - The International Federation of Gynecology and Obstetrics
PMB - Postmenopausal bleeding
ROC - Receiver operating characteristic
STARD - Standards for Reporting Diagnostic accuracy studies
TVUS - Transvaginal ultrasonography.
AUTHOR CONTRIBUTIONS
The manuscript was drafted by WY and CB. MZ and LQ leaded the study’s conceptualization and design. CB was mainly responsible for collecting and evaluating data, and DJ, LZ, WY and LL were involved in this process. CB, DJ, LZ, WY and LL participated in the evaluation of clinical data. WY and WL were mainly responsible for analyzing and interpretating the data, also participating in data and information visualization. LP, MZ and LQ revised the manuscript critically for important intellectual content and approved final version to be published. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the Gansu Provincial Maternity and Child-care Hospital, Gansu, China (IRB No.: 2022-GSFY-49), and it was conducted in accordance with the Declaration of Helsinki.
Written informed consent was obtained from all the participants prior to the publication of this study.
CONFLICT OF INTEREST
Authors WY, WL, and LP were employed by the company Beijing Origin-Poly Bio-Tec Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors in Gansu Provincial Maternity and Child-care Hospital declare they have no commercial or financial relationships that could be construed as a potential conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
This work was supported by the Lanzhou Science and Technology Project (No.2022-5-92) and Gansu Province Natural Science Foundation (No.23JRRA1380).
References
- Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial cancer trends by race and histology in the USA: Projecting the number of new cases from 2015 to 2040. J Racial Ethn Health Disparities 2016 doi: 10.1007/s40615-016-0292-2
- [CrossRef] [PubMed] [Google Scholar]
- Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565-74.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical wait time: A new health indicator in women with endometrial cancer. Gynecol Oncol. 2016;141:511-5.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term survival of endometrioid endometrial cancer patients. Arch Med Sci. 2010;6:937-44.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic evaluation of the endometrium in postmenopausal bleeding: An evidence-based approach. Maturitas. 2011;68:155-64.
- [CrossRef] [PubMed] [Google Scholar]
- Association of endometrial cancer risk with postmenopausal bleeding in women: A systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-22.
- [CrossRef] [PubMed] [Google Scholar]
- 734: The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic strategies for postmenopausal bleeding. Obstet Gynecol Int. 2010;2010:850812.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity of cervico-vaginal cytology in endometrial carcinoma: A systematic review and meta-analysis. Cancer Cytopathol. 2020;128:792-802.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med. 2018;10:eaap8793.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of methylation-driven genes prognosis signature and immune microenvironment in uterus corpus endometrial cancer. Cancer Cell Int. 2021;21:365.
- [CrossRef] [PubMed] [Google Scholar]
- The STARD statement for reporting studies of diagnostic accuracy: Explanation and elaboration. Clin Chem. 2003;49:7-18.
- [CrossRef] [Google Scholar]
- The ATAC adjuvant breast cancer trial in postmenopausal women: Baseline endometrial subprotocol data. BJOG. 2003;110:1099-106.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness and appropriateness in the application of office hysteroscopy. J Formos Med Assoc. 2019;118:1480-7.
- [CrossRef] [PubMed] [Google Scholar]
- Outpatient hysteroscopy: An observational study of patient acceptability. Medicina (Kaunas). 2004;40:1207-10.
- [Google Scholar]
- A simple cervicovaginal epigenetic test for screening and rapid triage of women with suspected endometrial cancer: Validation in several cohort and case/control sets. J Clin Oncol. 2022;40:3828-38.
- [CrossRef] [PubMed] [Google Scholar]
- British Gynaecological Cancer Society (BGCS) uterine cancer guidelines: Recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2022;270:50-89.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound detection of endometrial cancer in women with postmenopausal bleeding: Systematic review and meta-analysis. Gynecol Oncol. 2020;157:624-33.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of preoperative serum CA-125 levels in patients with uterine cancer: The Asian experience 2000 to 2012. Obstet Gynecol Sci. 2013;56:281-8.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated epigenomics analysis reveals a DNA methylation panel for endometrial cancer detection using cervical scrapings. Clin Cancer Res. 2017;23:263-72.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic values from integrated analysis of the nomogram based on RNA-binding proteins and clinical factors in endometrial cancer. Clin Med Insights Oncol. 2022;16 doi: 10.1177/11795549221123620
- [CrossRef] [PubMed] [Google Scholar]
- Combining copy number, methylation markers, and mutations as a panel for endometrial cancer detection via intravaginal tampon collection. Gynecol Oncol. 2020;156:387-92.
- [CrossRef] [PubMed] [Google Scholar]
- Combined genetic mutations and DNA-methylated genes as biomarkers for endometrial cancer detection from cervical scrapings. Clin Epigenet. 2019;11:170.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial cancer detection using a cervical DNA methylation assay (MPap) in women with abnormal uterine bleeding: A multicenter hospital-based validation study. Cancers (Basel). 2022;14:4343. doi: 10.3390/cancers14174343
- [CrossRef] [PubMed] [Google Scholar]








